Copyright
©The Author(s) 2015.
World J Clin Oncol. Dec 10, 2015; 6(6): 299-311
Published online Dec 10, 2015. doi: 10.5306/wjco.v6.i6.299
Published online Dec 10, 2015. doi: 10.5306/wjco.v6.i6.299
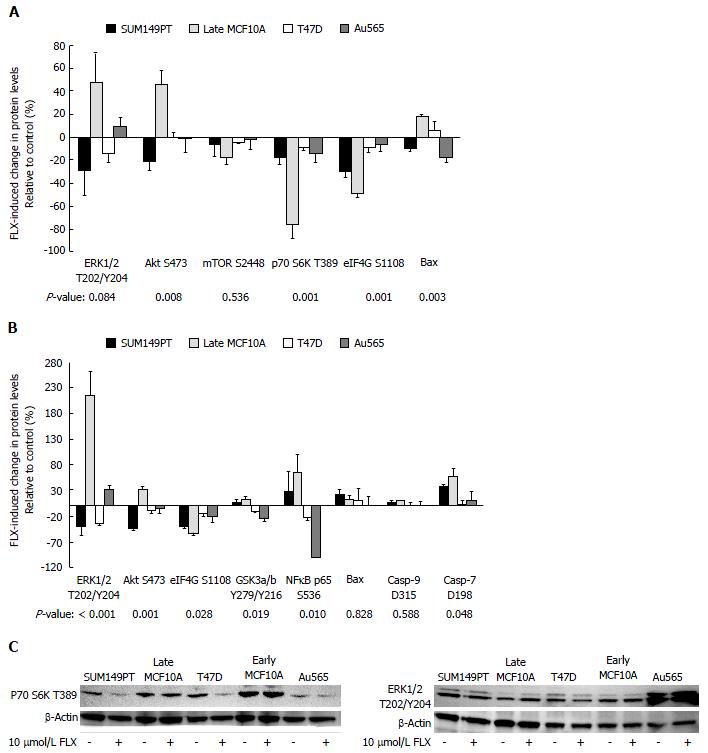
Figure 1 Expression levels of proteins along the phosphoinositide 3-kinase/Akt/mammalian target of rapamcyin, mitogen-activated protein kinase/extracellular signal-regulated kinase, and apoptosis pathways vary by cell lines.
Statistically significant and differentially expressed proteins, following (A) 24 h and (B) 48 h treatment of 10 μmol/L FLX, were indicated with stars; (C) Expression of few selected proteins across cell lines after 48 h of 10 μmol/L fluoxetine was confirmed by Western blotting. For detection of p70 S6K and ERK1/2 activation, cell extracts were loaded at 100 μg and 30 μg per lane, respectively. MEK: Mitogen-activated protein kinase; ERK: Extracellular signal-regulated kinase; FLX: Fluoxetine; p70 S6K: p70 S6 Kinase; GSK3: Glycogen synthase kinase 3; eIF4G: Eukaryotic translation initiation factor 4G.
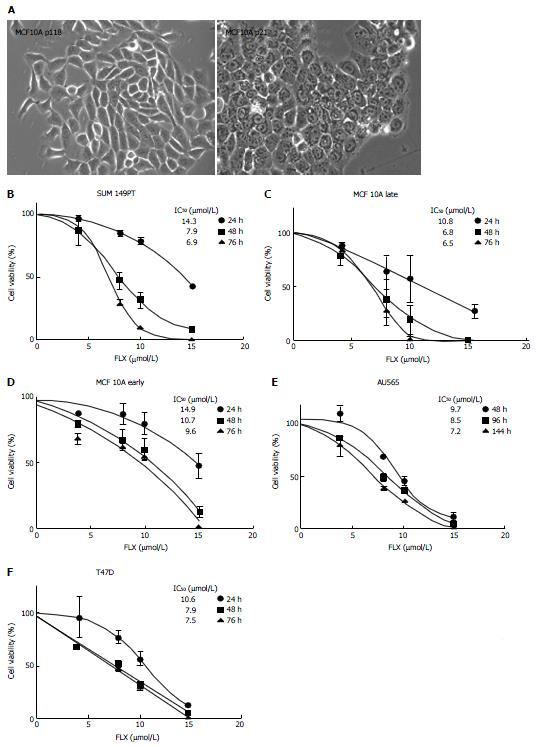
Figure 2 Time-dependent and concentration-dependent inhibition of cell growth induced by fluoxetine.
