Copyright
©2011 Baishideng Publishing Group Co.
World J Clin Oncol. Jan 10, 2011; 2(1): 8-27
Published online Jan 10, 2011. doi: 10.5306/wjco.v2.i1.8
Published online Jan 10, 2011. doi: 10.5306/wjco.v2.i1.8
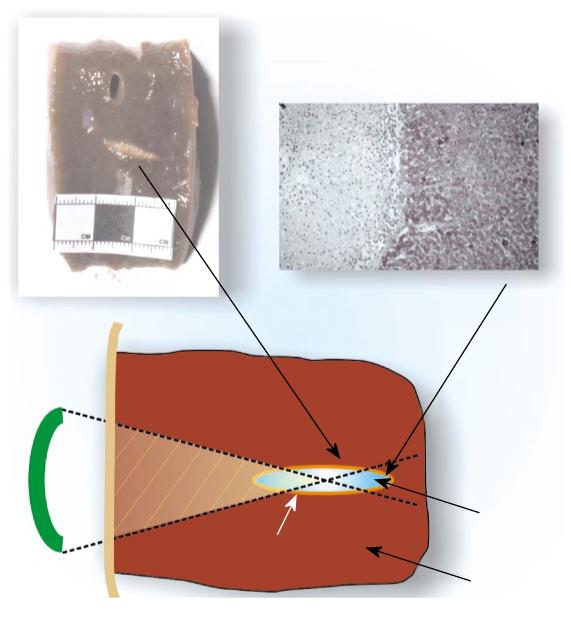
Figure 1 The high intensity focused ultrasound beam passes through overlying skin and other tissues without harming them and is focused to necrose a localized tumor region, which may lie deep within the body.
There is a very sharp boundary between dead and live cells at this contour.
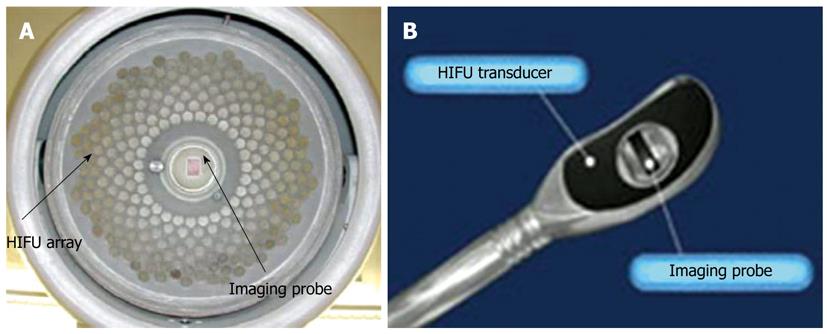
Figure 2 The structure of (A) an extracorporeal (FEP-BY02) and (B) a transrectal (Ablatherm) high intensity focused ultrasound transducer.
HIFU: High intensity focused ultrasound.
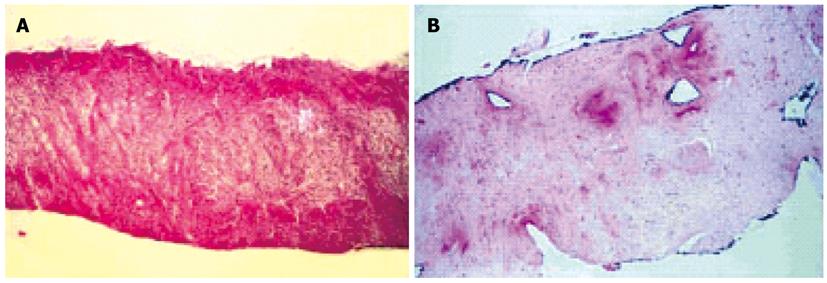
Figure 3 Complete destruction of the glandular tissue due to coagulation necrosis lesion which reaches the capsula and the periprostatic fat 48 h after high intensity focused ultrasound treatment (A) and the necrotic prostatic tissue is replaced by a fibrotic tissue, including the capsula, 3 mo after high intensity focused ultrasound therapy (B).
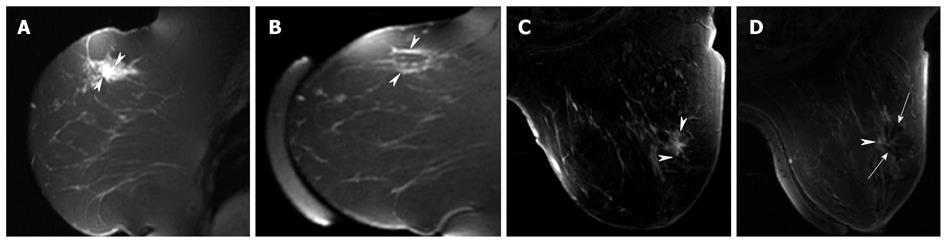
Figure 4 Sagittal contrast-enhanced T1-weighted fat-saturated magnetic resonance sagittal (A) and axial (C) images of a 1.
