Copyright
©The Author(s) 2022.
World J Clin Oncol. Jun 24, 2022; 13(6): 505-519
Published online Jun 24, 2022. doi: 10.5306/wjco.v13.i6.505
Published online Jun 24, 2022. doi: 10.5306/wjco.v13.i6.505
Figure 1 Diagram of the administration schedule for the determination of cell viability or cell sensitivity to chemotherapy.
PX: Paclitaxel; NIC: Nicotine.
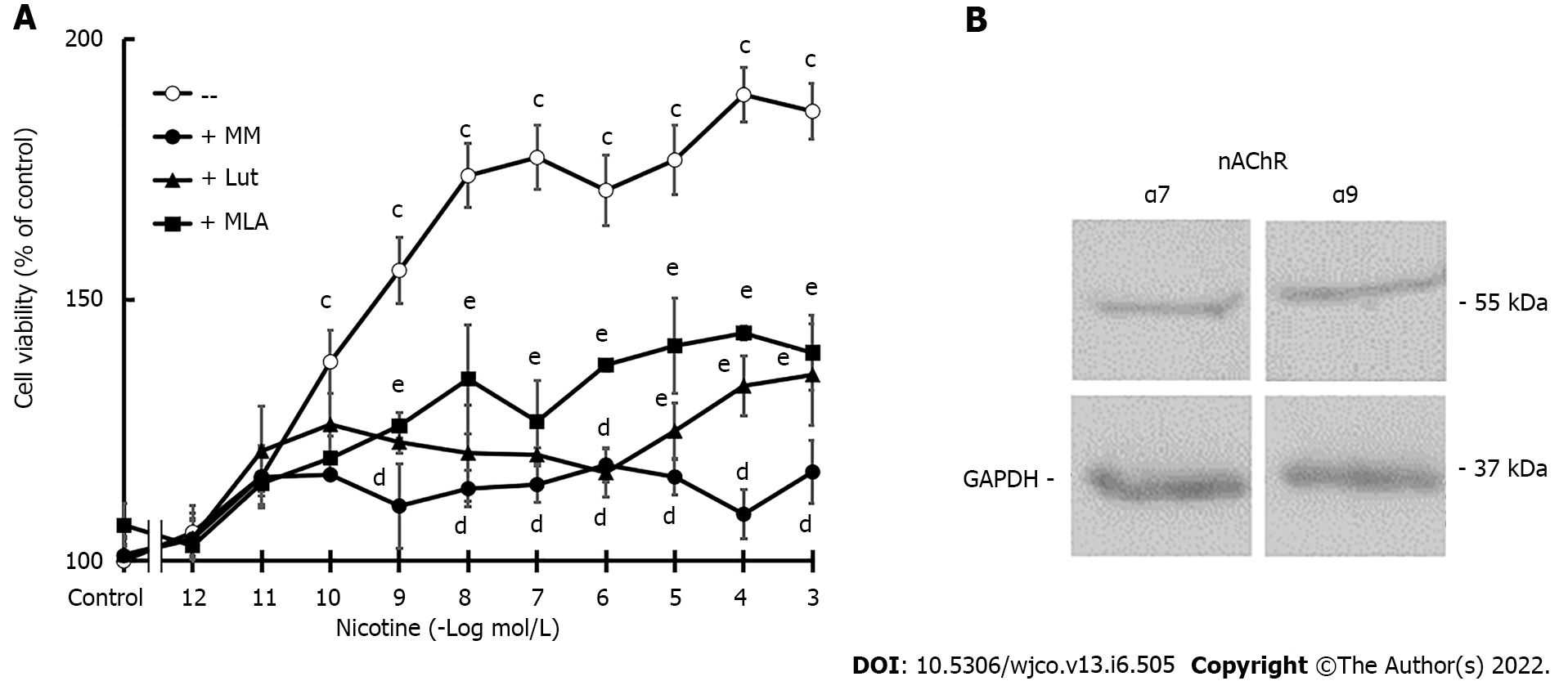
Figure 2 MDA-MB-231 cell viability.
