Copyright
©2011 Baishideng Publishing Group Co.
World J Clin Oncol. May 10, 2011; 2(5): 195-202
Published online May 10, 2011. doi: 10.5306/wjco.v2.i5.195
Published online May 10, 2011. doi: 10.5306/wjco.v2.i5.195
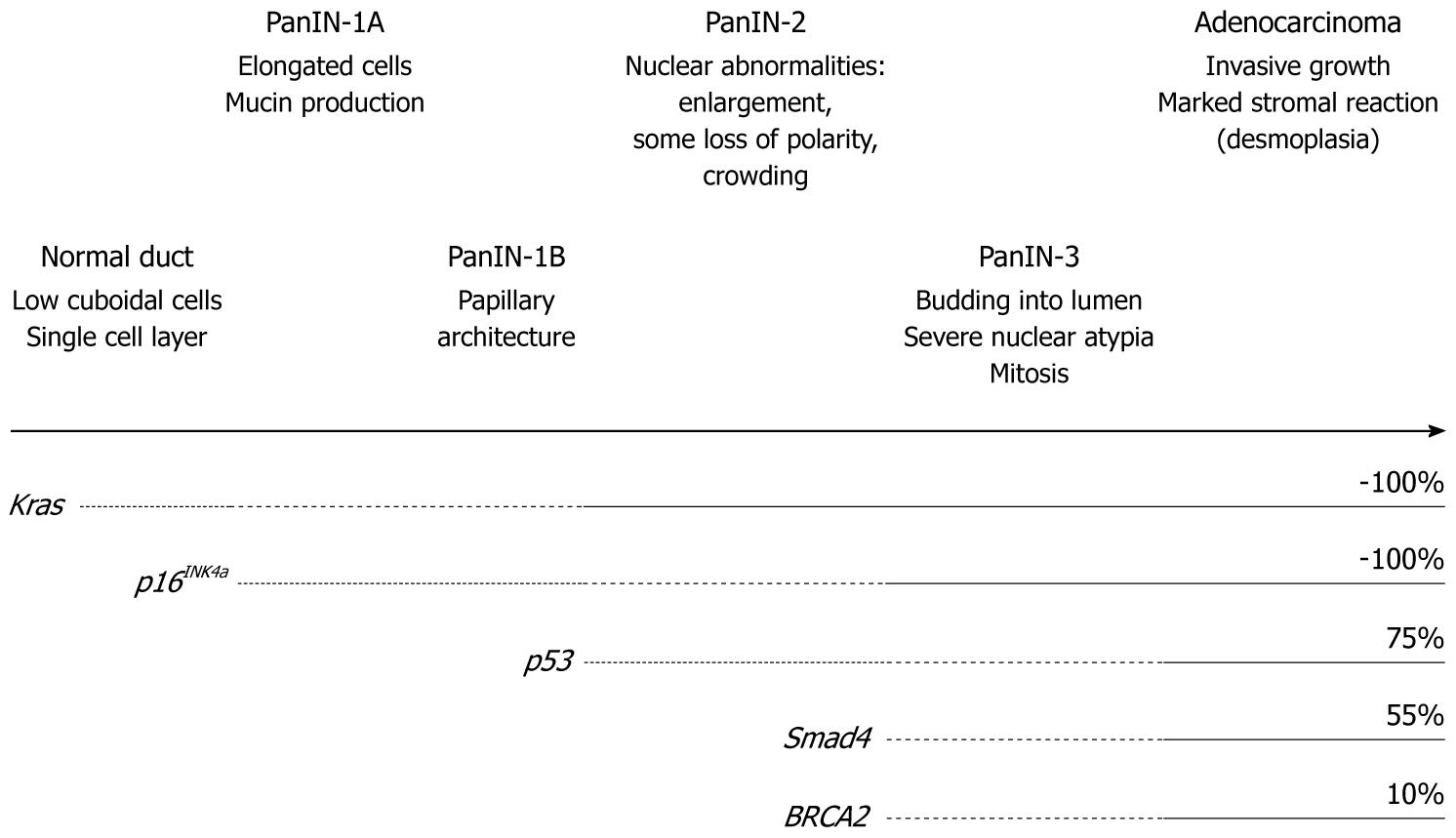
Figure 1 Multi-step carcinogenesis hypothesis of pancreatic cancer.
As genetic alterations of Kras, p16INK4a, p53, Smad4 and BRCA2 accumulate, the pre-cancer lesion pancreatic intraepithelial neoplasia (PanIN) occurs and progresses from low-grade (1A, 1B) to high-grade (2, 3) and to invasive cancer. The frequency of each genetic alteration at the invasive cancer stage is also shown.
