Copyright
©The Author(s) 2022.
World J Clin Oncol. May 24, 2022; 13(5): 323-338
Published online May 24, 2022. doi: 10.5306/wjco.v13.i5.323
Published online May 24, 2022. doi: 10.5306/wjco.v13.i5.323
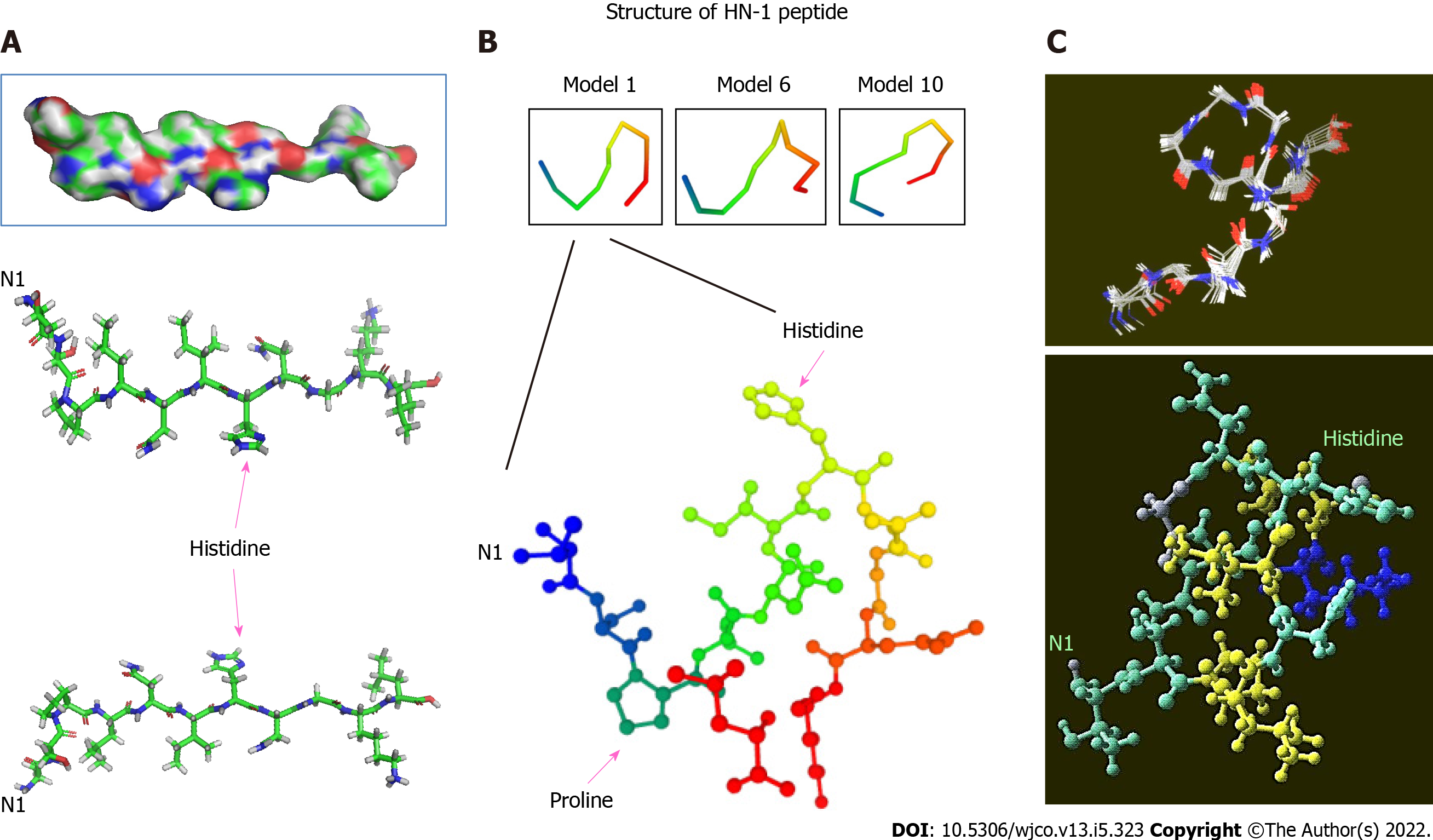
Figure 1 Three-dimensional structure of HN-1 peptide.
