Copyright
©The Author(s) 2024.
World J Clin Oncol. Aug 24, 2024; 15(8): 1078-1091
Published online Aug 24, 2024. doi: 10.5306/wjco.v15.i8.1078
Published online Aug 24, 2024. doi: 10.5306/wjco.v15.i8.1078
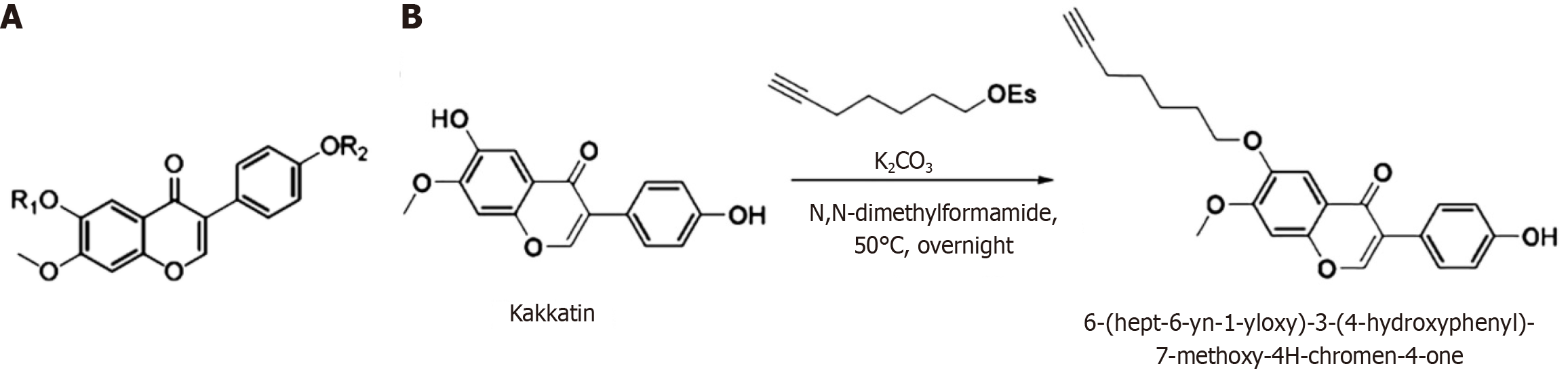
Figure 1 Molecular structure and preparation process of 6-(hept-6-yn-1-yloxy)-3-(4-hydroxyphenyl)-7-methoxy-4H-chromen-4-one.