A: Concentration-response curves of nicotine on cell viability in the absence or presence of nicotinic antagonists: mecamylamine [non-selective for nicotinic acetylcholine receptors (nAChRs)], methyllycaconitine (selective for α7 nAChRs), or luteolin (selective for α9 nAChRs) at a concentration of 10-6 mol/L. Values are the mean ± SD of five experiments performed in duplicate. cP < 0.001 vs control; dP < 0.001 vs nicotine; eP < 0.001 vs control or nicotine; B: Western blot assay to detect α7 and α9 nAChR expression. Molecular weights are indicated on the right. The expression of glyceraldehyde 3-phosphate dehydrogenase was used as the loading control. One representative experiment of three is shown. MM: Mecamylamine; MLA: Methyllycaconitine; Lut: Luteolin; nAChRs: Nicotinic acetylcholine receptors; GAPDH: Glyceraldehyde 3-phosphate dehydrogenase.
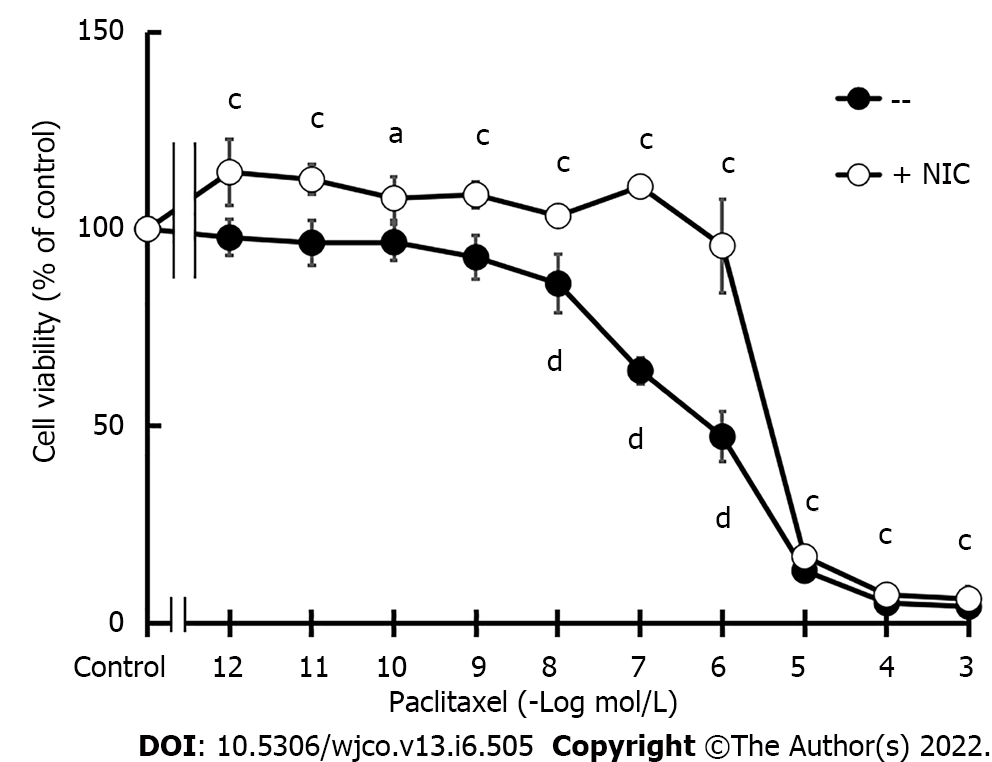
Figure 3 MDA-MB-231 cell viability.
Concentration-response curves of paclitaxel on cell viability in the absence or presence of nicotine (NIC) (10-10 mol/L). Values are the mean ± SD of six experiments performed in duplicate. aP < 0.05; cP < 0.001 vs Control; dP < 0.001 vs +NIC. NIC: Nicotine.
