Copyright
©The Author(s) 2025.
World J Clin Oncol. Feb 24, 2025; 16(2): 95642
Published online Feb 24, 2025. doi: 10.5306/wjco.v16.i2.95642
Published online Feb 24, 2025. doi: 10.5306/wjco.v16.i2.95642
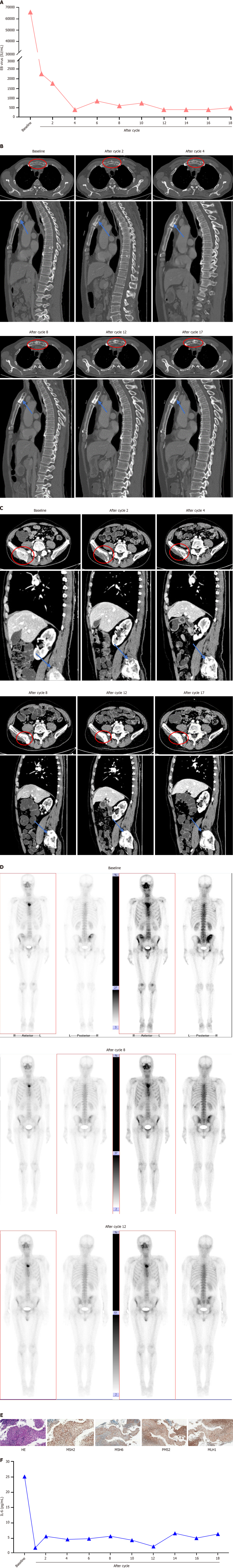
Figure 1 Examinations.
A: The Epstein–Barr virus DNA changes during PRaG treatment; B: Computed tomography (CT) evaluation of sternal metastases at baseline and during treatment; C: CT evaluation of right iliac metastases at baseline and during treatment; D: Bone scans of the patient at baseline and during treatment; E Initial pathology and immunohistochemistry of the patient hematoxylin and eosin stain, mutS homolog (MSH) 2, MSH6, PMS1 homolog 2, and mutL homolog 1; F: The interleukin-6 changes during PRaG treatment. EB: Epstein–Barr; IL: Interleukin; HE: Hematoxylin and eosin; MSH2: MutS homolog 2; MSH6: MutS homolog 6; PMS2: PMS1 homolog 2; MLH1: MutL homolog 1.
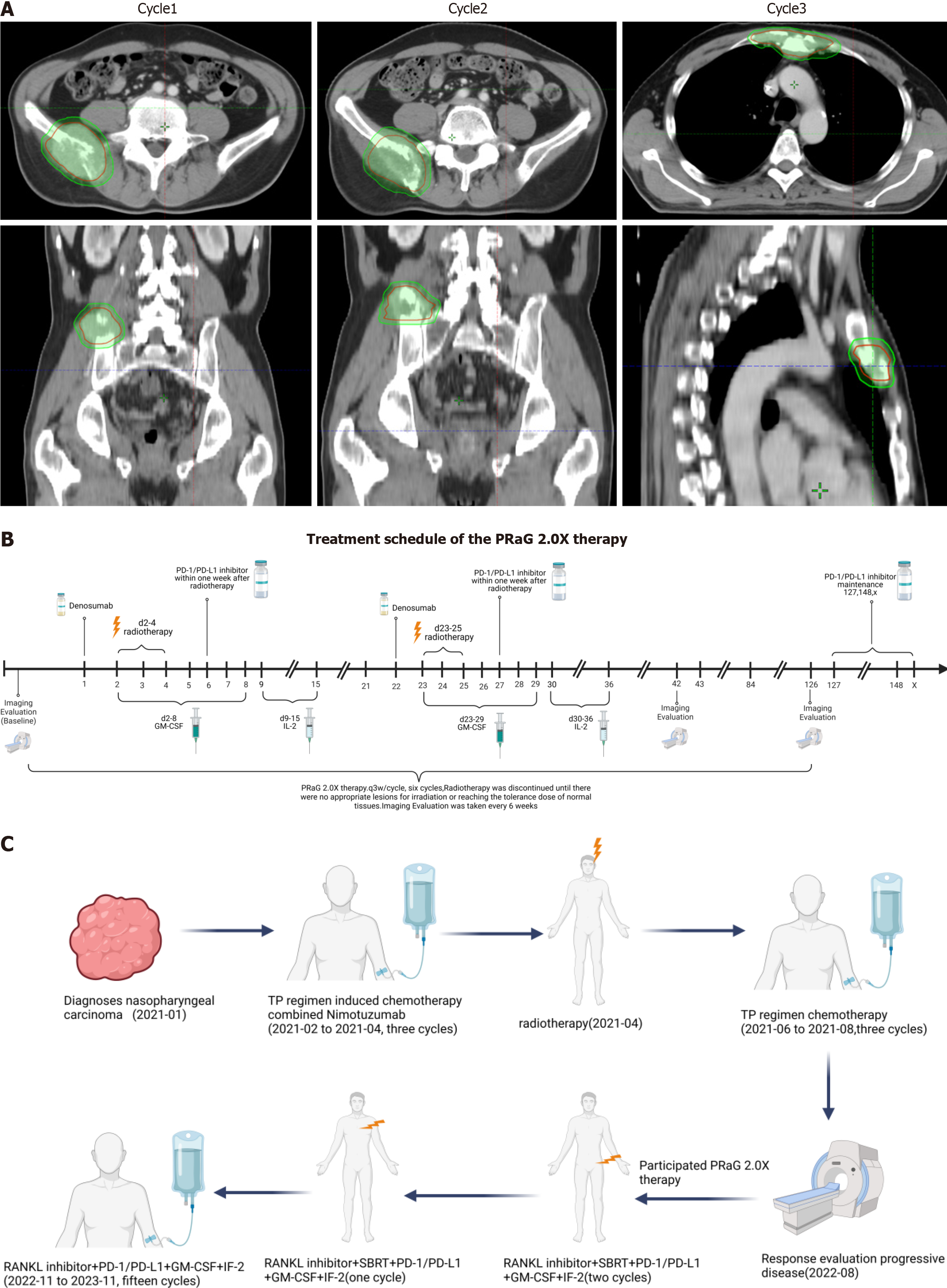
Figure 2 Treatment.
