Copyright
©The Author(s) 2025.
World J Clin Oncol. Aug 24, 2025; 16(8): 108112
Published online Aug 24, 2025. doi: 10.5306/wjco.v16.i8.108112
Published online Aug 24, 2025. doi: 10.5306/wjco.v16.i8.108112
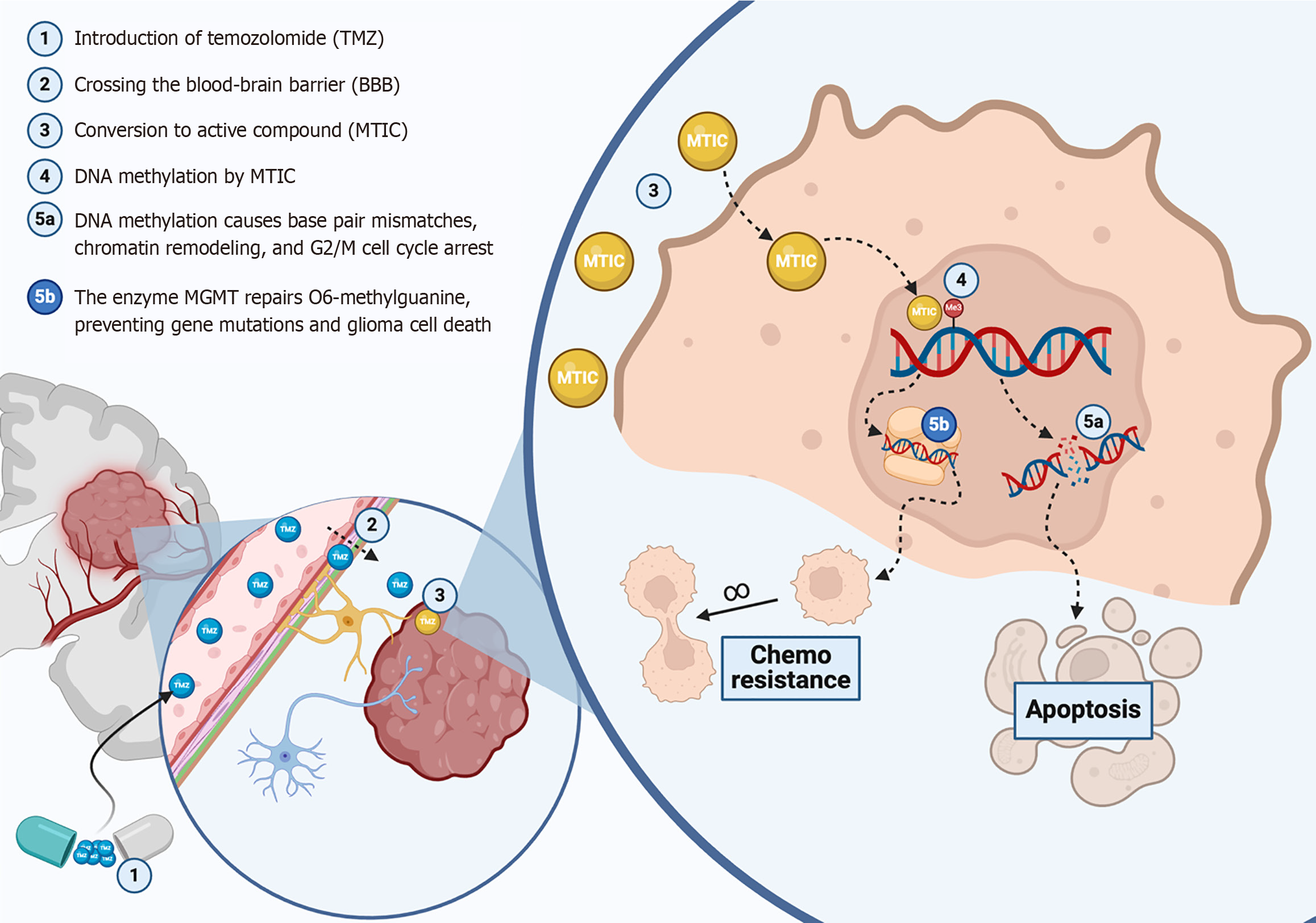
Figure 1 The basic mechanism of action of temozolomide in the treatment of gliomas.
