Copyright
©2011 Baishideng Publishing Group Co.
World J Clin Oncol. Aug 10, 2011; 2(8): 311-325
Published online Aug 10, 2011. doi: 10.5306/wjco.v2.i8.311
Published online Aug 10, 2011. doi: 10.5306/wjco.v2.i8.311
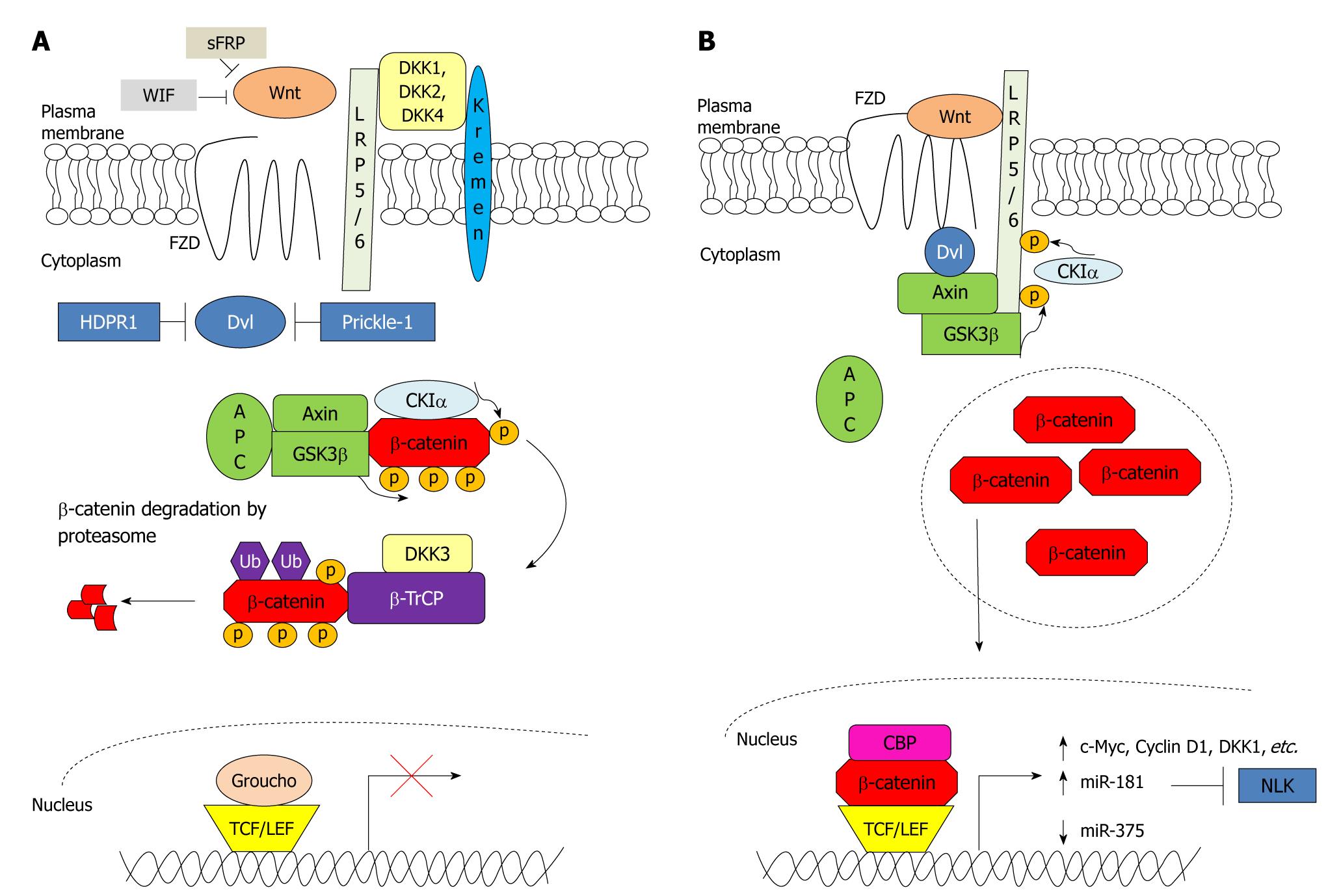
Figure 1 Wnt/β-catenin signalling in the absence and presence of Wnt stimulus.