A: Bright field images of late and early passage of MCF10A cells were shown in 20 × magnification; B-F: The IC50 values for the dose-response curves for each cell line were also indicated; cell viability for each cell line was measured spectrophotometrically by means of MTT assay. Data represented the average of optical densities normalized to untreated samples ± SEM of 6 measurements for each concentration. MTT: 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; FLX: Fluoxetine.
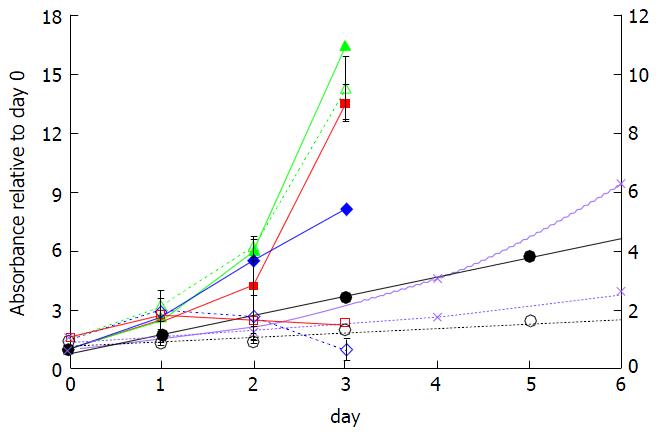
Figure 3 Effect of incubation time on microwave theory and techniques reduction by cell lines in the absence (solid lines) and presence (dotted lines) of 10 μmol/L fluoxetine.
Reduction of MTT by viable Late MCF10A (diamonds), SUM149PT (squares), Early MCF10A (triangles), T47D (circles), and Au565 cells (crosses) was monitored at absorbance of 570 nm and normalized to day 0. The ordinate axis on the right represents the absorbance values for T47D and Au565 cells. MTT: 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide.
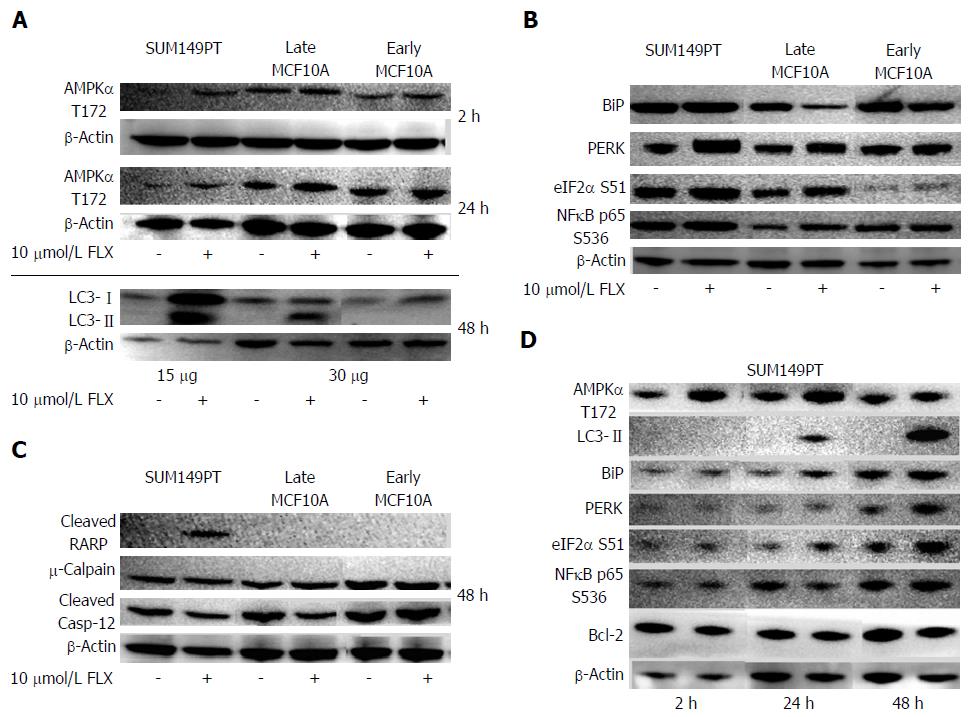
Figure 4 Key proteins along the autophagy, unfolded protein response, and apoptosis pathways were examined across cell lines after 10 μmol/L fluoxetine treatment at various times.
