Copyright
©The Author(s) 2021.
World J Clin Oncol. Aug 24, 2021; 12(8): 646-655
Published online Aug 24, 2021. doi: 10.5306/wjco.v12.i8.646
Published online Aug 24, 2021. doi: 10.5306/wjco.v12.i8.646
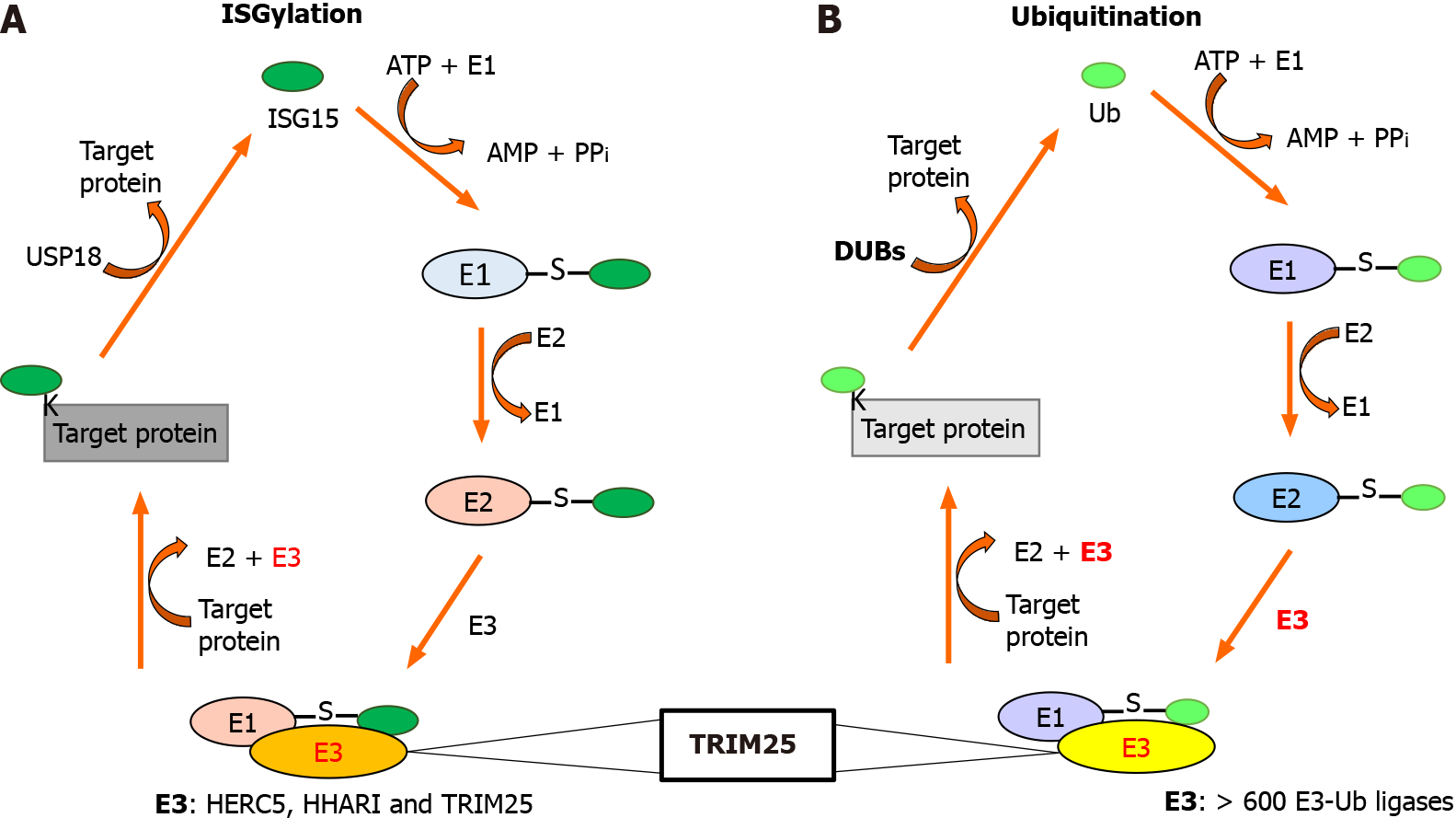
Figure 1 TRIM25 is an E3-ligase common for interferon-stimulated gene 15 and ubiquitin proteins.
