Copyright
©The Author(s) 2023.
World J Clin Oncol. Dec 24, 2023; 14(12): 606-619
Published online Dec 24, 2023. doi: 10.5306/wjco.v14.i12.606
Published online Dec 24, 2023. doi: 10.5306/wjco.v14.i12.606
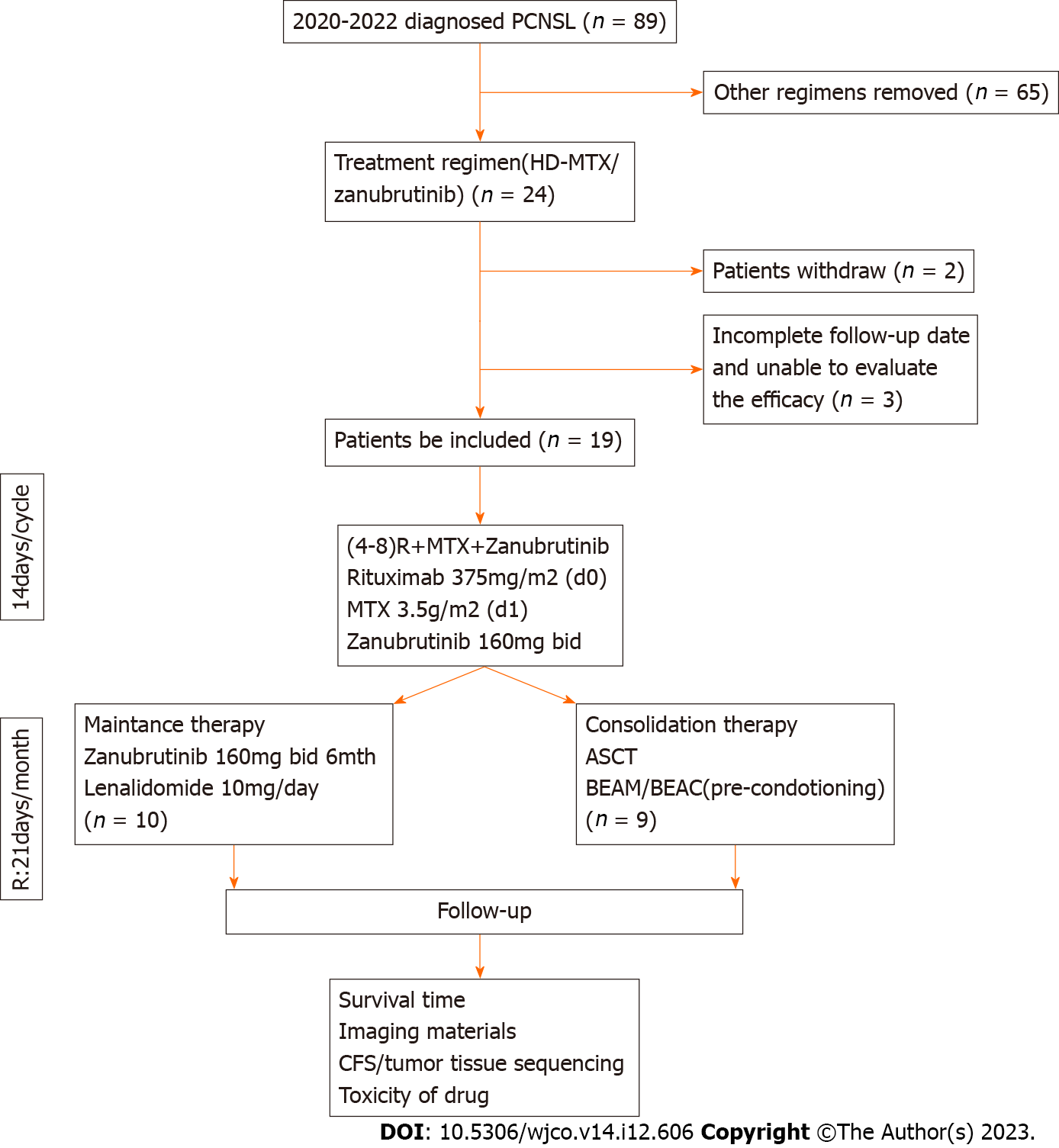
Figure 1 Patient acquisition flow diagram.
PCNSL: Primary central nervous system lymphoma; HD-MTX: High-dose methotrexate; ASCT: Autologous stem cell transplantation; BEAM: Carmustine, etoposide, cytarabine, and melphalan; BEAC: Carmustine, etoposide, cytarabine, and cyclophosphamide; CFS: Cancer Fatigue Scale. R:21 days/month: Lenalidomide for maintenance therapy.