A: Molecular structure of the kakkatin derivative; B: Process preparation diagram of 6-(hept-6-yn-1-yloxy)-3-(4-hydroxyphenyl)-7-methoxy-4H-chromen-4-one.
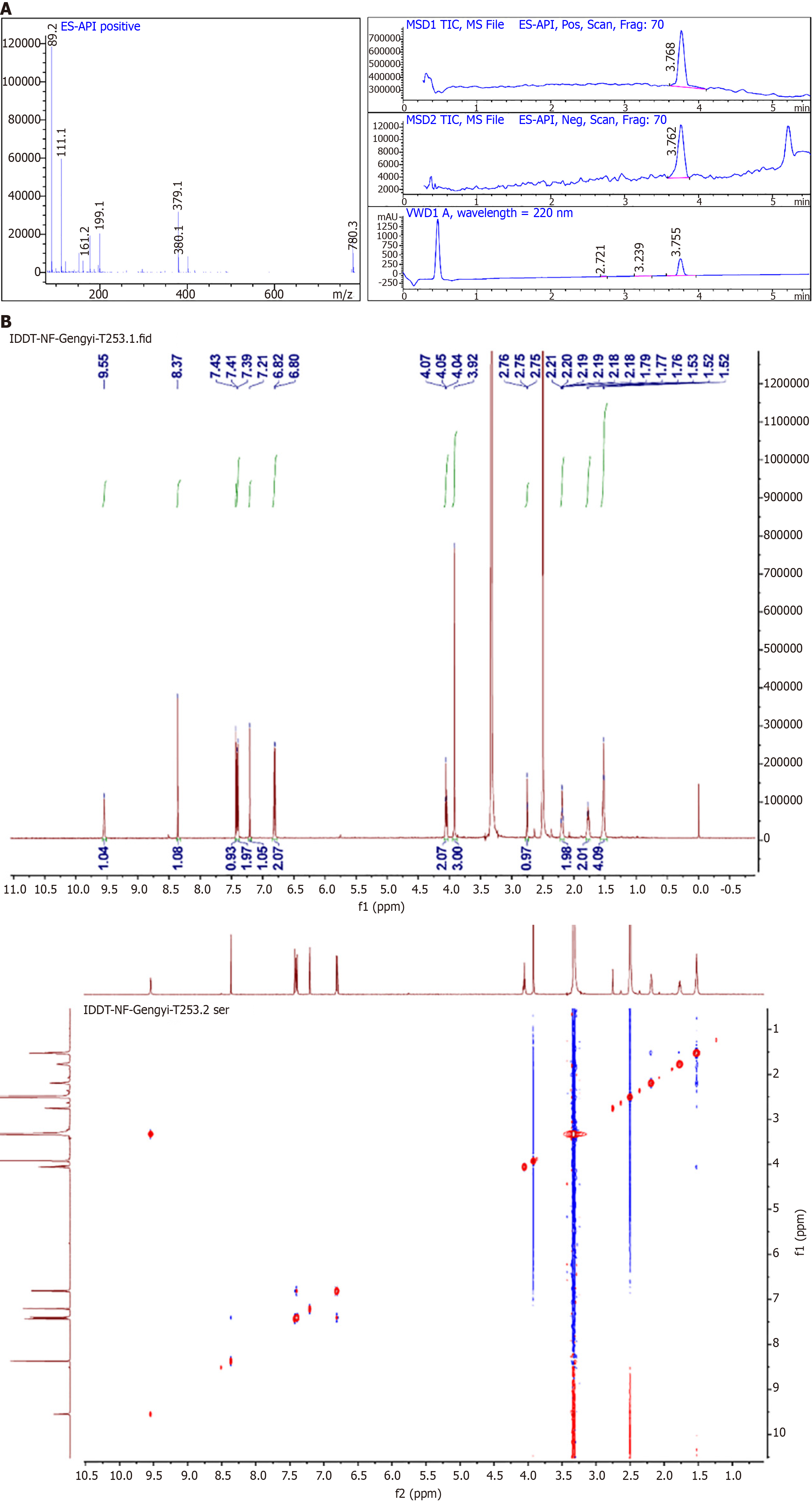
Figure 2 Characterization of 6-(hept-6-yn-1-yloxy)-3-(4-hydroxyphenyl)-7-methoxy-4H-chromen-4-one.
A: Liquid chromatography-mass spectrometry of 6-(hept-6-yn-1-yloxy)-3-(4-hydroxyphenyl)-7-methoxy-4H-chromen-4-one (HK); B: Nuclear magnetic hydrogen spectroscopy of HK.
