Copyright
©2014 Baishideng Publishing Group Inc.
World J Clin Oncol. Aug 10, 2014; 5(3): 393-405
Published online Aug 10, 2014. doi: 10.5306/wjco.v5.i3.393
Published online Aug 10, 2014. doi: 10.5306/wjco.v5.i3.393
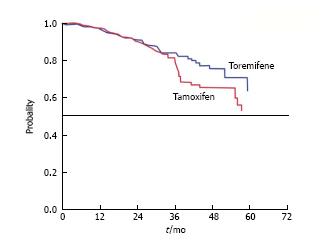
Figure 1 Time from randomization to recurrence in estrogen-receptor positive invasive breast cancer patients receiving adjuvant toremifene or tamoxifen[33].
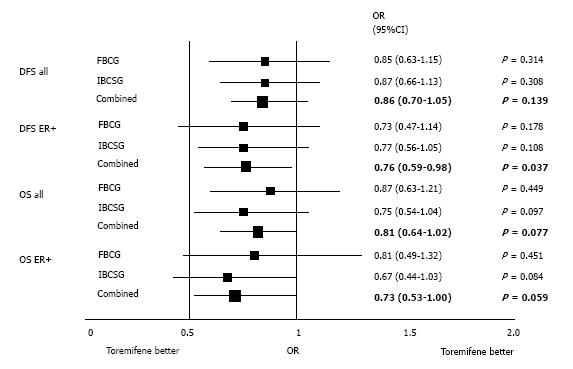
Figure 2 Disease-free survival and overall survival in patients receiving adjuvant toremifene and tamoxifen: meta-analysis of the Finnish Breast Cancer Group[30] and International Breast Cancer Study Group[34] (data on file).
DFS: Disease-free survival; OS: Overall survival; IBCSG: International Breast Cancer Study Group; FBCG: Finnish Breast Cancer Group.
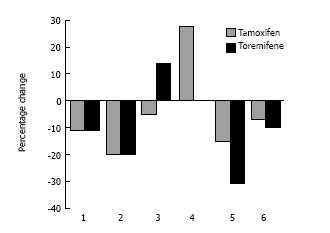
Figure 3 Percentage change in lipid parameters after one year with toremifene and tamoxifen[47].
1: Total cholesterol; 2: low-density lipoprotein (LDL) cholesterol; 3: high-density lipoprotein (HDL) cholesterol; 4: Triglycerides; 5: LDL:HDL; 6: Apo lipoprotein-B.
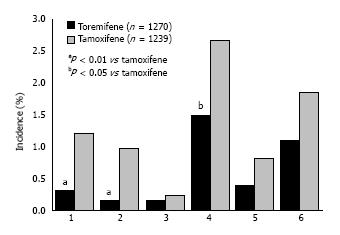
Figure 4 Incidence of serious vascular events in patients randomized to toremifene or tamoxifen adjuvant therapy in post-menopausal women[17].
1: Total cerebrovascular events; 2: Stroke; 3: Transient ischaemic attack; 4: Total thromboembolic events; 5: Pulmonary embolism; 6: Deep vein thrombosis.
- Citation: Mustonen MV, Pyrhönen S, Kellokumpu-Lehtinen PL. Toremifene in the treatment of breast cancer. World J Clin Oncol 2014; 5(3): 393-405
- URL: https://www.wjgnet.com/2218-4333/full/v5/i3/393.htm
- DOI: https://dx.doi.org/10.5306/wjco.v5.i3.393