A, D: Critical effector of autophagy, AMPK, was activated after 2 h and 24 h of treatment in SUM149PT. Autophagy in cell lines was detected by the presence of cleaved LC3 (LC3-II) bands; B, C: The balance, level, and duration of ER stress sensors (B) and effectors of UPR (C) may ultimately dictate whether a cell lives or dies; D: protein levels were monitored for up to 48 h in SUM149PT after fluoxetine treatment, showing concurrent induction of UPR and autophagy by 24 h. Blots shown were representative of at least 3 different experiments. The amounts of total cell extracts loaded for each cell line were indicated in the bottom panels in (A) for cleaved LC3 detection, while total loading in all cell lines for AMPKα detection was 100 μg. In panels B, C, and D, the amount of cell extracts loaded in each lane was 30 μg, 100 μg and 50 μg, respectively. Since the Abcam antibody for cleaved LC3 detection was very sensitive in panel A, we chose another manufacturer (cell signaling technology) to detect the same cleaved protein in panel D without the problem of overexposure during chemiluminescence. UPR: Unfolded protein response; ER: Endoplasmic reticulum; AMPK: Adenosine monophosphate kinase.
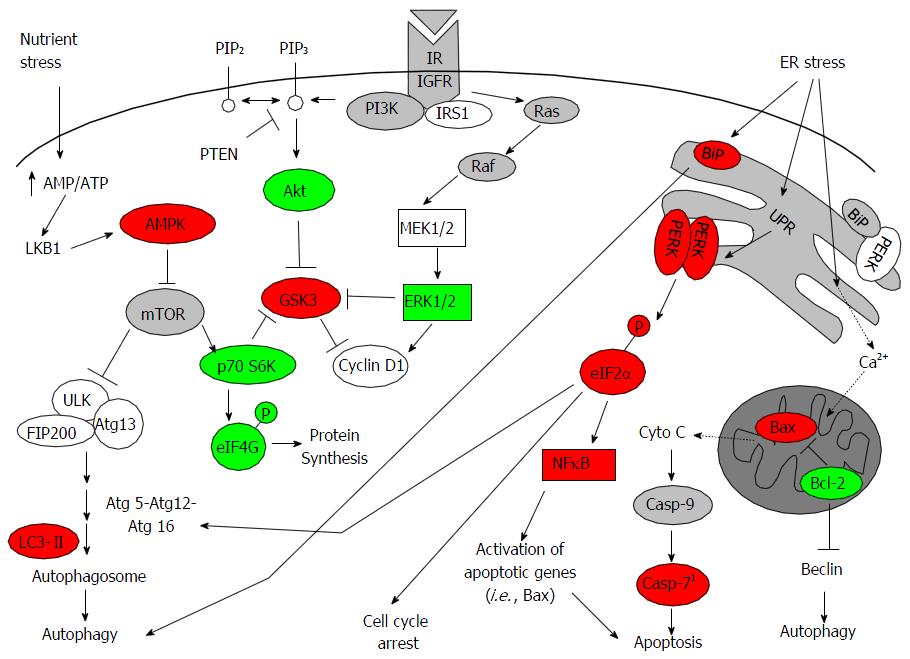
Figure 5 Integrated signaling network modulated by fluoxetine in inflammatory triple negative breast cancer line SUM149PT.
Proteins with increased and decreased expression levels after FLX treatment were highlighted in red and green, respectively. This proposed that model of FLX-induced cellular stress results in excessive UPR that leads to cell growth inhibition, autophagy, and potentially selective promotion of NFκB-mediated transcription of apoptotic genes, such as Bax. Oligomerization of Bax promotes the release of cytochrome C into cytosol and subsequent activation of caspase-9 and downstream effectors of apoptosis, such as caspase-7[38,40]. Alternatively, Ca2+ released during ER stress has been shown to promote cytochrome C release. AMPK: Adenosine monophosphate kinase; UPR: Unfolded protein response; FLX: Fluoxetine; p70 S6K: p70 S6 Kinase; ER: Endoplasmic reticulum.
- Citation: Bowie M, Pilie P, Wulfkuhle J, Lem S, Hoffman A, Desai S, Petricoin E, Carter A, Ambrose A, Seewaldt V, Yu D, Ibarra Drendall C. Fluoxetine induces cytotoxic endoplasmic reticulum stress and autophagy in triple negative breast cancer. World J Clin Oncol 2015; 6(6): 299-311
- URL: https://www.wjgnet.com/2218-4333/full/v6/i6/299.htm
- DOI: https://dx.doi.org/10.5306/wjco.v6.i6.299