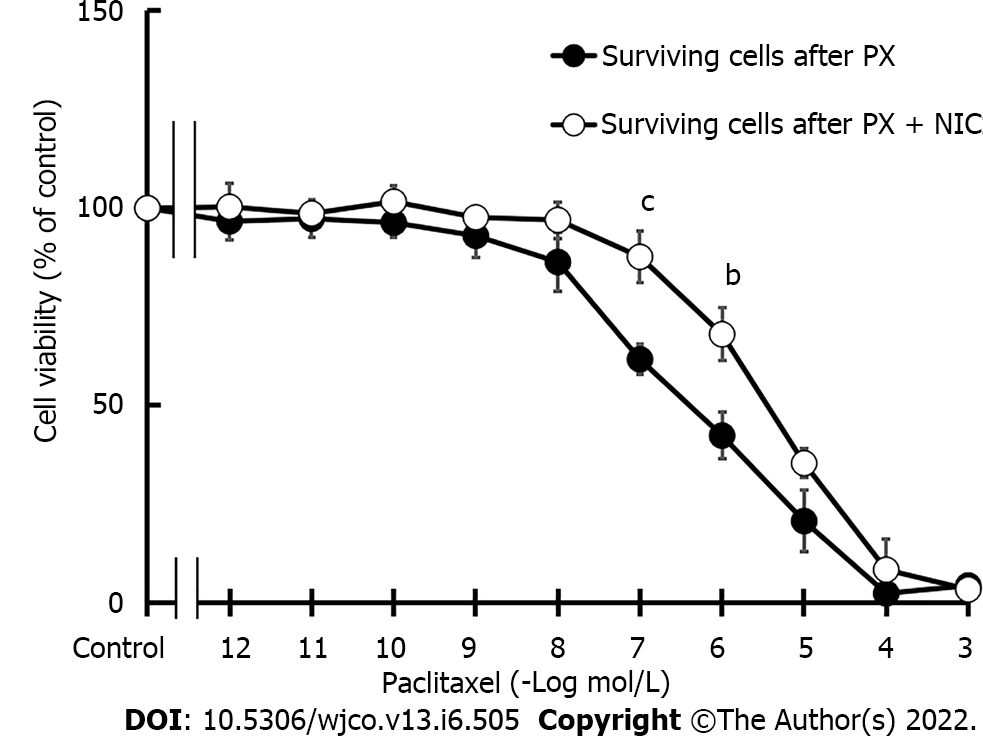
Figure 4 Sensitivity of MDA-MB-231 cells to chemotherapy.
Concentration-response curves of paclitaxel (PX) on the viability of surviving cells after three cycles of PX treatment (10-7 mol/L) in the absence or presence of nicotine (10-10 mol/L). Values are the mean ± SD of three experiments performed in duplicate. bP < 0.01; cP < 0.001 vs surviving cells after three cycles of PX treatment. PX: Paclitaxel; NIC: Nicotine.
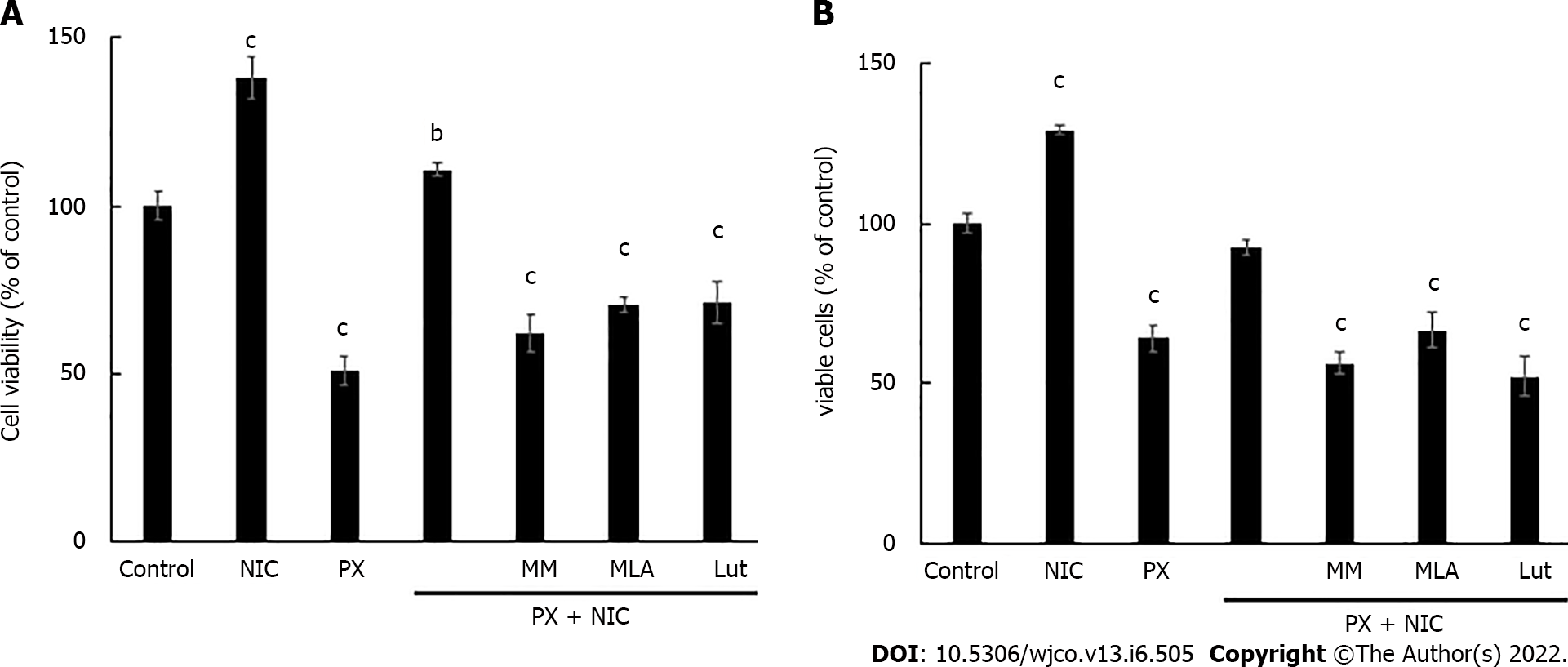
Figure 5 Effect of nicotine on paclitaxel treatment.
A: Viability determination of MDA-MB-231 cells treated with nicotine (10-10 mol/L) and paclitaxel (10-7 mol/L) alone or in combination, in the absence or presence of