Published online Aug 6, 2017. doi: 10.4292/wjgpt.v8.i3.174
Peer-review started: January 20, 2017
First decision: May 3, 2017
Revised: May 18, 2017
Accepted: July 14, 2017
Article in press: July 15, 2017
Published online: August 6, 2017
Processing time: 194 Days and 7.4 Hours
Gastrointestinal (GI) dysmotility is a common problem in the critically ill population. It can be a reflection and an early sign of patient deterioration or it can be an independent cause of morbidity and mortality. GI dysmotility can be divided for clinical purposes on upper GI dysmotility and lower GI dysmotility. Upper GI dysmotility manifests by nausea, feeding intolerance and vomiting; its implications include aspiration into the airway of abdominal contents and underfeeding. Several strategies to prevent and treat this condition can be tried and they include prokinetics and post-pyloric feeds. It is important to note that upper GI dysmotility should be treated only when there are clinical signs of intolerance (nausea, vomiting) and not based on measurement of gastric residual volumes. Lower GI dysmotility manifests throughout the spectrum of ileus and diarrhea. Ileus can present in the small bowel and the large bowel as well. In both scenarios the initial treatment is correction of electrolyte abnormalities, avoiding drugs that can decrease motility and patient mobilization. When this fails, in the case of small bowel ileus, lactulose and polyethylene glycol solutions can be useful. In the case of colonic pseudo obstruction, neostigmine, endoscopic decompression and cecostomy can be tried when the situation reaches the risk of rupture. Diarrhea is also a common manifestation of GI dysmotility and the most important step is to differentiate between infectious sources and non-infectious sources.
Core tip: This manuscript presents the case for a cautious look at the gastrointestinal (GI) system during critical illness. GI dysfunction can be an early sign of decompensation, but unfortunately is often overlooked due to the natural tendency to gravitate towards the cardiovascular, respiratory and renal systems when looking for decompensation signs. It is our intention to bring attention to this system and help the clinician in using the GI tract as an early marker for decompensation and also to identify and treat potential GI complications common in this population.
- Citation: Vazquez-Sandoval A, Ghamande S, Surani S. Critically ill patients and gut motility: Are we addressing it? World J Gastrointest Pharmacol Ther 2017; 8(3): 174-179
- URL: https://www.wjgnet.com/2150-5349/full/v8/i3/174.htm
- DOI: https://dx.doi.org/10.4292/wjgpt.v8.i3.174
The gastrointestinal tract is a vast organ system with many key functions during normal state and physiology. Its functions include digestion and absorption of nutrients, immunomodulation, excretion of fluids, electrolyte balance and hormonal control[1]. These functions are integral for maintenance of homeostasis in health, adaptation in sickness and also as a source of disease.
Acute gastrointestinal injury (AGI) can occur as the result of the gastrointestinal tract been a bystander during periods of critical illness with possible grim consequences. The mechanisms responsible of this injury are diverse and include cytokines and ischemia-reperfusion injury. Observational studies have linked AGI with increased mortality and longer ICU-LOS[2].
AGI common manifestations include: Delayed gastric emptying, ileus, malabsorption, diarrhea, GI hemorrhage and GI bleed[3]. Due to this GI dysmotility in the ICU should be addressed seriously and systematically since it could be the manifestation of GI tract failure as well as manifestation of disease.
For the purpose of this review we would like to divide the problem in upper GI dysmotility and lower GI dysmotility.
Upper GI dysmotility is usually manifested as delayed gastric emptying, regurgitation and ultimately aspiration. These are signs and symptoms that should never be disregarded since they point out at AGI; the difficult questions would be how aggressive should we be monitoring and treating delayed gastric emptying? What is the optimal method of monitoring? What is the optimal treatment?
Delayed gastric emptying is a common occurrence in the critically ill[4], multiple factors are associated to decreased gastric emptying (Table 1) and once develops there has been concern that this may be linked to aspiration pneumonia and worse outcomes[5].
| Factors associated with decreased gastric emptying |
| Hyperglycemia |
| Opiates |
| Elevated intracranial pressure |
| Electrolyte abnormalities |
| Ischemia |
| Hypoxia |
| Sepsis |
| Burns |
| Abdominal surgery |
| Hyperosmolar formulas |
The challenge for the clinician is to find a way to monitor and prevent significant dysmotility leading to reflux and aspiration.