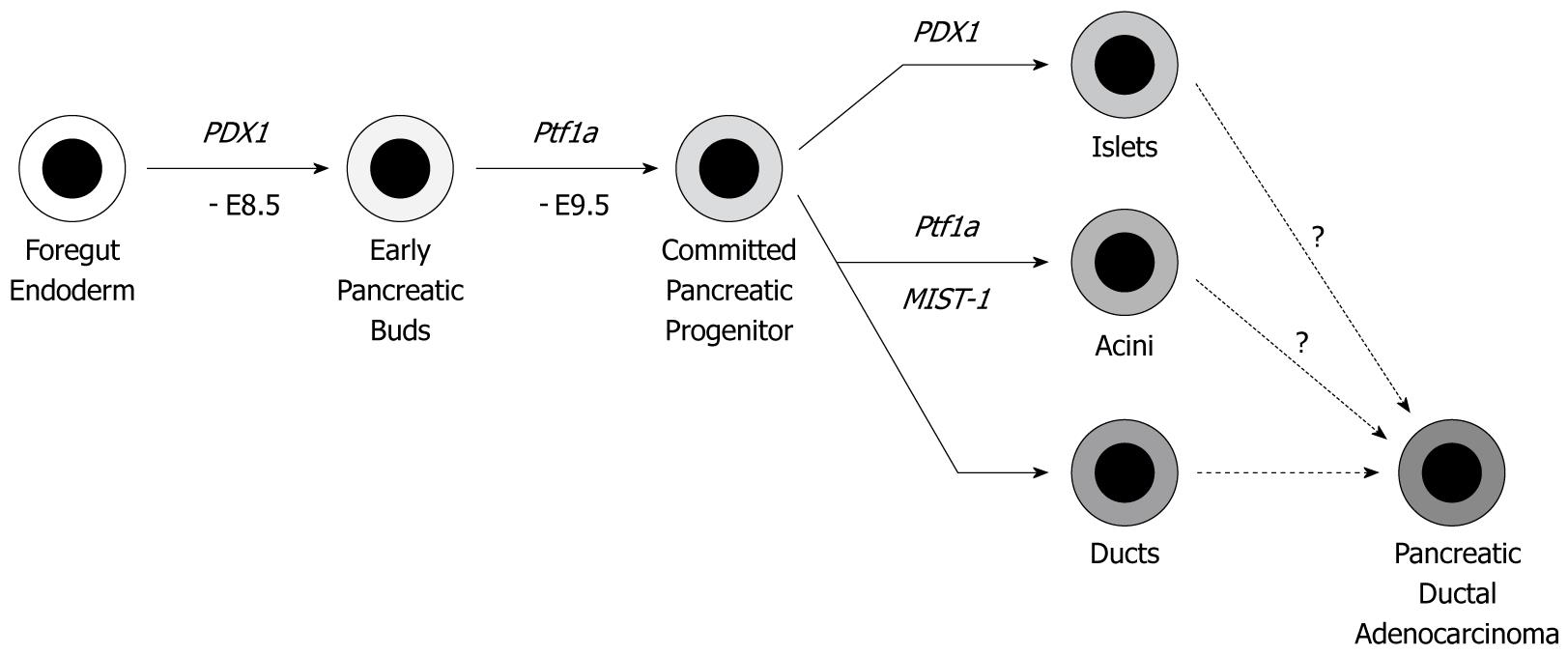
Figure 2 Cell differentiation program in the pancreas.
PDX1 and Ptf1a genes are expressed on E8.5-9.5 d, which determines the cell fate in the pancreas epithelium. By using the PDX1 or Ptf1a gene promoter-induced Cre-loxP system, all three lineages (islet, acini and duct) have the designed genetic alterations. The cell of origin of pancreatic ductal adenocarcinoma (PDAC) is still under discussion. Experimentally, PDAC can be derived from all three lineages.