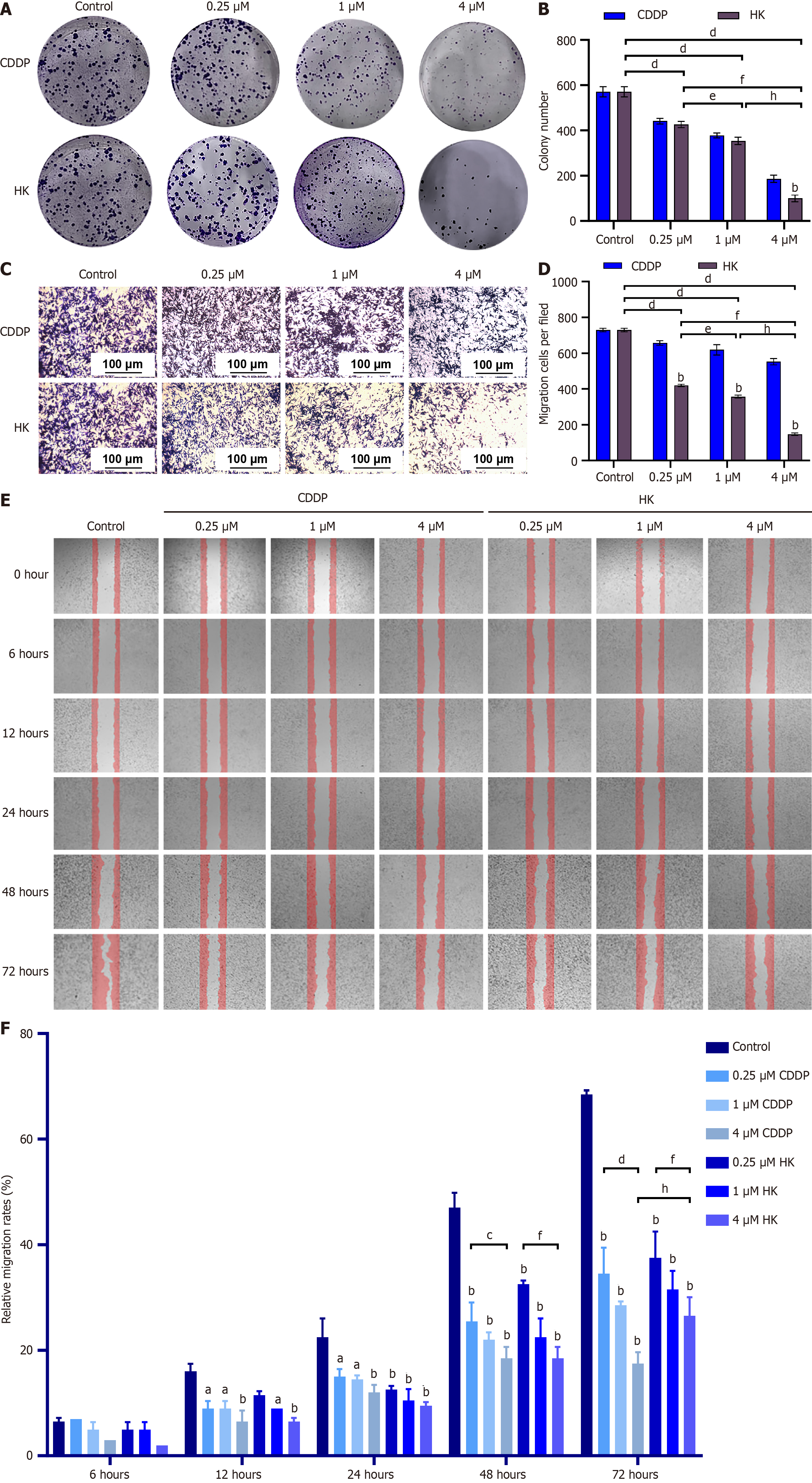
Figure 3 The 6-(hept-6-yn-1-yloxy)-3-(4-hydroxyphenyl)-7-methoxy-4H-chromen-4-one inhibited the proliferation, invasion and metastasis of hepatocellular carcinoma SMMC-7721 cells.
A: Cloning assay evaluated the effect of 6-(hept-6-yn-1-yloxy)-3-(4-hydroxyphenyl)-7-methoxy-4H-chromen-4-one (HK) on the proliferation of SMMC-7721 cells; B: Colony formation assay to quantify cell proliferation; C: Transwell assay to detect the inhibitory effect of HK on the invasion of SMMC-7721 cells; D: Transwell assay to quantify SMMC-7721 cells; E: Scratch assay to detect the inhibitory effect of HK on the lateral migration of SMMC-7721 cells; F: Scratch assay to quantify SMMC-7721 cells. aP < 0.05; bP < 0.01; cP < 0.05; dP < 0.01; eP < 0.05; fP < 0.01; gP < 0.05; hP < 0.01. CDDP: Cisplatin; HK: 6-(hept-6-yn-1-yloxy)-3-(4-hydroxyphenyl)-7-methoxy-4H-chromen-4-one.