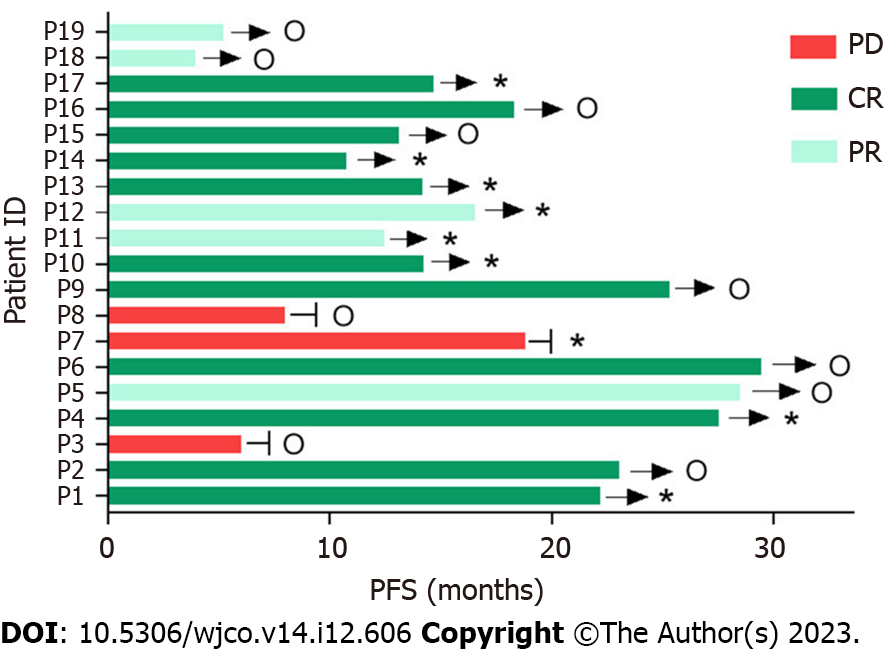
Figure 2 Clinical response and progression-free survival of all patients.
o: using a zanubrutinib-based maintenance regimen. *: Using ASCT as a consolidation regimen; →: Ongoing; -: PD; ID: Identification number; PD: Progressive disease; CR: Complete response; PR: Partial response; PFS: Progression-free survival; ASCT: Autologous stem cell transplantation.
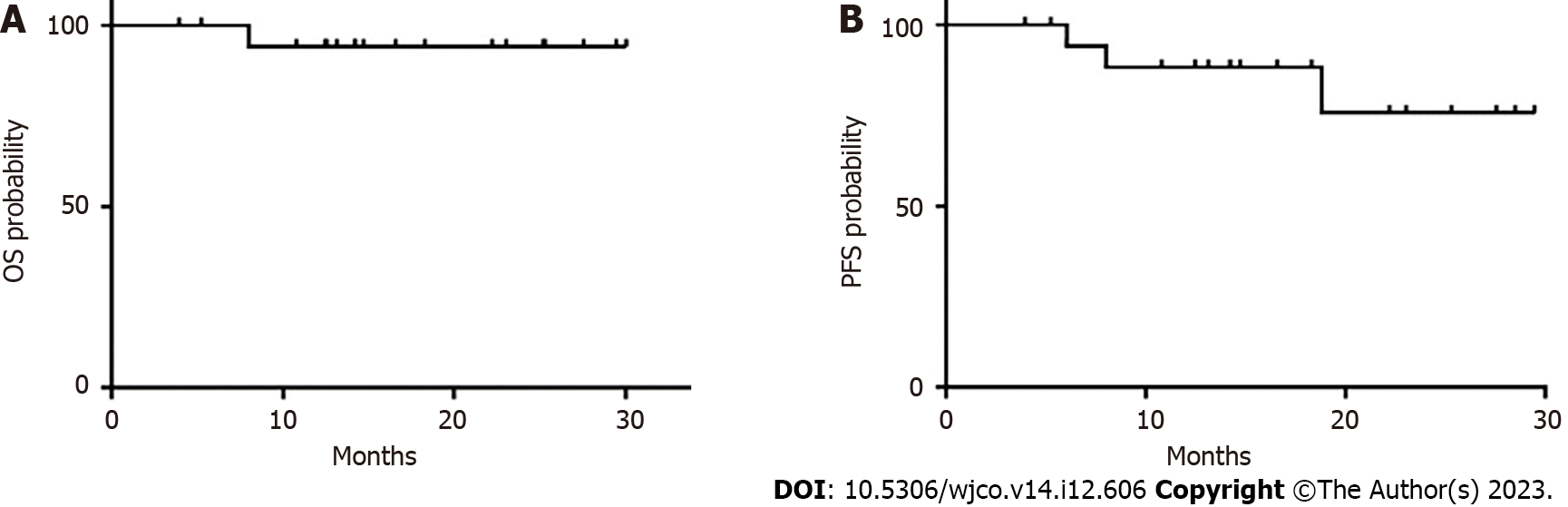
Figure 3 Kaplan-Meier curve for overall survival and progression-free survival.