A: A 3D model of HN-1 peptide generated using PyMol molecular graphics system, version 1.2r3pre, Schrödinger, LLC. All graphics depict an identical configuration with the bottom two panels in the opposite orientation; B: An ensemble of de novo conformations generated by PEP-FOLD (INSERM, France) in RPBS bioinformatics web portal: https://bioserv.rpbs.univ-paris-diderot.fr/services/PEP-FOLD/; C: A 3-dimensional profile of the lowest energy structure obtained for HN-1 peptide was viewed using Raswin computer modeling software. All structures were generated using TSPLNIHNGQKL as the raw input peptide sequence. N1: The N-terminal residue.
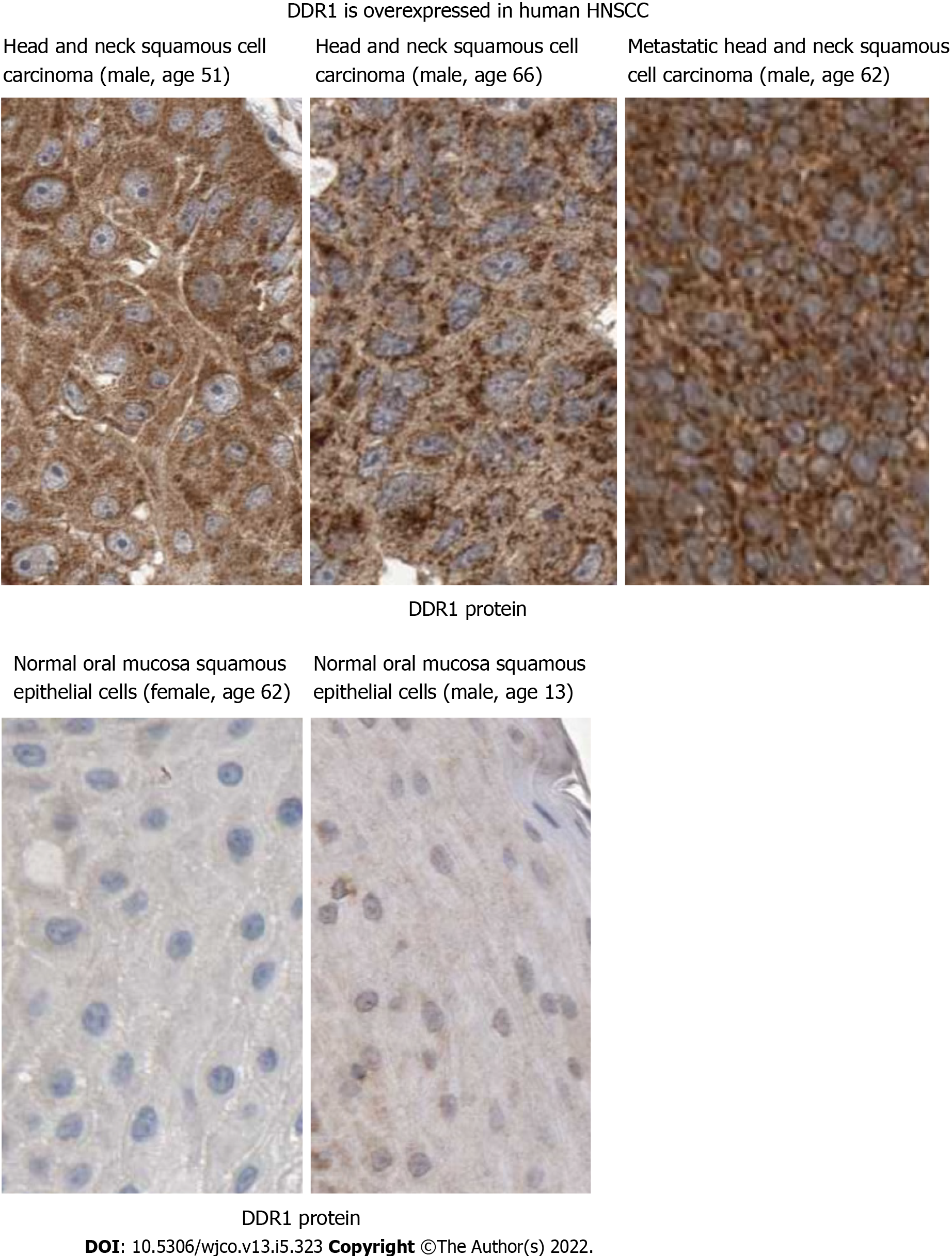
Figure 2 Discoidin domain receptor 1 may mediate HN-1 endocytosis.