A: Wnt/β-catenin signalling is regulated by several antagonists to prevent the formation of frizzled (FZD)-Wnt-low-density lipoprotein receptor-related protein 5/6 (LRP5/6) complex. Secreted frizzled-related protein (sFRP) and Wnt inhibitory factor (WIF) bind directly to Wnt, whereas dickkopfs (DKKs) bind to LRP5/6. Furthermore, human homologue of Dapper (HDPR1) and Prickle-1 inhibit the action of dishevelled (Dvl). In the absence of Wnt stimulus, β-catenin is first primed for phosphorylation by casein kinase Iα (CKIα) followed by phosphorylation by glycogen synthase kinase 3β (GSK3β) at three residues. The phosphorylated β-catenin is targeted for ubiquitination by β-transducin repeat-containing protein (β-TrCP) and is subsequently degraded by the proteasome. In the nucleus, T-cell factor (TCF)/lymphoid enhancer factor (LEF) represses transcription of the Wnt/β-catenin pathway target genes by interacting with co-repressor Groucho; B: Wnt binds to and activates FZD and LRP5/6 receptors. Dvl is recruited to the plasma membrane and binds to FZD. This results in the recruitment of Axin and GSK3β to LRP5/6. LRP5/6 is then phosphorylated by CKIα and GSK3β, resulting in an inactivation of the destruction complex and leading to β-catenin accumulation in the cytoplasm. β-catenin then subsequently translocates to the nucleus where it binds with TCF/LEF and other co-activators e.g. CREB binding protein (CBP) to mediate transcription of genes and microRNAs responsible for proliferation and growth. APC: Adenomatous polyposis coli; NLK: Nemo-like kinase; p: Phosphorylated; Ub: Ubiquitinated.
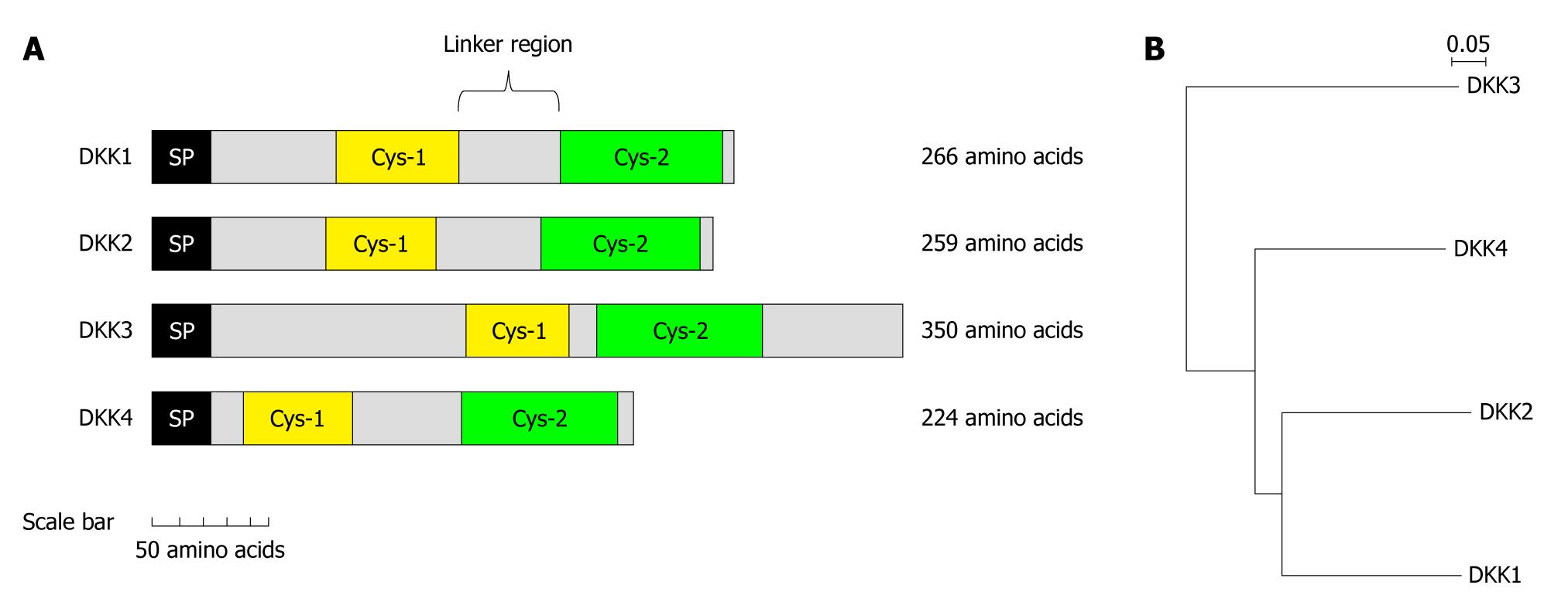
Figure 2 Domain structure and phylogenetic tree of human dickkopf proteins.
A: Each dickkopf (DKK) contains two cysteine-rich domains, each of which is separated by a linker region of various lengths. The amino-terminal cysteine-rich domain (Cys-1) domains are unique to each DKK, whereas the carboxyl-terminal cysteine-rich domain (Cys-2) domains are conserved among all members of the DKK family; B: Phylogenetic tree of the DKK proteins. Amino acid sequences were aligned by the Clustal W program, and the phylogenetic tree was constructed by the neighbour joining method. The scale bar indicates the estimated number of substitutions per 50 amino acids. SP: Signal peptide.
- Citation: Fatima S, Lee NP, Luk JM. Dickkopfs and Wnt/β-catenin signalling in liver cancer. World J Clin Oncol 2011; 2(8): 311-325
- URL: https://www.wjgnet.com/2218-4333/full/v2/i8/311.htm
- DOI: https://dx.doi.org/10.5306/wjco.v2.i8.311