Temozolomide reaches high-grade glioma cells after passing through the blood–brain barrier. The active molecule (MTIC), which methylates DNA bases, splits double-strand DNA, and triggers apoptosis. O6-methylguanine-DNA methyltransferase and other DNA repair pathways are in opposition to MTIC action. MTIC: 5-(3-methyltriazen-1-yl)-imidazole-4-carboxamide; TMZ: Temozolomide; MGMT: O6-methylguanine-DNA methyltransferase; BBB: Blood-brain barrier. This figure was created by BioRender.com (Supplementary material).
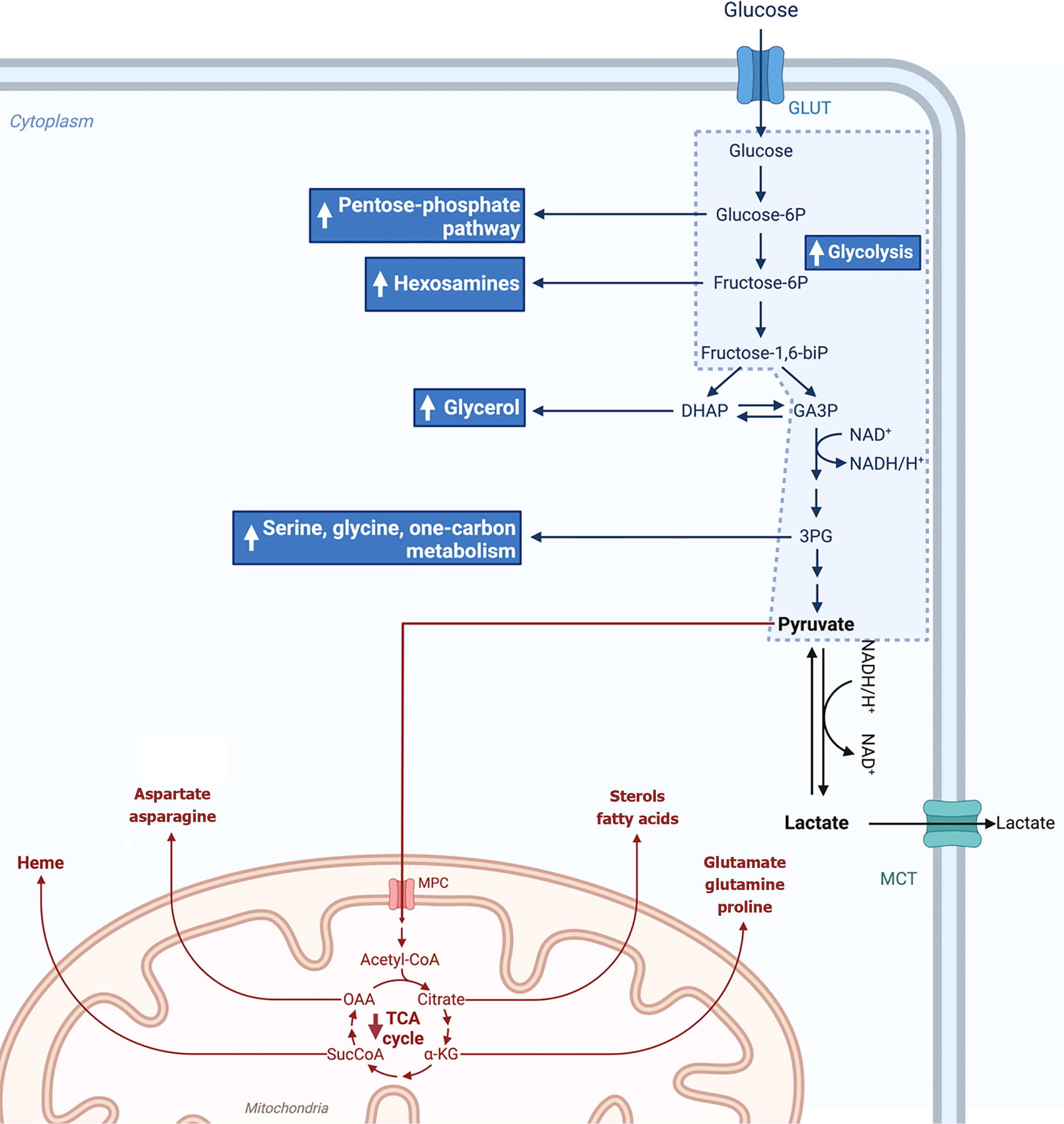
Figure 2 Illustration of glucose metabolism metabolized in a tumoral cell.