A: The target area of the patient was irradiated by three radiation treatments during the PRaG 2.0X treatment; B: PRaG 2.0X treatment process. The patient received three cycles of RANKL inhibitor combined with radiotherapy and immunotherapy and 15 cycles of RANKL inhibitor combined with immunotherapy for consolidation therapy, including multiple imaging evaluations; C: The entire diagnosis and treatment process of the patient since being diagnosed with nasopharyngeal carcinoma. PD-1: Programmed cell death protein 1; PD-L1: Programmed death receptor-ligand 1; IL: Interleukin; GM-CSF: Granulocyte-macrophage colony-stimulating factor.
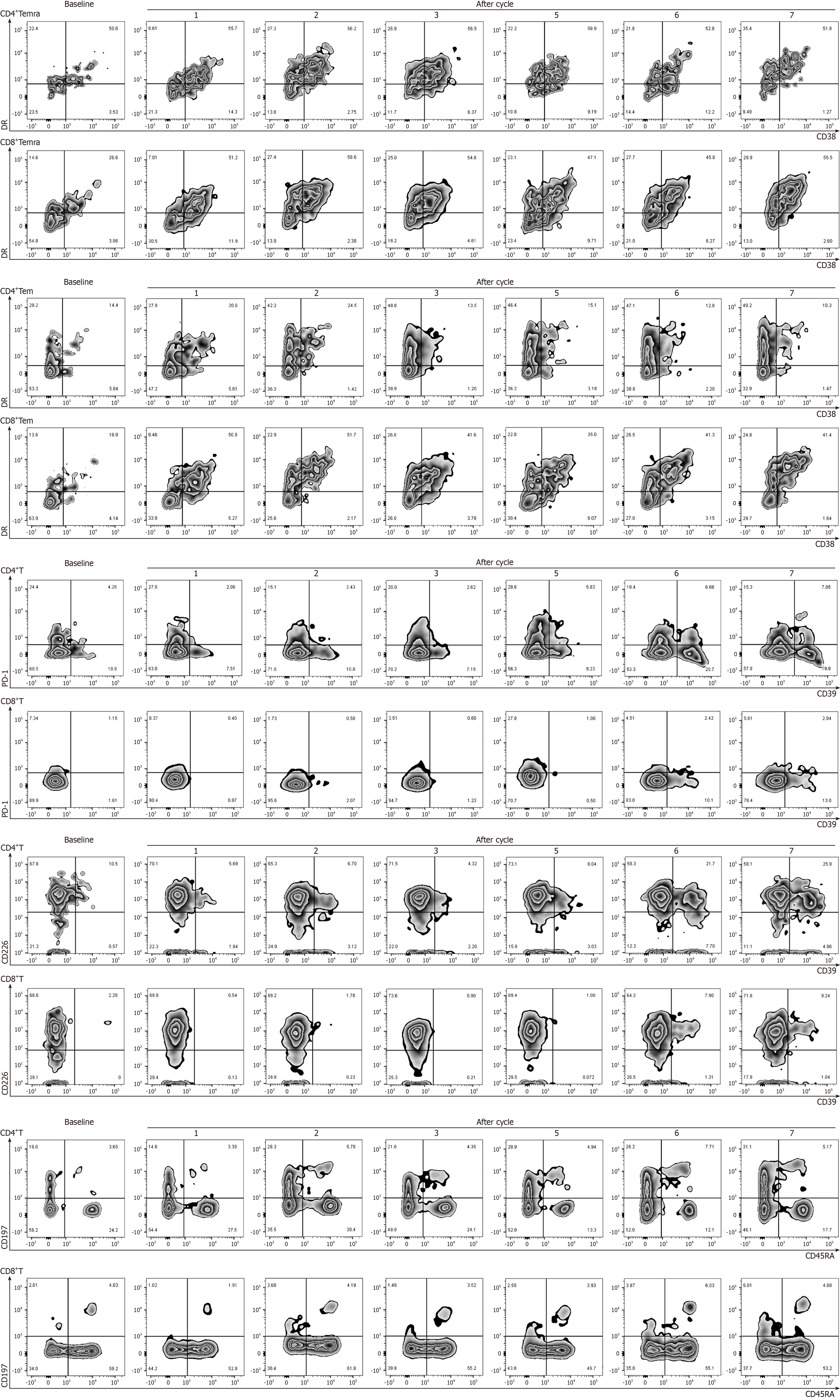
Figure 3 Percentage of peripheral blood lymphocytes during PRaG treatment.
The percentage of CD4+T cells and CD8+T cell subsets increased to varying degrees during PRaG treatment.
- Citation: Chen WW, Kong YH, Zhang LY. Denosumab combined with immunotherapy, radiotherapy, and granulocyte-macrophage colony-stimulating factor for the treatment of metastatic nasopharyngeal carcinoma: A case report. World J Clin Oncol 2025; 16(2): 95642
- URL: https://www.wjgnet.com/2218-4333/full/v16/i2/95642.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i2.95642