Discoidin domain receptor 1 (DDR1) protein is upregulated in human head and neck squamous cell carcinoma. Immunohistochemical analysis of DDR1 was conducted by comparing tumor vs normal tissues in Human Protein Atlas database (http://www.proteinatlas.org/). The results showed positive cytoplasmic and membranous staining. Bar: 25 micrometers; DDR1: Discoidin domain receptor 1.
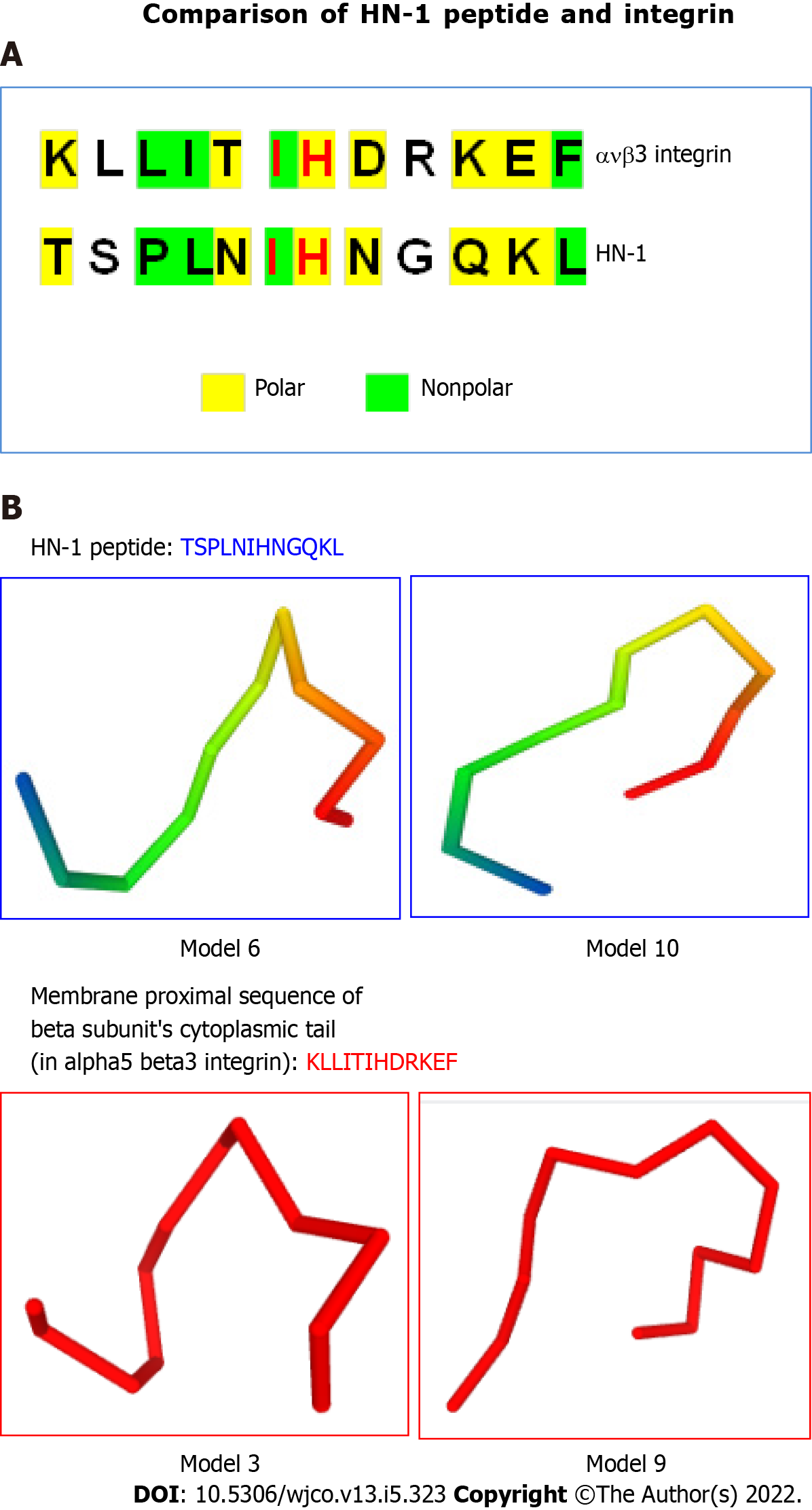
Figure 3 HN-1 peptide exhibits similarity to integrin peptide.
A: The similarities between the HN-1 sequence (TSPLNIHNGQKL) and a stretch of amino acids in alpha5 beta3 integrin (KLLITIHDRKEF) are highlighted. As HN-1 peptide lacks the recognition motif (RGD) of integrin, HN-1 may interact with an "integrin-like" molecule. HN-1 is internalized by attached cells but not by suspended cells. As tissue culture plates are typically coated with collagen, collagen-binding DDR1 receptor (expressed by adherent but not suspended cells) may represent the receptor for HN-1 consistent with that DDR1 is overexpressed in human head and neck cancer (also breast cancer) targeted by HN-1. avb3: alpha5 beta3; B: Comparison of 3D models generated by PEP-FOLD (INSERM, France) in RPBS bioinformatics web portal: https://bioserv.rpbs.univ-paris-diderot.fr/services/PEP-FOLD/ TSPLNIHNGQKL: HN-1 peptide (top panels); KLLITIHDRKEF, membrane proximal sequence of beta subunit’s cytoplasmic tail in alpha5 beta3 integrin (bottom panels); TSPLNIHNGQKL: Thr-Ser-Pro-Leu-Asn-Ile-His-Asn-Gly-Gln-Lys-Leu; KLLITIHDRKEF: Lys-Leu-Leu-Ile-Thr-Ile-His-Asp-Arg-Lys-Glu-Phe.