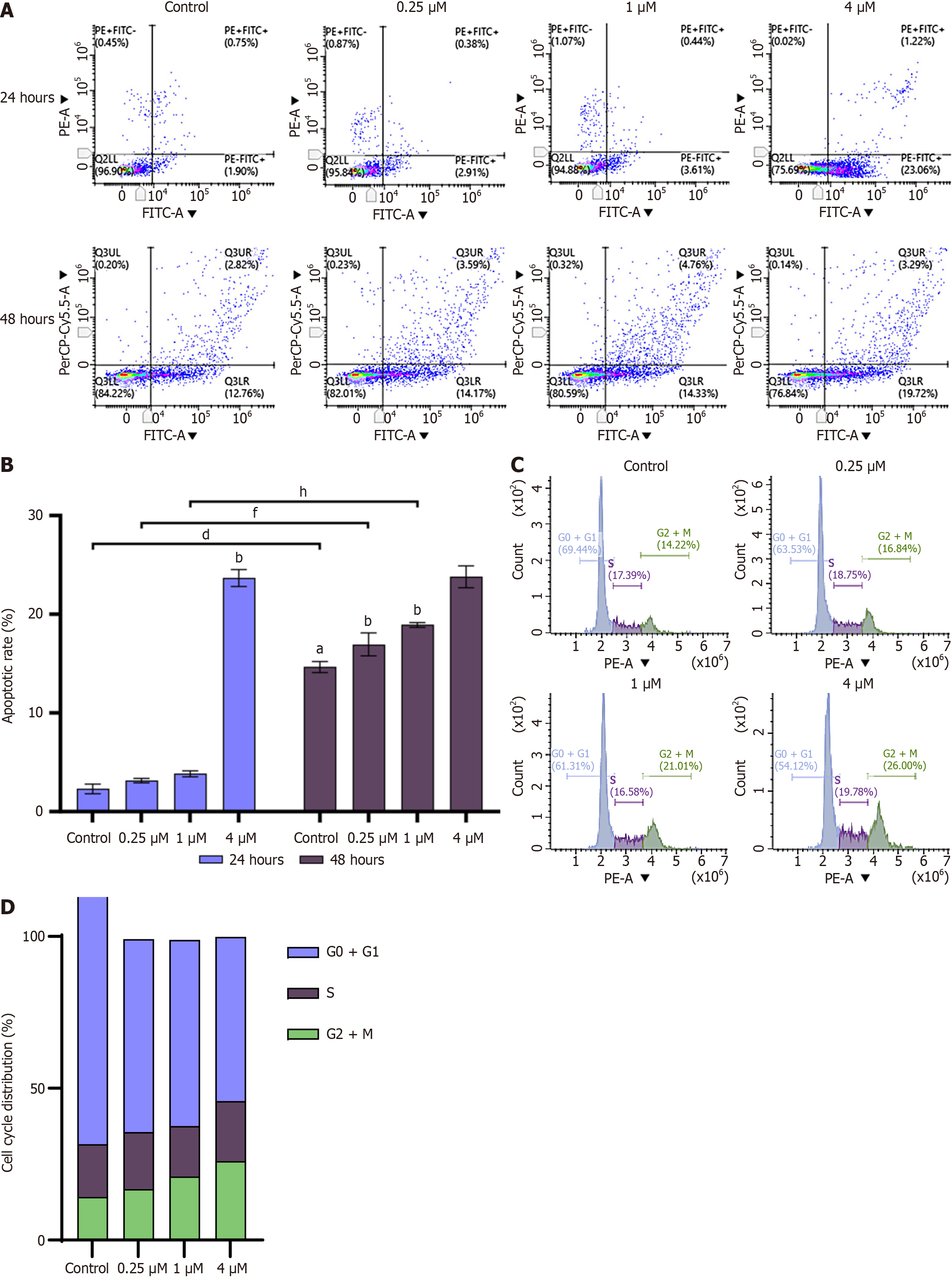
Figure 4 The 6-(hept-6-yn-1-yloxy)-3-(4-hydroxyphenyl)-7-methoxy-4H-chromen-4-one induces apoptosis and cycle arrest in hepato