8-cm poorly differentiated invasive ductal carcinoma in a 44-year-old woman before MRgFUS. An irregular enhancing mass is seen in the upper outer quadrant of the right breast (arrow heads). Three days after magnetic resonance-guided focused US surgery (MRgFUS), minimal strikes of enhancement are seen without mass like enhancement in the sagittal image (arrow heads in B), which may represent hyperemia due to reactive inflammation or residual tumor. On the axial image (D), dark signal void area is seen at the site of the prior enhancing mass (long arrows). At histopathology, about 50% of the carcinoma and adjacent normal tissue showed thermal effects and the remaining portion of the carcinoma appeared viable.
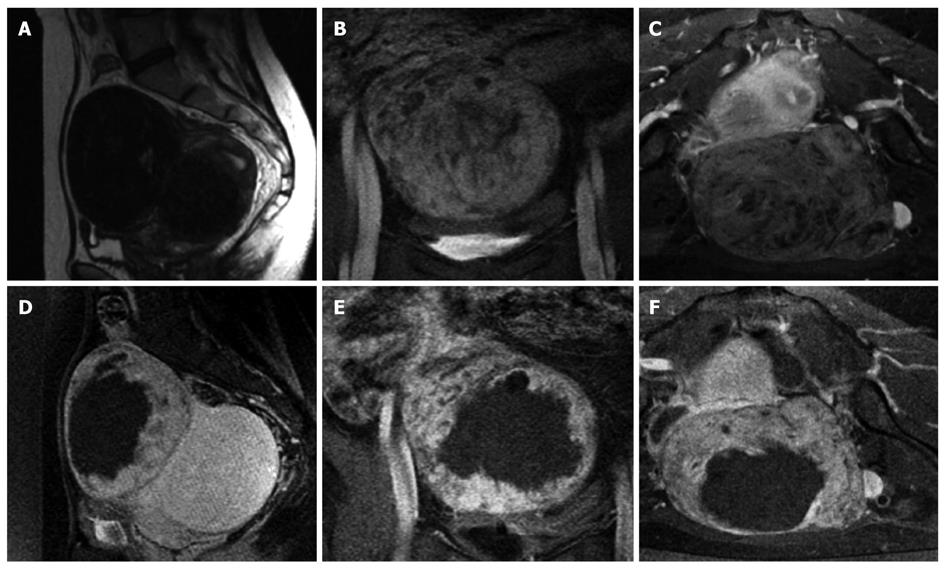
Figure 5 Images of a uterine fibroid pretreatment and posttreatment with MRgFUS.
Top, Sagittal T2 fast spin-echo (A), coronal spoiled gradient-recalled echo sequence (SPGR) postgadolinium (B), and axial SPGR postgadolinium (C) are obtained pretreatment. The low SI homogenous fibroid depicted in Figure 3A demonstrates slight heterogenous enhancement pretreatment (Figure 3B, C). Bottom, Sagittal SPGR post-gadolinium (D), coronal SPGR post-gadolinium (E), and axial SPGR post-gadolinium (F) are obtained immediately post-treatment. A new large nonperfused area is identified, consistent with treatment-induced necrosis.
Figure 6 Dynamic contrast-enhanced gradient-echo T1-weighted magnetic resonance images (180/6.
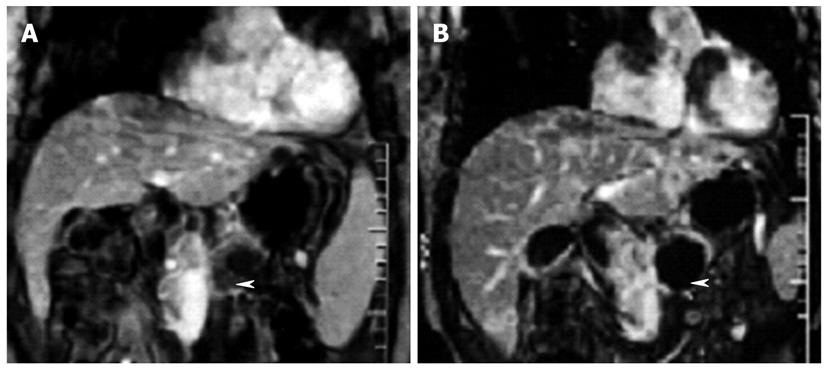
0, 90° flip angle, 128 × 256 matrix, 10-mm-thick sections, 2-mm intersection gap, one signal acquired, and 18-s acquisition time) obtained with breath holding in 48-year-old man who underwent high-intensity focused ultrasound ablation for advanced pancreatic cancer. The tumor was 4.5 cm × 4.5 cm in diameter and located in the body of the pancreas. A: Image obtained before high-intensity focused ultrasound shows the blood supply in the pancreatic lesion (arrowhead); B: Image obtained 2 wk after high-intensity focused ultrasound shows no evidence of contrast enhancement in the treated lesion (arrowhead), which is indicative of complete coagulation necrosis in the pancreatic cancer.