A: The ISGylation system; B: The Ubiquiti
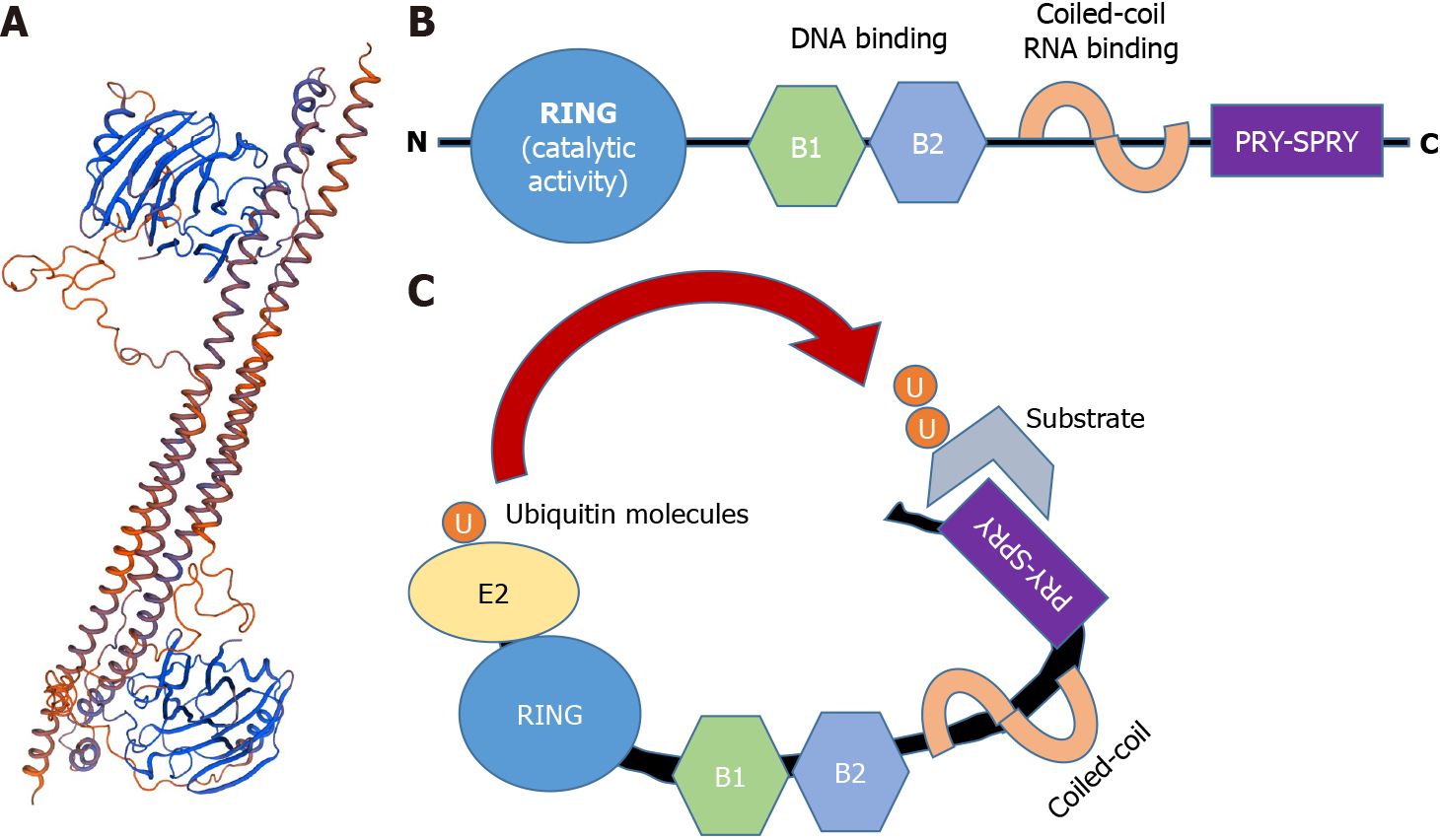
Figure 2 Structure of TRIM25.