A: overall survival; B: progression-free survival. OS: Overall survival; PFS: Progression-free survival.
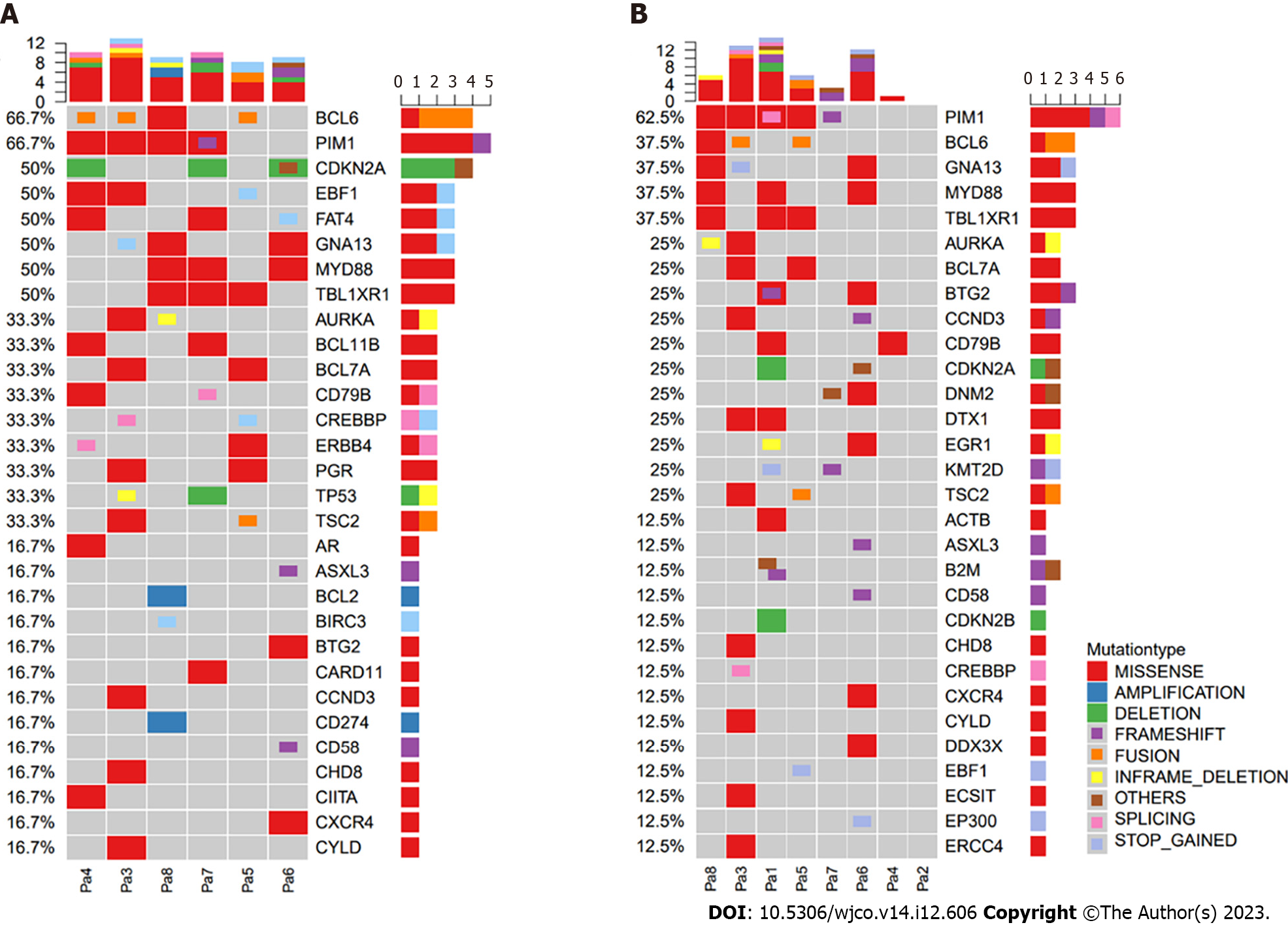
Figure 4 Gene alterations detected in tumor tissue and cerebrospinal fluid.
A: Tumor tissue; B: Cerebrospinal fluid. CSF: Cerebrospinal fluid; P: Patient.