The Warburg effect, also known as aerobic glycolysis, marks the process of glucose conversion to pyruvate and then to lactate, despite cancer cells having a fully functional respiratory chain. In addition to increasing the amount of lactate and protons secreted into the extracellular space, an enhanced glycolytic pathway also makes glycolysis intermediates more readily available. This enables the increased flow of substrates to the one-carbon cycle and pentose phosphate pathway, which supports lipid and nucleotide synthesis and preserves redox homeostasis. 1,3-biPG: 1,3-biphosphoglycerate; 2-PG: 2-phosphoglicerate; 3-PG: 3-phosphoglicerate; ADP: Adenosine diphosphate; aKG: Alpha-ketoglutarate; ALDO: Aldolase; ATP: Adenosine triphosphate; DHAP: Dihydroxyacetone phosphate; ENO: Enolase; FAD+: Flavin adenine dinucleotide; G6PD: Glucose-6-phosphate dehydrogenase; GA3P: Glyceraldehyde 3-phosphate; GAPDH: Glyceraldehyde 3-phosphate; GDP: Guanosine diphosphate; GLUT1: Glucose transporter 1; GSH: Glutathione; GTP: Guanosine triphosphate; HCy: Homocysteine; HK: Hexokinase, LDH: Lactate dehydrogenase; MCT4: Monocarboxylate transporter 4; Met: Methionine; NAD+: Nicotinamide adenine dinucleotide; NADP: Nicotinamide adenine dinucleotide phosphate; OAA: Oxaloacetic acid; PEP: Phosphoenolpyruvate; PFK: Phosphofructokinase; PGAM: Phosphoglycerate mutase; PGI: Phosphoglucoisomerase; PGK: Phosphoglycerate kinase; PK: Pyruvate kinase; SucCoa: Succinyl-coA; THF: Tetrahydrofolate. This figure was created by BioRender.com (Supplementary material).
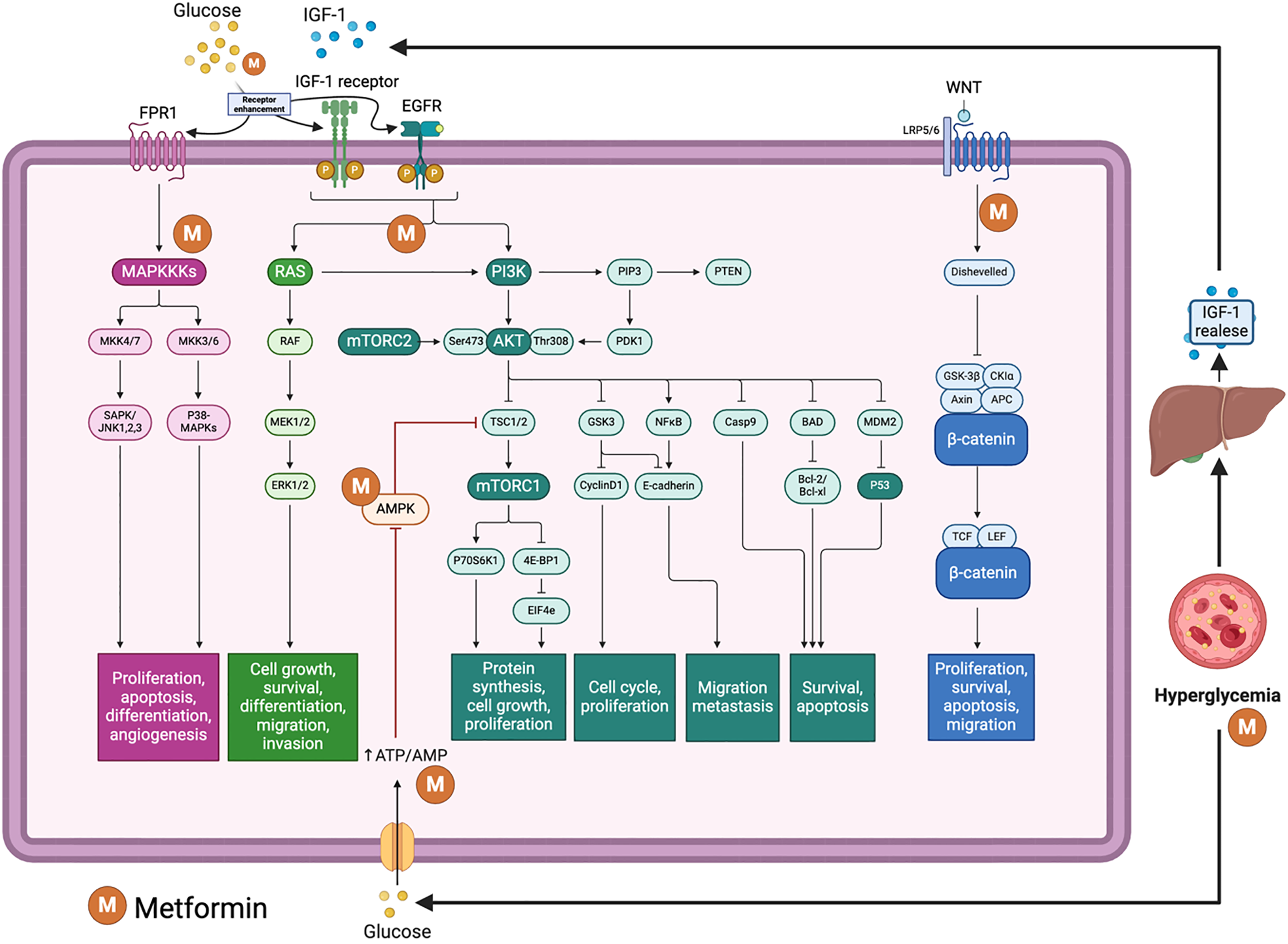
Figure 3 Pathways related to glucose and metformin effects on glioblastoma.
Figure illustrates the complex signaling pathways influenced by glucose and metformin in the context of glioblastoma, a type of aggressive brain tumor. Glucose enters the cell and contributes to hyperglycemia, which can indirectly promote tumor growth. Hyperglycemia stimulates the release of insulin-like growth factor 1 (IGF-1) from the