A: Crystal structure of the human TRIM25 coiled-coil and PRYSPRY domains. (DNA sequence retrieved from NCBI. Gene, https://www.ncbi.nlm.nih.gov/gene/7706#gene-expression 21/01/21, and 3D model created with Swiss Model: https://swissmodel.expasy.org/); B: Schematic representation of TRIM25, including the conserved RING, B boxes (B1 and B2), the coiled-coil domain and the C-terminal variable domain (CTD) PRY-SPRY; C: RING and CTD domains bind to the ubiquitin-loaded E2 and the substrate, respectively. Consequently, both molecules get closer, therefore favoring substrate ubiquitination.
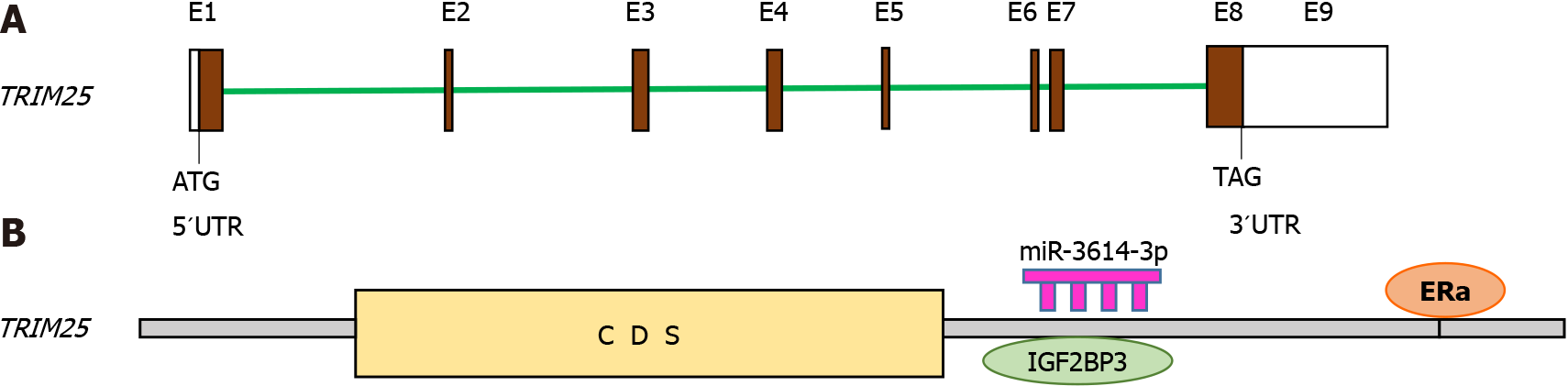
Figure 3 Structure of TRIM25 gene and its regulation.
The structure of the TRIM25 gene is shown in (A) Regulation of TRIM25 expression by ERα, (B) miRNA-3614-3p, and IGF2BP3 through its 3´UTR region.
- Citation: Tecalco-Cruz AC, Abraham-Juárez MJ, Solleiro-Villavicencio H, Ramírez-Jarquín JO. TRIM25: A central factor in breast cancer. World J Clin Oncol 2021; 12(8): 646-655
- URL: https://www.wjgnet.com/2218-4333/full/v12/i8/646.htm
- DOI: https://dx.doi.org/10.5306/wjco.v12.i8.646