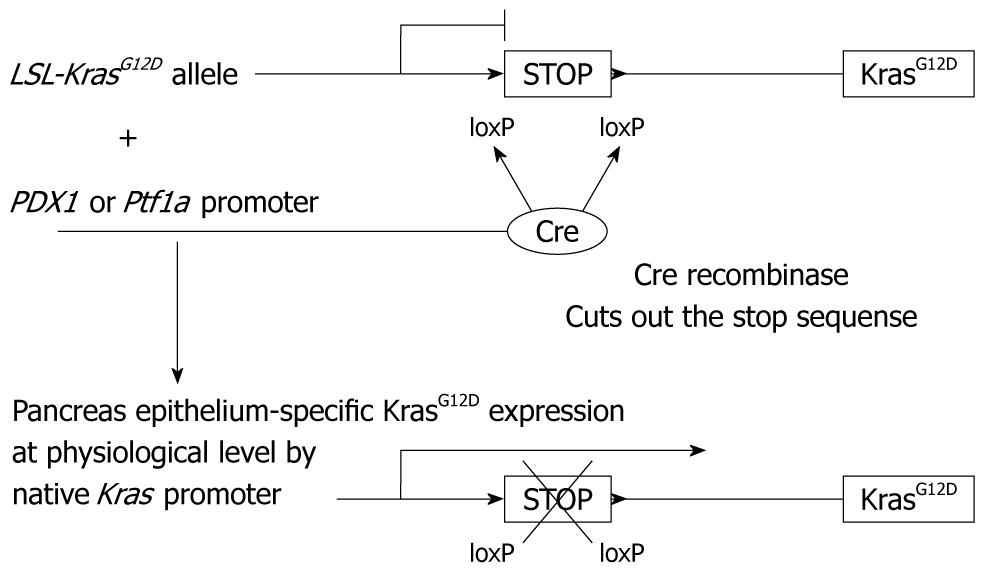
Figure 3 Pancreas epithelium-specific KrasG12D expression.
Cre recombinase is expressed under the promoter of PDX1 or Ptf1a gene, which is pancreas epithelium-specific. Without cre recombinase expression, a stop sequence between the loxP sites prevents mutant KrasG12D expression. In cells where only cre recombinase is expressed, the stop sequence is cut out, which turns on the pancreas epithelium-specific KrasG12D expression at a physiological level under the native Kras promoter.
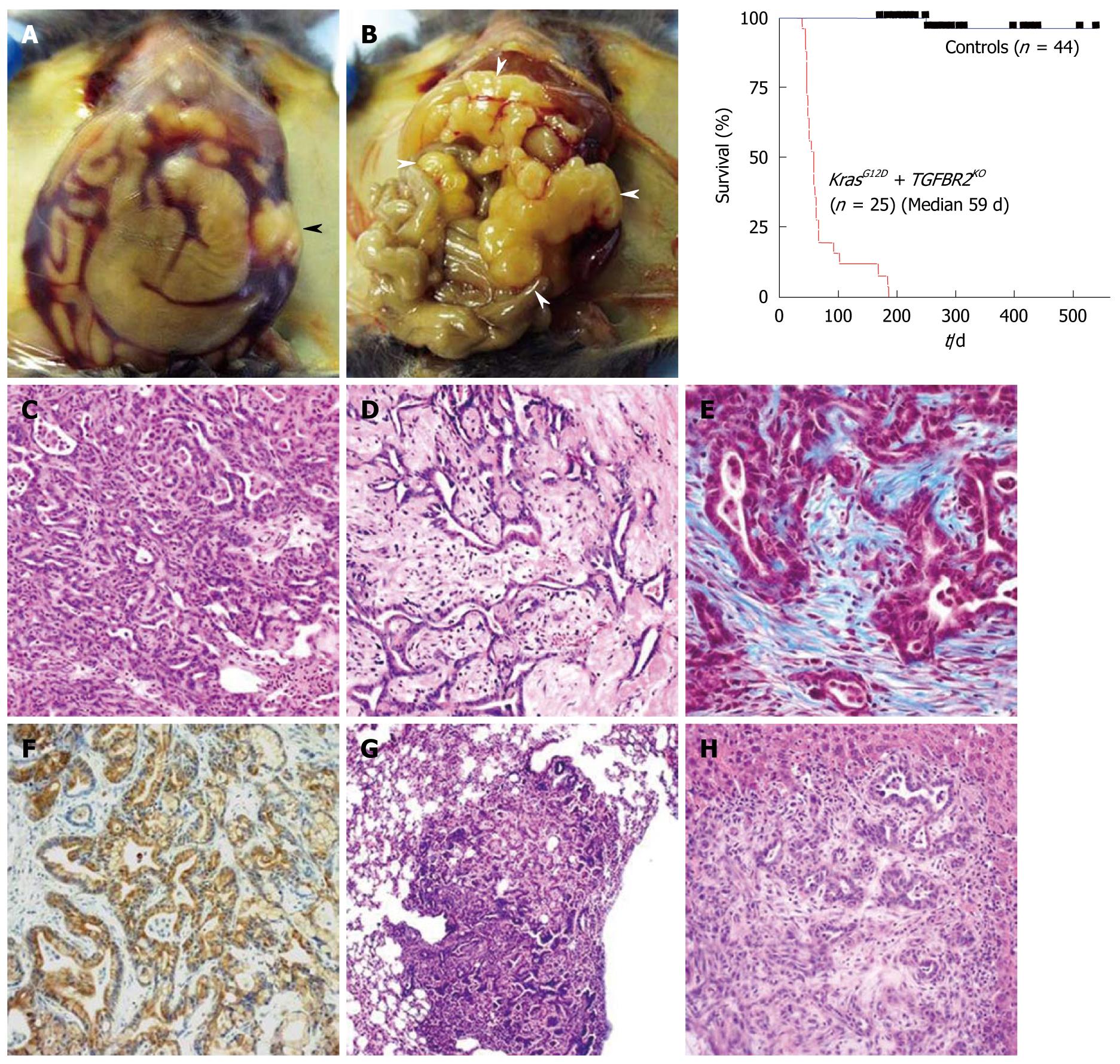
Figure 4 Endogenous KrasG12D plus transforming growth factor-β receptor 2 knockout pancreatic ductal adenocarcinoma.