Multiple direct and indirect methods of measuring gastric emptying have been studied (Table 2). Scintigraphy is the gold standard but is not practical or readily available in the ICU setting. Unfortunately all of the other indirect methods have limitations and the availability is limited and we are left with an imperfect surrogate of gastric emptying measurement: The gastric residual volume (GRV)[6], and also with a promising alternative: The 13C-octanoate breath test.
| Methods of measuring gastric emptying |
| Scintigraphy |
| Paracetamol absorption |
| Carbohydrate absorption |
| Isotope breath test |
| Ultrasound and MRI |
| Gastric residual volumes |
The gastric residual volume has been used as an indirect surrogate of gastric emptying. Several limitations of using the GRV have been described. A normal patient’s endogenous secretions can confuse this measurement since a patient can produce up to 4500 mL a day of saliva, gastric secretions and duodenal reflux[7].
Other limitations are technical and they include[8]: (1) a lack of standardization on the quantity of a normal GRV, 15 mL to 500 mL has been described as an upper limit; (2) location of the tip of the tube; (3) different volumes depending on the bore of the catheter; and (4) inconsistent frequency of measurements.
Several small studies have looked into the correlation of different volumes of GRV (150-250 mL), and it has been shown to be a sensitive marker for delayed gastric emptying when compared to scintigraphy and acetaminophen absorption test, but, the negative predictive value was low, thus a lot of the patients with a negative test still had abnormal gastric emptying. More importantly having an abnormal GRV did not correlate to any significant clinical outcome[9,10].
The clinical impact from checking GRV is underfeeding and early enteral nutrition has been shown to improve outcomes of critically ill patients, on the other hand checking GRV has not been shown to decrease vomiting or aspiration. In a 205 patients study, subjects were divided in two groups, one group had feedings held if a GRV were > 250 mL, the second group did not have GRV checked. Patients in the non GRV group achieved higher delivery of EN plus vomiting episodes and clinical aspiration events were not statistically different than the patient’s in the GRV group[11].
Based on this data we do not recommend monitoring of GRV in the critically ill patient, but this does not mean that we should not address gastric intolerance manifested as nausea and/or vomiting.
The octonoate breath test has been developed as a non-invasive technique that is less cumbersome than scintigraphy since does not require patient transportation outside of the intensive care unit. It has been studied against scintigraphy in the critically ill population undergoing mechanical ventilation. In this test, carbon-13 (a non-radioactive isotope) is added to a test meal of 100 mL of octanoic acid. 13C-Octanoic acid is not absorbed in the stomach but is rapidly absorbed by the duodenum and then metabolized in the liver to produce 13CO2. Once the test meal is given, the 13CO2 enrichment of the exhaled air is measured with an isotope ratio mass spectrometer at standard times for 3 to 6 h; due to the properties of the isotope this measurement is reflective of gastric emptying. The biggest study to date showed that this test had an 89% sensitivity and a 67% specificity in identifying delayed gastric emptying when compared to scintigraphy, giving it a 92% PPV and a 57% NPV. Also the authors also concluded that the wide confidence interval (45%-88%) made it a good option to test gastric emptying in the research setting but not in a real life clinical setting[12]. Other limitations include the high cost and size of spectrometer units[13].
Interventions to prevent and treat gastric dysmotility include: The use of continuous feeding vs intermittent bolus feeding, post-pyloric feeding and prokinetics.
Continuous infusions of enteral feeds have the theoretical advantage of decreasing the amount of regurgitation and aspiration compared to intermittent boluses, unfortunately the evidence is scant. Small trials[14,15] suggest a decreased incidence of elevated gastric residuals and due to this more success in meeting caloric needs with the continuous methods but there is no difference in hard clinical outcomes. The current recommendations of the American Society of Parenteral and Enteral Nutrition (ASPEN) are to choose continuous feedings on those patients that are intolerant to bolus feeding and those that are high risk for aspiration[16].
Another possible solution would be to place the enteral feeding tube past the pylorus to prevent regurgitation and aspiration of gastric contents. A recent meta-analysis showed that there was a decrease in the incidence of pneumonias, but there was no significant difference in nutritional outcomes, length of stay or hospital mortality[17]. But, placing a post-pyloric tube can be technically difficult and delay initiation of enteral nutrition, due to that the ASPEN guidelines suggest to use the gastric route routinely and favor the post-pyloric route to patients at high risk of aspiration or those that showed intolerance.
The use of prokinetics has been associated with decreased GRV but no significant change in length of stay or mortality[18]. The most commonly studied agents include erythromycin at a dose of 3-7 mg/kg per day and metoclopramide at a dose of 10 mg every 4 h. If one chooses to use these agents, we must be aware of the side effects that include QT prolongation and diarrhea with both agents and tardive dyskinesia in the case of metoclopramide.
Lower GI dysmotility can be manifested in the ICU as ileus, acute colonic pseudo obstruction and diarrhea.