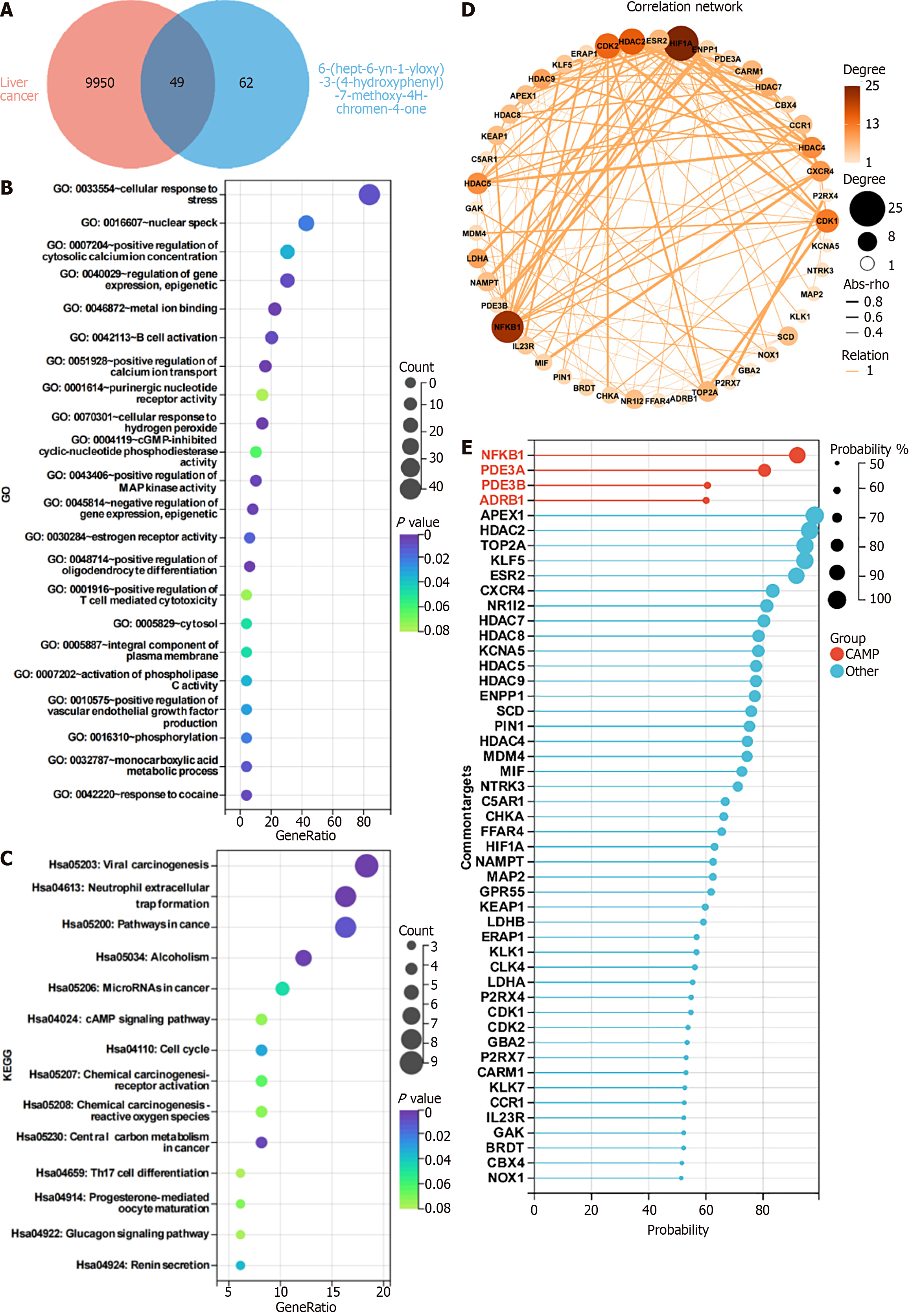
Figure 5 Network pharmacological effects of 6-(hept-6-yn-1-yloxy)-3-(4-hydroxyphenyl)-7-methoxy-4H-chromen-4-one and hepatocellular carcinoma SMMC-7721 cells.