liver, which binds to the IGF-1 receptor (IGF-1R) on the cell membrane, activating downstream signaling pathways that include the RAS/MAPK and PI3K/AKT/mTOR pathways. Epidermal growth factor receptor (EGFR) is another receptor that activates similar downstream signaling cascades, further promoting cell growth and survival. The MAPK pathway, activated by both IGF-1R and EGFR, involves several kinases (MAPKKK, MAPKK, MAPK) and leads to cell proliferation, differentiation, and angiogenesis. The PI3K activation leads to the phosphorylation of AKT, which in turn activates mTORC1 and mTORC2 complexes; mTORC1 promotes protein synthesis, cell growth, and proliferation, while mTORC2 supports cell survival and cytoskeletal organization. AKT activation also influences cell cycle regulation and apoptosis through various downstream targets. WNT signaling leads to the stabilization and nuclear translocation of β-catenin, which influences gene expression related to proliferation, survival, and migration. Metformin, a common diabetes medication, activates AMPK, inhibiting mTORC1, thus reducing protein synthesis and cell growth, and promotes glucose uptake, counteracting hyperglycemia. Metformin directly inhibits mitochondrial complex I, leading to increased AMP/ATP ratio and subsequent AMPK activation. It also inhibits IGF-1R and EGFR signaling, reducing the downstream effects on cell growth and survival pathways, and impacts the WNT/β-catenin pathway by reducing β-catenin levels, which may suppress tumor proliferation and survival. This figure was created by BioRender.com (Supplementary material).
- Citation: Begagić E, Džidić-Krivić A, Bečulić H, Pugonja R, Ljevaković A, Bašić B, Nuhović A, Milanović E, Hadžić S, Bećirović E, Buljubašić L, Bećirović M, Pojskić M. Glucose and antidiabetic therapy in temozolomide resistance in glioblastoma. World J Clin Oncol 2025; 16(8): 108112
- URL: https://www.wjgnet.com/2218-4333/full/v16/i8/108112.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i8.108112