A, B: Macroscopic appearances and a survival curve of the endogenous KrasG12D plus transforming growth factor-β receptor 2 (Tgfbr2) knockout pancreatic ductal adenocarcinoma (PDAC) mice; A: Abdominal distension and bloody ascites are observed. Black arrowhead indicates the tumor; B: The whole pancreas is occupied by tumor and enlarged (white arrowheads). Jaundice is also observed here; C-H: Microscopic appearance of the endogenous KrasG12D plus Tgfbr2 knockout PDAC; C, D: Differentiated ductal adenocarcinoma with abundant stroma is observed in HE sections; E: Marked fibrosis and desmoplasia is observed. The blue color indicates fibrosis in trichrome blue staining; F: Positive immunostaining of cytokeratin 19 indicates a ductal phenotype tumor; G, H: The tumor has a metastatic potential to the lung (G) and liver (H).
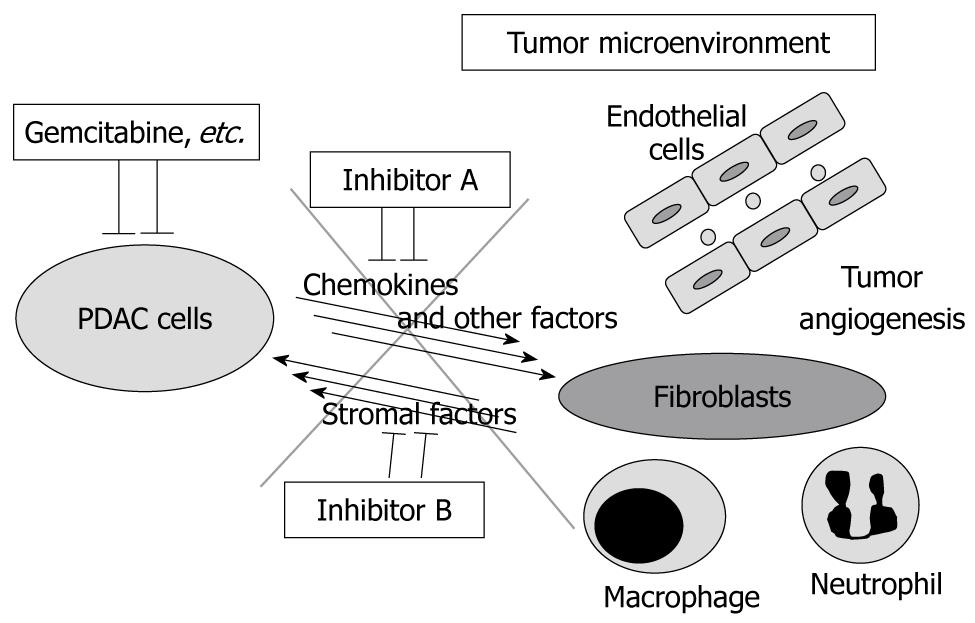
Figure 5 Tumor-stromal interaction as a therapeutic target of pancreatic ductal adenocarcinoma.
In pancreatic ductal adenocarcinoma (PDAC) tissue, PDAC cells and stromal cells are interacting with each other by certain factors including chemokines, which might have tumor-promoting effects. The tumor microenvironment contains, for example, fibroblasts, macrophages, neutrophils and vascular endothelial cells. The combination of conventional chemo reagents (e.g. gemcitabine) and the inhibition of the tumor-stromal interaction might be a more effective therapeutic strategy for PDAC.
- Citation: Ijichi H. Genetically-engineered mouse models for pancreatic cancer: Advances and current limitations. World J Clin Oncol 2011; 2(5): 195-202
- URL: https://www.wjgnet.com/2218-4333/full/v2/i5/195.htm
- DOI: https://dx.doi.org/10.5306/wjco.v2.i5.195