A: Cross-Venn diagram of 6-(hept-6-yn-1-yloxy)-3-(4-hydroxyphenyl)-7-methoxy-4H-chromen-4-one (HK)-related targets and SMMC-7721 cells; B: Gene Ontology enrichment analysis of 49 potential targets of HK in the treatment of SMMC-7721 cells; C: Kyoto Encyclopedia of Genes and Genomes enrichment analysis of 49 potential targets of HK in the treatment of SMMC-7721 cells; D: Correlation network analysis of 49 potential targets of HK in the treatment of SMMC-7721 cells; E: Correlation of 49 targets with HK. GO: Gene Ontology; KEGG: Kyoto Encyclopedia of Genes and Genomes.
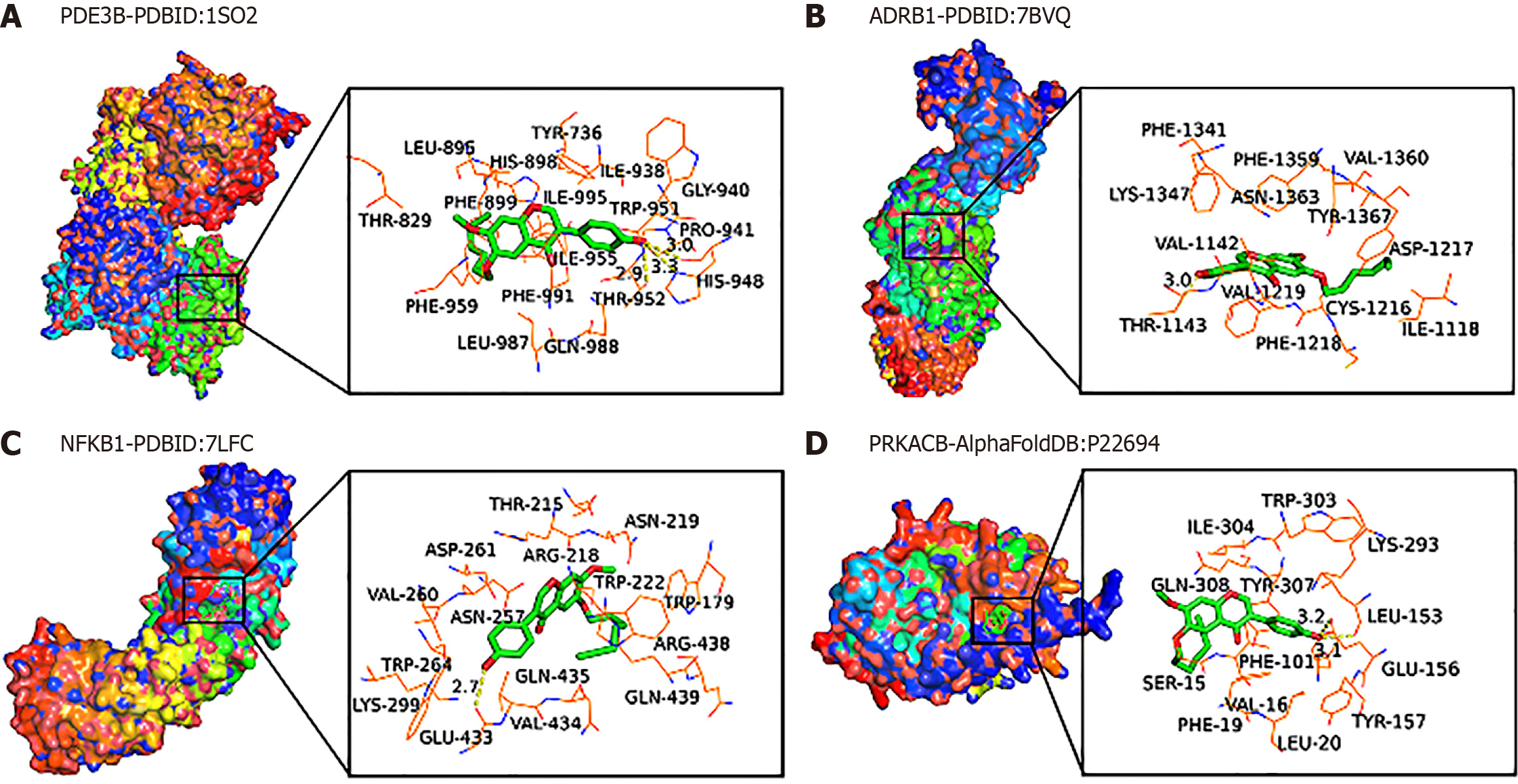
Figure 6 Molecular docking diagram of 6-(hept-6-yn-1-yloxy)-3-(4-hydroxyphenyl)-7-methoxy-4H-chromen-4-one and genes.
