Copyright
©The Author(s) 2021.
World J Clin Oncol. Nov 24, 2021; 12(11): 1009-1022
Published online Nov 24, 2021. doi: 10.5306/wjco.v12.i11.1009
Published online Nov 24, 2021. doi: 10.5306/wjco.v12.i11.1009
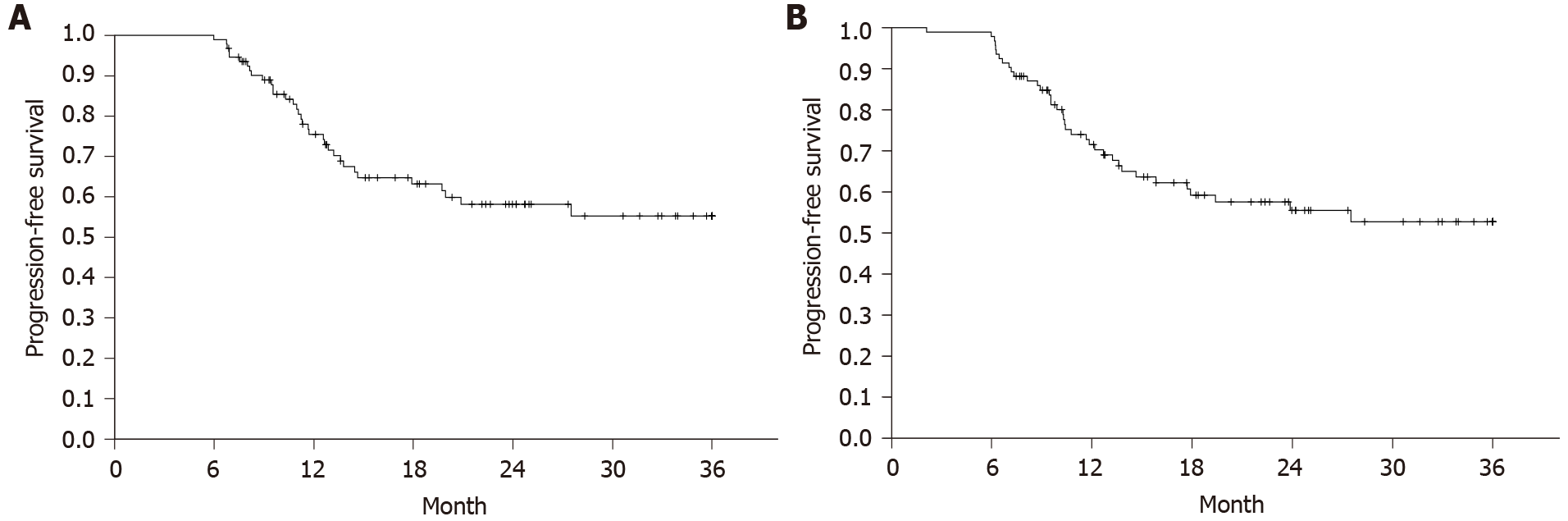
Figure 1 Progression-free survival according to the CHAARTED and STAMPEDE definitions of progressive disease and Swedish national guidelines.
Time from date of diagnosis to last follow-up/death. Censored at 36 mo. A: CHAARTED and STAMPEDE; B: Swedish national guidelines.
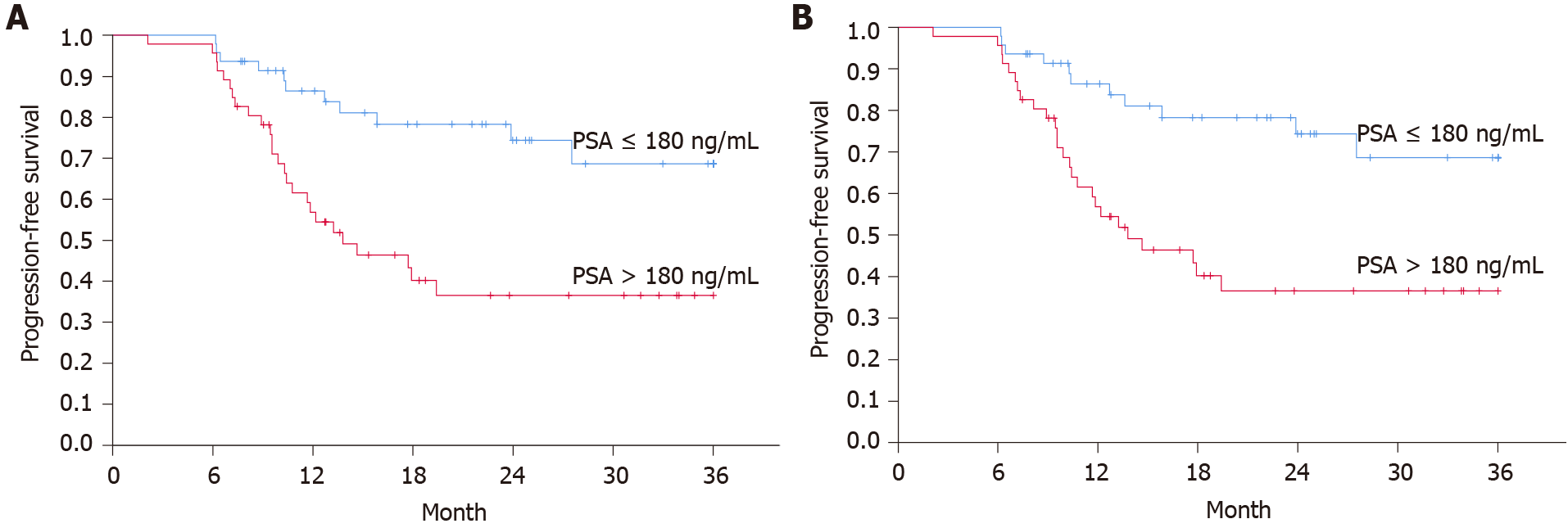
Figure 2 Progression-free survival for subgroups defined by PSA over/under median.
A: Progression-free survival (PFS) according to the CHAARTED and STAMPEDE definitions of progressive disease (log-rank: P = 0.0027); B: PFS according to the Swedish national guidelines (log-rank: P = 0.0018). Time from date of diagnosis to last follow-up/death. Censored at 36 mo.
- Citation: Isaksson J, Green H, Papantoniou D, Pettersson L, Anden M, Rosell J, Åvall-Lundqvist E, Elander NO. Real-world evaluation of upfront docetaxel in metastatic castration-sensitive prostate cancer. World J Clin Oncol 2021; 12(11): 1009-1022
- URL: https://www.wjgnet.com/2218-4333/full/v12/i11/1009.htm
- DOI: https://dx.doi.org/10.5306/wjco.v12.i11.1009