Copyright
©The Author(s) 2025.
World J Clin Oncol. Aug 24, 2025; 16(8): 108768
Published online Aug 24, 2025. doi: 10.5306/wjco.v16.i8.108768
Published online Aug 24, 2025. doi: 10.5306/wjco.v16.i8.108768
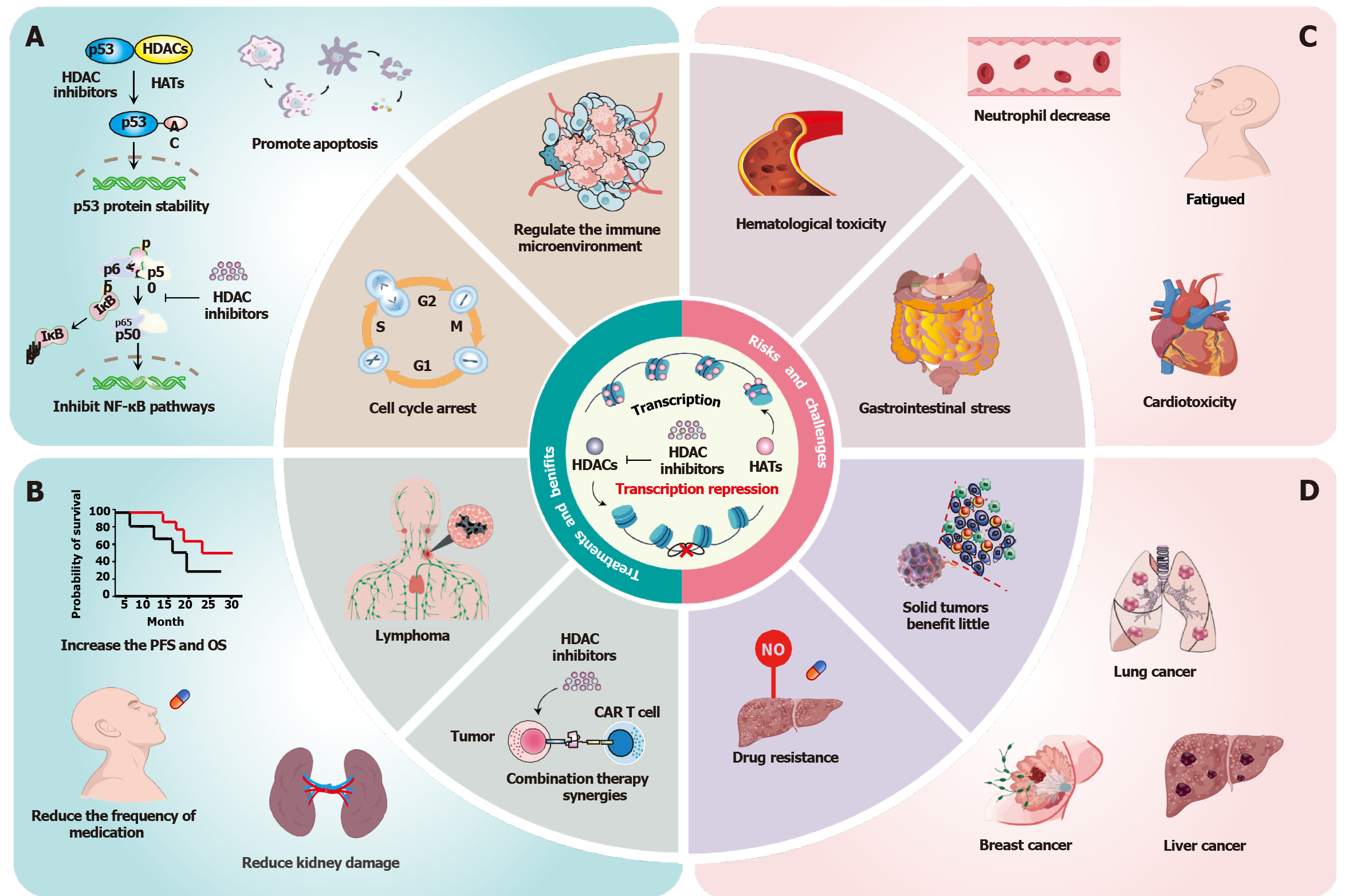
Figure 1 The dual-edged sword of histone deacetylase inhibitors in cancer therapy.
A: Mechanistic basis of histone deacetylase inhibitors (HDACis) antitumor activity. HDACis suppress tumor cell proliferation and promote caspase-dependent apoptosis through stabilization of p53 signaling, induce cell cycle arrest, and suppress NF-κB nuclear translocation; B: Clinical advantages in haematological malignancies [especially T-cell lymphoma (TCL) and multiple myeloma]. The overall response rate (ORR) and disease control rate (DCR) of patients with peripheral TCL receiving tucidinostat monotherapy were 39.06% and 64.45%, and the 5-year survival rate was improved. Furthermore, HDACis in combination with other therapeutic agents lower the conventional chemotherapeutic dose and reduce the damage to the kidneys; C: Treatment-limiting toxicities. HDACis treatment class-related risks, including cardiotoxicity, hematologic toxicity, myelosuppression, and diarrhoea. Cardiotoxicity mainly includes QTc prolongation, reduced left ventricular ejection fraction, isolated cases, and ventricular tachyarrhythmias. Dose-dependent hematologic toxicity manifests as grade 3/4 thrombocytopenia (38% incidence) and neutropenia (42% incidence). Furthermore, HDACi treatment also leads to increased fatigue in patients; D: Challenges in solid tumor management. One of the primary challenges is limited efficacy in solid tumors, such as non-small cell lung cancer (ORR = 9%), breast cancer (progression-free survival = 2.1 months), and hepatocellular carcinoma (DCR = 18%). Another critical challenge is multidrug resistance, which is mediated by ABC transporter upregulation and compensatory HDAC6 overexpression. HDAC: Histone deacetylase; PFS: Progression-free survival; OS: Overall survival.
- Citation: Xiao S, Xu XZ, Liao M, Song DD, Tang JF, Zhou CF. Potential risks of histone deacetylase inhibitors in cancer therapeutics and feasible combination therapeutic strategies. World J Clin Oncol 2025; 16(8): 108768
- URL: https://www.wjgnet.com/2218-4333/full/v16/i8/108768.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i8.108768