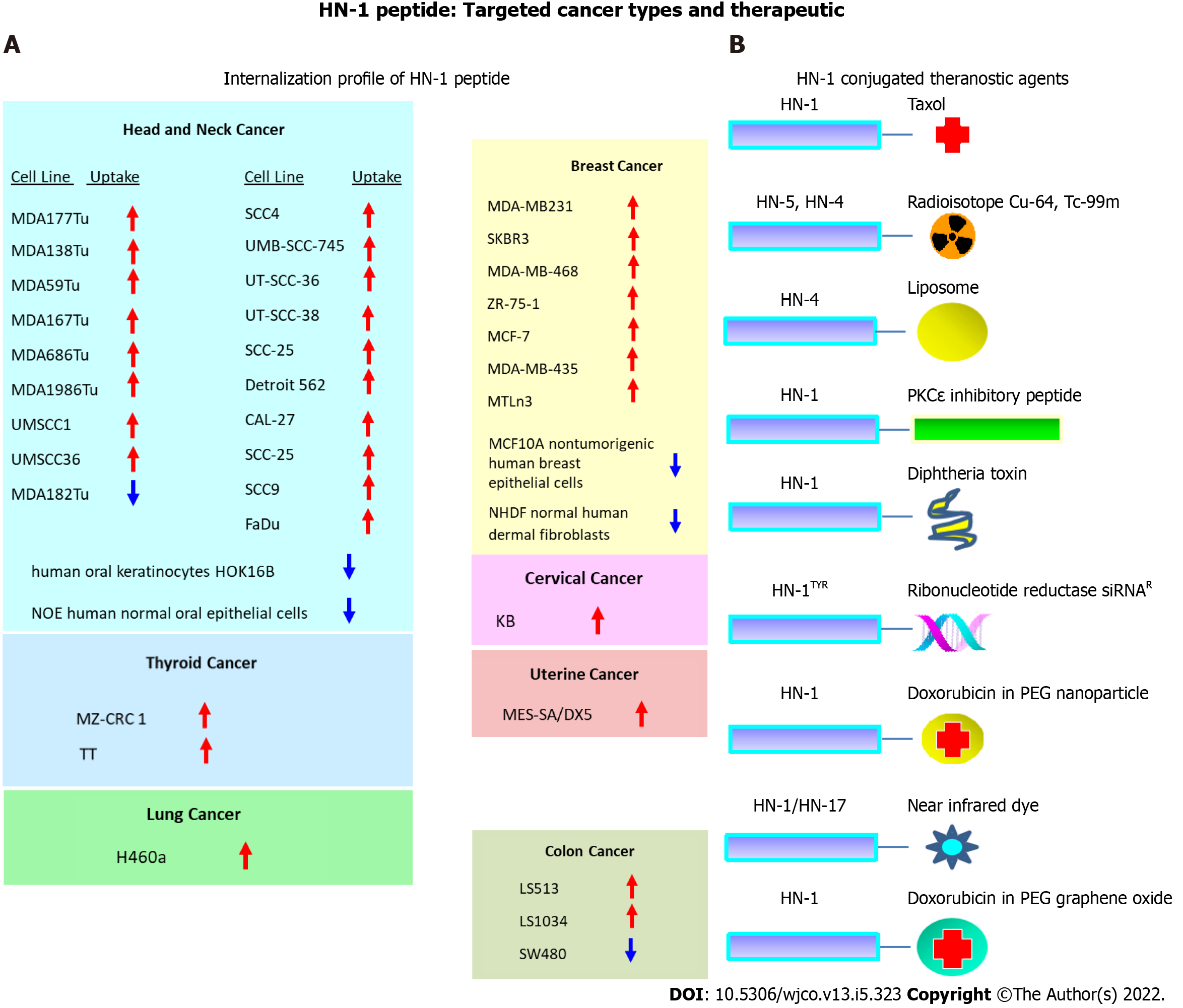
Figure 4 The repertoire of targeted cancers and therapeutic application of HN-1 peptide.
A: HN-1 uptake profile. The internalization of HN-1 peptide by various human cancer cells vs the corresponding normal human cells was compared. HN-1 uptake: red arrow (internalized), blue arrow (undetectable); B: Previously developed HN-1 based conjugates for therapy or diagnosis. Note: whether HN-17 peptide (consisting of a permutated version of HN-1 sequence) enters cells via the same route as HN-1 peptide or through a distinct route is not known. HOK: Human oral keratinocyte; NOE: Normal oral epithelial; NHDF: Normal human dermal fibroblasts; Cu: Copper; Tc: Technetium; PKCe: Protein kinase C epsilon; siRNA: Small interfering RNA; PEG: Polyethylene glycol.
- Citation: Hong FU, Castro M, Linse K. Tumor specifically internalizing peptide ‘HN-1’: Targeting the putative receptor retinoblastoma-regulated discoidin domain receptor 1 involved in metastasis. World J Clin Oncol 2022; 13(5): 323-338
- URL: https://www.wjgnet.com/2218-4333/full/v13/i5/323.htm
- DOI: https://dx.doi.org/10.5306/wjco.v13.i5.323