nicotinic antagonists: mecamylamine [non-selective for nicotinic acetylcholine receptors (nAChRs)], methyllycaconitine (selective for α7 nAChRs), or luteolin (selective for α9 nAChRs) at a concentration of 10-6 mol/L; B: Determination of percentage of living MDA-MB-231 cells treated with the same drug combinations as those shown in Figure 5A. Values are the mean ± SD of four experiments performed in duplicate. bP < 0.01; cP < 0.001 vs control, considered as 100%. MM: Mecamylamine; MLA: Methyllycaconitine; Lut: Luteolin; PX: Paclitaxel; NIC: Nicotine.
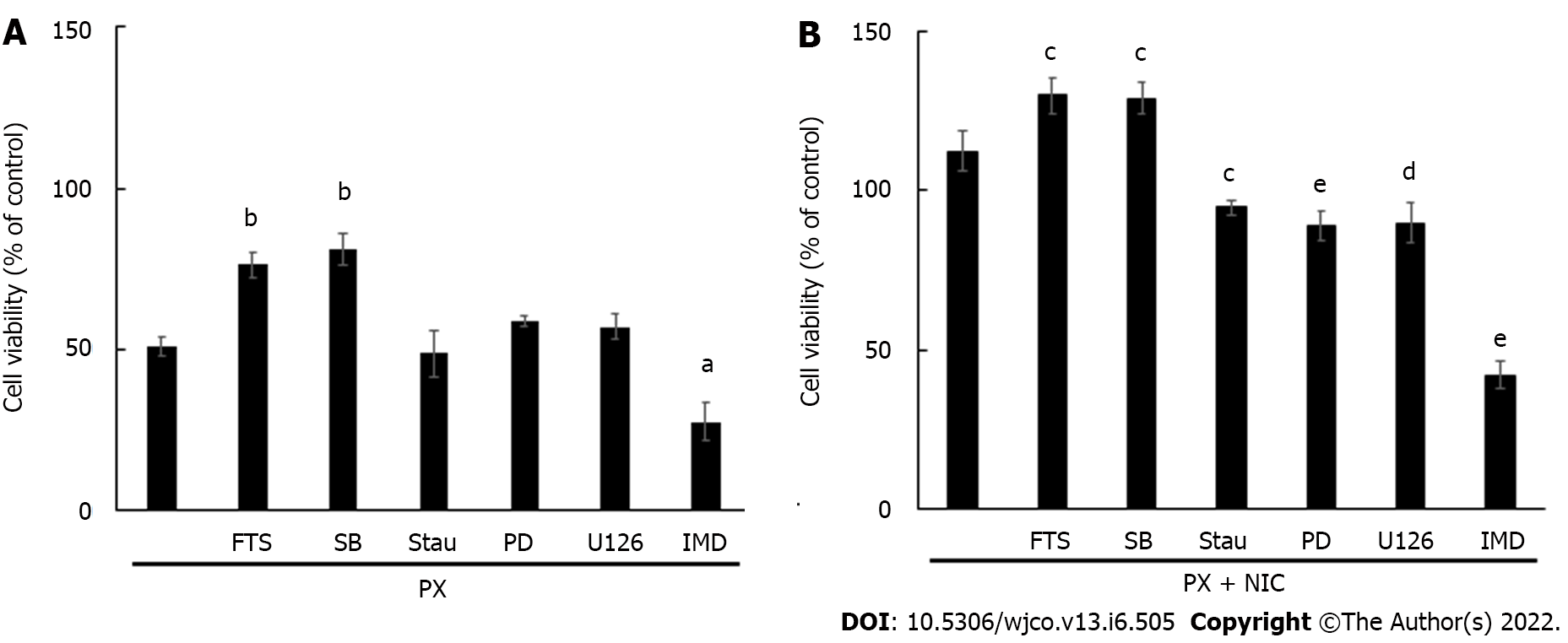
Figure 6 Effect of paclitaxel and nicotine on MDA-MB-231 cell viability.
A: Cells were treated with paclitaxel (PX) (10-7 mol/L) and the mediators were evaluated in the absence or presence of the kinase inhibitors for: PKC (staurosporine, 10-8 mol/L), MEK (PD098059 PD, 10-5 mol/L), Ras (S-trans, trans-farnesylthiosalicylic acid, 10-6 mol/L), ERK1/2 (U126, 10-5 mol/L), p38MAPK (SB203580, 10-5 mol/L) or IKKβ (IMD354, 5 × 10-8 mol/L); B: Cells were treated with the combination of PX and nicotine (NIC) (10-10 mol/L) as well as with the same inhibitors as those shown in Figure 6A. Values are the mean ± SD of four experiments performed in duplicate. aP < 0.05; bP < 0.01 vs PX. cP < 0.05; dP < 0.01; eP < 0.001 vs PX+NIC. FTS: S-trans, trans-farnesylthiosalicylic acid; SB: SB203580; Stau: Staurosporine; PD: PD098059; PX: Paclitaxel; NIC: Nicotine.