A: Molecular docking between PDE3B and 6-(hept-6-yn-1-yloxy)-3-(4-hydroxyphenyl)-7-methoxy-4H-chromen-4-one (HK); B: Molecular docking between ARBD1 and HK; C: Molecular docking between NFKB1 and HK; D: Molecular docking between PRKACB and HK.
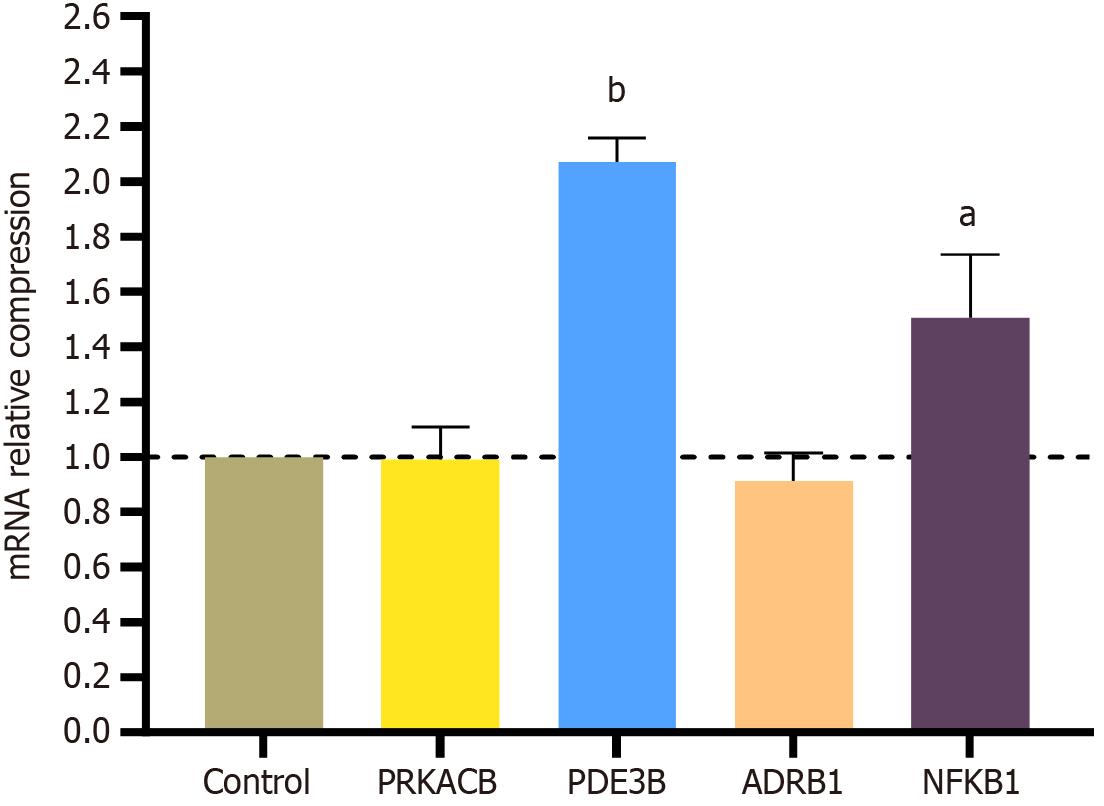
Figure 7 Real-time PCR assays validation.
aP < 0.05; bP < 0.01.
- Citation: Jiang YY, Dong HH, Zhou WT, Luo JZ, Wei X, Huang YQ. Preparation of kakkatin derivatives and their anti-tumor activity. World J Clin Oncol 2024; 15(8): 1078-1091
- URL: https://www.wjgnet.com/2218-4333/full/v15/i8/1078.htm
- DOI: https://dx.doi.org/10.5306/wjco.v15.i8.1078