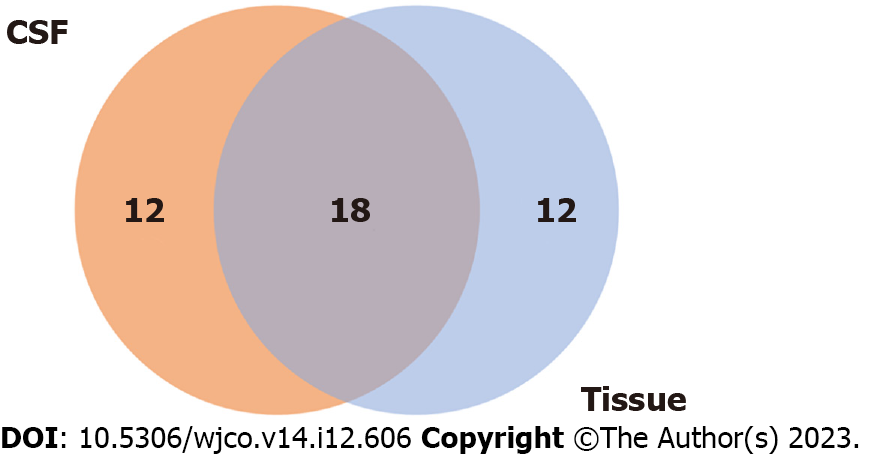
Figure 5 Concordance between baseline primary tumor tissue and cerebrospinal fluid samples for mutation detection.
CSF: Cerebrospinal fluid.
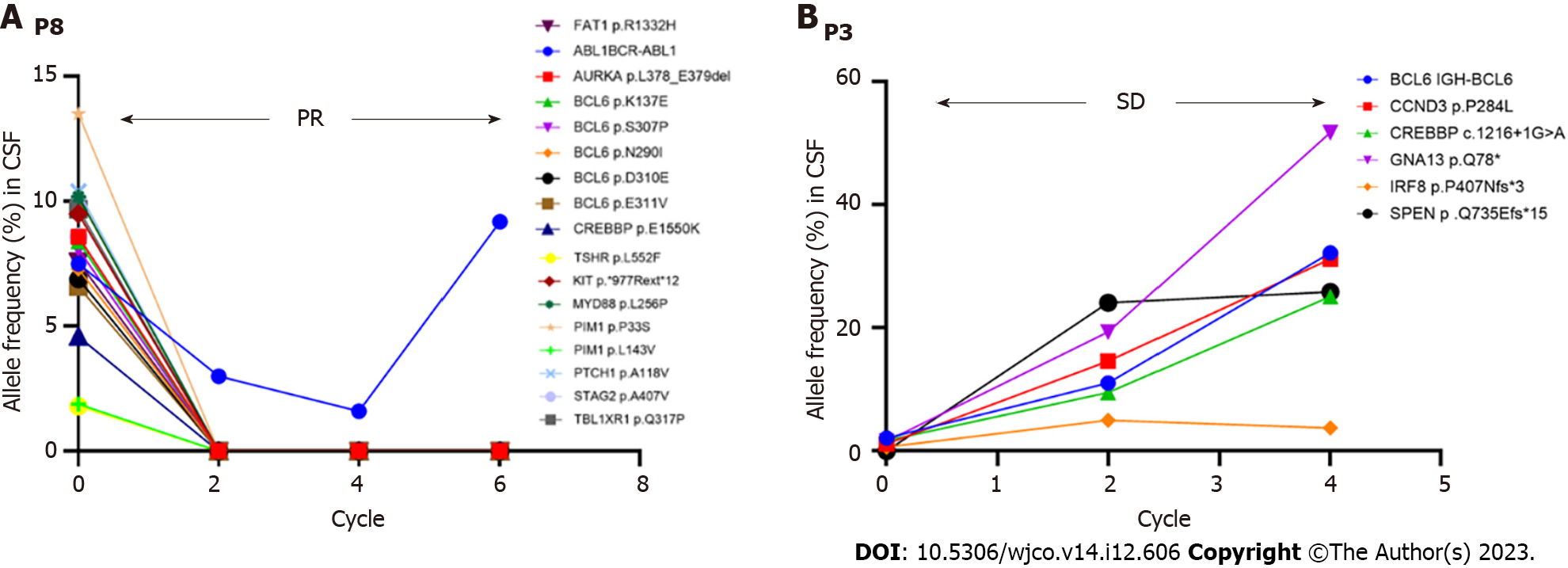
Figure 6 Disease monitoring during therapy by evaluating cerebrospinal fluid circulating tumor DNA.
A: P8; B: P3. PR: Partial response; SD: Stable disease; P8: Patient 8; P3: Patient 3.
- Citation: Wang N, Chen FL, Pan L, Teng Y, Wei XJ, Guo HG, Jiang XM, Huang L, Liu SC, Liang ZL, Li WY. Clinical outcomes of newly diagnosed primary central nervous system lymphoma treated with zanubrutinib-based combination therapy. World J Clin Oncol 2023; 14(12): 606-619
- URL: https://www.wjgnet.com/2218-4333/full/v14/i12/606.htm
- DOI: https://dx.doi.org/10.5306/wjco.v14.i12.606