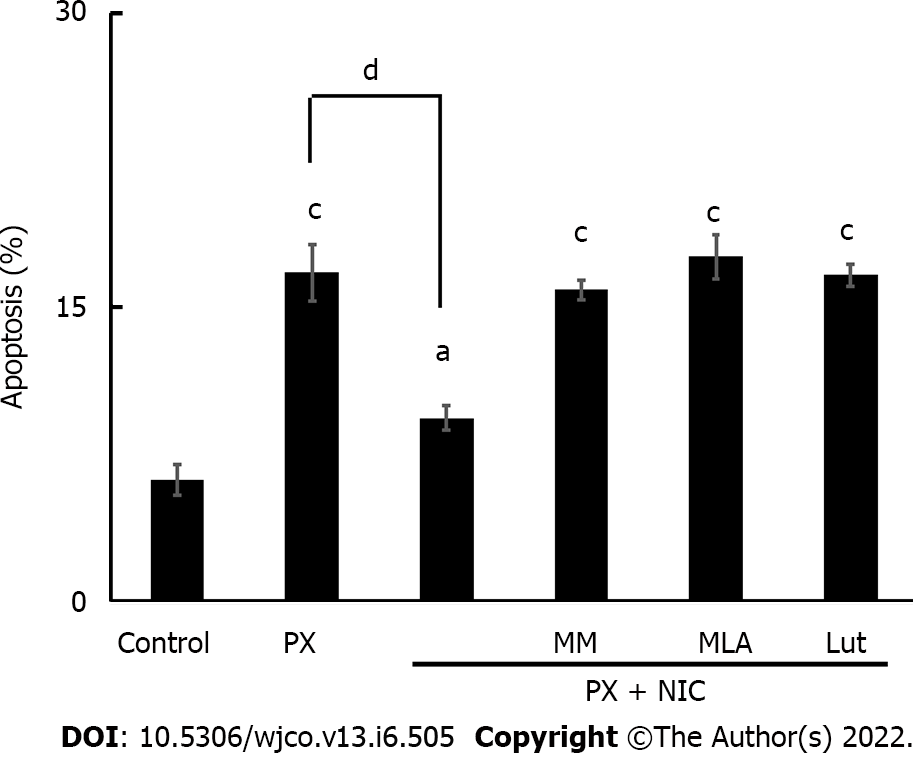
Figure 7 Effect of nicotine on paclitaxel-induced apoptosis in MDA-MB-231 cells.
Tumor cells were treated with paclitaxel (10-7 mol/L) in the absence or presence of the following nicotinic antagonists: mecamylamine [non-selective for nicotinic acetylcholine receptors (nAChRs)], methyllycaconitine (selective for α7 nAChRs), or luteolin (selective for α9 nAChRs) at a concentration of 10-6 mol/L. The percentage of apoptotic cells was determined by flow cytometry. Values are the mean ± SD of four experiments performed in duplicate. aP < 0.05; bP < 0.01; cP < 0.001 vs control; dP < 0.001. MM: Mecamylamine; MLA: Methyllycaconitine; Lut: Luteolin; PX: Paclitaxel; NIC: Nicotine.
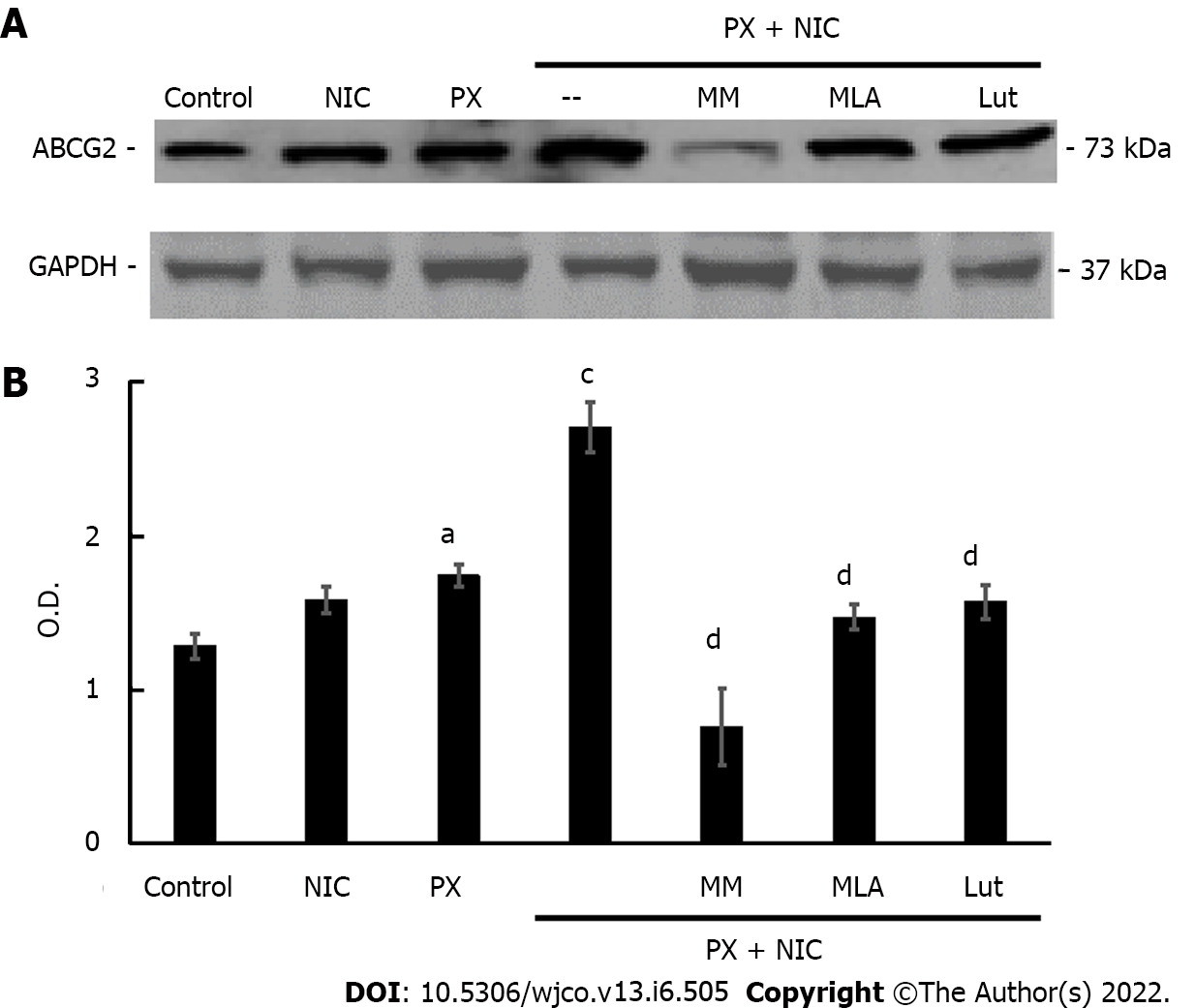
Figure 8 Effect of nicotine on paclitaxel-induced expression of ATP binding cassette transporter G2 protein in MDA-MB-231 cells.
A: ATP binding cassette transporter G2 expression was determined by Western blot assays in cells treated with paclitaxel (10-7 mol/L), nicotine (10-10 mol/L) or both, in the absence or presence of the nicotinic antagonists mecamylamine [non-selective for nicotinic acetylcholine receptors (nAChRs)], methyllycaconitine (selective for α7 nAChRs) or luteolin (selective for α9 nAChRs) at a concentration of 10-6 mol/L. Molecular weights are indicated on the right; B: The densitometric analysis of the bands is expressed as optical density units relative to the expression of glyceraldehyde 3-phosphate dehydrogenase protein used as the loading control. One representative experiment of three is shown. Values are the mean ± SD of three experiments. aP < 0.05; cP < 0.001 vs Control; dP < 0.001 vs PX+NIC. ABCG2: ATP “binding cassette” G2 drug transporter; O.D.: Optical density; MM: Mecamylamine; MLA: Methyllycaconitine; Lut: Luteolin; nAChRs: Nicotinic acetylcholine receptors; GAPDH: Glyceraldehyde 3-phosphate dehydrogenase; PX: Paclitaxel; NIC: Nicotine.