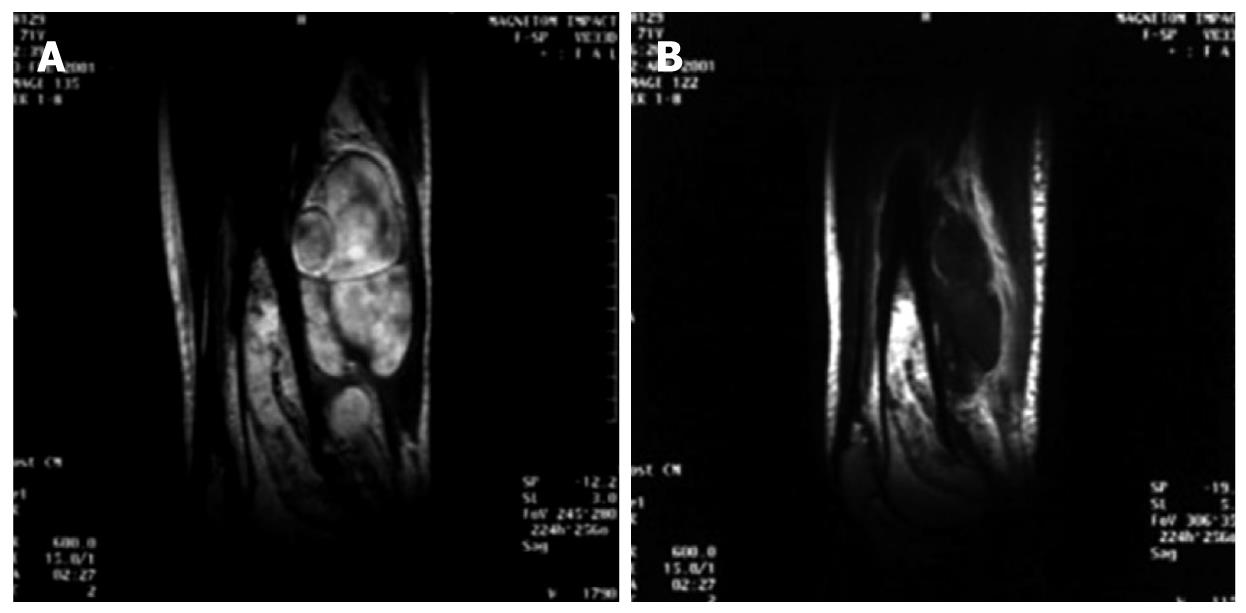
Figure 7 Contrast-enhanced magnetic resonance images (A) before and (B) 14-d after high intensity focused ultrasound ablation in a 45-year-old patient with osteosarcoma at the upper right tibia.
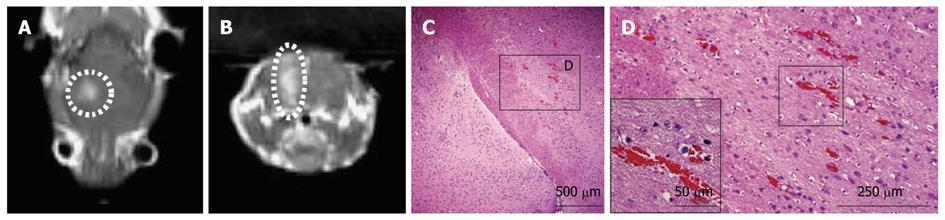
Figure 8 The axial (A) and the coronal magnetic resonance images (B) are presented with a tissue block showing the blood-brain barrier disruption from the mice, overview of the half hemisphere of a mouse with focused ultrasound-induced blood-brain barrier disruption (C) and high magnification of the lesions with severe damage (D).
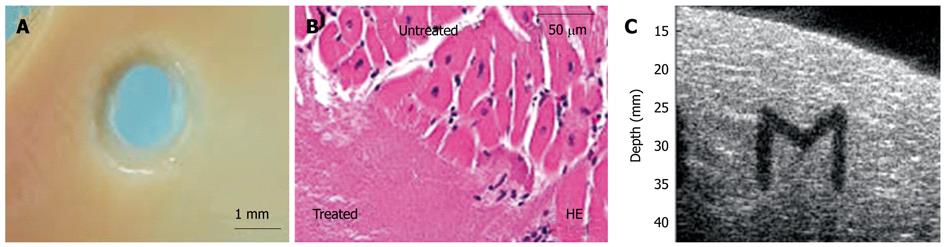
Figure 9 Representative (A) image and (B) histology of tissue erosion after histotripsy, and (C) “M” shape lesion generated by histotripsy shown in ultrasound imaging.
- Citation: Zhou YF. High intensity focused ultrasound in clinical tumor ablation. World J Clin Oncol 2011; 2(1): 8-27
- URL: https://www.wjgnet.com/2218-4333/full/v2/i1/8.htm
- DOI: https://dx.doi.org/10.5306/wjco.v2.i1.8