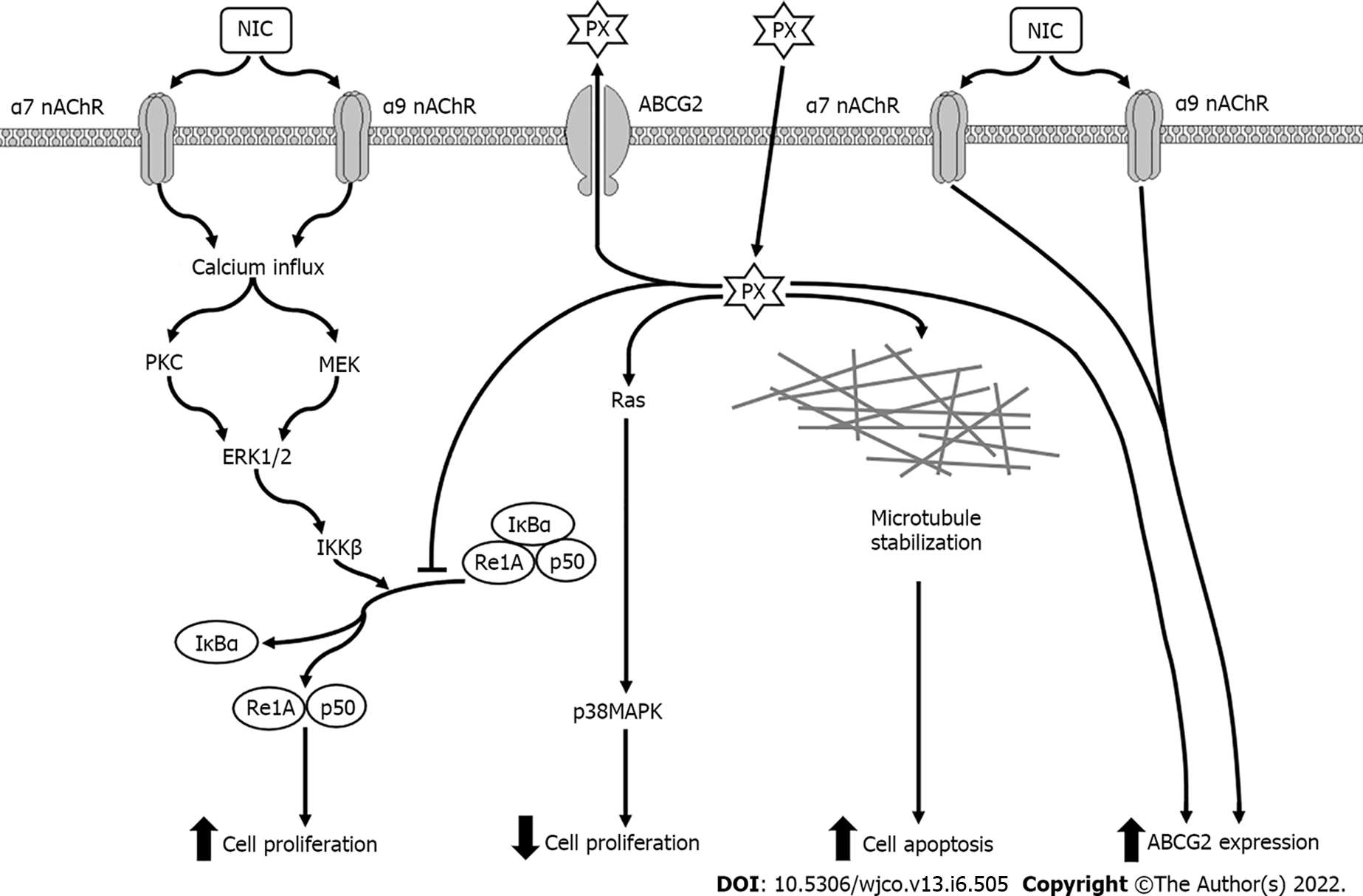
Figure 9 Possible signal transduction pathways in MDA-MB-231 cells activated by paclitaxel in the absence or presence of nicotine.
NIC: Nicotine; PX: Paclitaxel; nAChR: Nicotinic acetylcholine receptors; ABCG2: ATP “binding cassette” G2 drug transporter; PKC: Protein kinase C; MEK: Mitogen-activated protein kinase kinase; ERK: Extracellular signal-regulated kinases; p38MAPK: p38 Mitogen-activated protein kinases; IKKβ: IκB kinase; IκBα: κB inhibitors.
- Citation: Español A, Sanchez Y, Salem A, Obregon J, Sales ME. Nicotinic receptors modulate antitumor therapy response in triple negative breast cancer cells. World J Clin Oncol 2022; 13(6): 505-519
- URL: https://www.wjgnet.com/2218-4333/full/v13/i6/505.htm
- DOI: https://dx.doi.org/10.5306/wjco.v13.i6.505