Unfortunately none of the usual tests used in the outpatient setting to evaluate motility disorders has been validated or found useful in the intensive care unit setting. The clinician is left with his clinical exam acumen and the usual routine tests performed the critically ill, this is why is important to suspect these disorders and look for them on our daily exam. We will describe the most common clinical presentations.
Ileus is defined as the absence of physiologic motility in the bowel, leading to a lack of progression of bowel contents through the gastrointestinal tract. A more specific definition has been described and this includes: Absence of a bowel movement for ≥ 3 d, treatment for constipation, and one of the following: (1) radiologic confirmed ileus; (2) feed intolerance; (3) abdominal distention; or (4) need for gastric decompression. This has to be differentiated from acute mechanical obstruction that may be a surgical emergency. It has been reported to occur in 20%-50% of the ICU population[18]. The average duration of the episode is 6.5 d and is associated with longer ICU stays as well as underfeeding[19].
The critically ill patient population is specially primed to develop ileus. Inflammation, narcotic use, vasopressor use and electrolyte imbalances makes them susceptible to a disequilibrium between sympathetic and parasympathetic forces. Common clinical entities that predispose to ileus include: Abdominal surgery, sepsis, pancreatitis, peritonitis, narcotic use, anticholinergic use, hypokalemia, hypomagnesemia, hyperglycemia, acidosis, hypoxia, hypothermia, renal failure and mechanical ventilation[20].
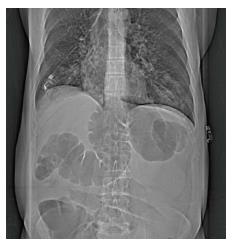
Ileus is usually manifested as inability to tolerate feeds, nausea, vomiting, abdominal distension, constipation and obstipation. The imaging studies show the presence of gas distension of bowel loops and air fluid levels within them (Figure 1). When severe enough it can develop into abdominal compartment syndrome, which is a life threatening emergency.
The basic management of ileus includes the correction of electrolyte abnormalities, avoidance of opioid agonists, avoidance of anticholinergic drugs, mobilization and early enteral feedings when possible.
Other therapies may include the use of gastric decompression, osmotive laxatives, opioid antagonists and promotility agents.
A double blinded study comparing the use of placebo vs polyethylene glycol vs lactulose in ICU patients with 3 or more days without a bowel movement showed that, both lactulose and polyethylene glycol are better in promoting defecation than placebo. Patients receiving polyethylene glycol had a lower incidence of acute intestinal pseudo obstruction. Early defecation was associated to a decreased LOS. Based on these findings is reasonable to start osmotive laxatives in this patient population[21].
The use of promotility agents in ileus seems more controversial. Erythromycin has been tried due to the theoretical effect on the motilin receptor. Despite this theoretical mechanism the trials have consistently failed to show any positive effect and its use comes with risk of a prolonged QT and arrhythmias. So we recommend against its use[22]. Metoclopramide has also been tried but results have been conflicting and no clear role exists for its use.
Acute colonic pseudo obstruction is a potentially fatal condition defined as an acute dilatation of the colon without a mechanical obstruction. Clinically is characterized by abdominal distension, commonly constipation, but flatus or stools may pass as well, an abdominal exam that may be benign but also it can present with exquisite abdominal tenderness, especially at the level of the cecum. The most feared complication would be perforation that usually happens in the cecum[23].
The pathophysiology is thought to be an imbalance between the parasympathetic/sympathetic signals. Clinical factors predisposing to this condition are multiple and include medications, surgery, critical illness, neurologic factors and metabolic factors (Table 3).
| Factors predisposing to Ogilvie’s syndrome |
| Medications |
| Opiates |
| Anticholinergics |
| Vasopressors |
| Calcium channel blockers |
| Cardiovascular factors |
| Shock |
| Heart failure |
| Critical illness |
| Severe sepsis |
| Pancreatitis |
| Mechanical ventilation |
| Hypoxemia |
| Post-operative state |
| Abdominal surgery |
| Peritonitis |
| Pelvic or hip fracture surgery |
| Metabolic factors |
| Hypokalemia |
| Renal failure |
| Hyperglycemia |
| Neurologic |
| Spinal cord lesions |
| Stroke |
The most important alternative diagnosis to rule out is toxic megacolon and mechanical obstruction. Mechanical obstruction can be easily ruled out by the presence of gas on all colonic segments on an abdominal plain film. If there is doubt a CT of the abdomen and pelvis with oral contrast can clarify the situation. Differentiating between Ogilvie’s and toxic megacolon can be more difficult. In the general population the most common cause of toxic megacolon is inflammatory bowel disease, in the critically ill the most common cause is C. difficile infection[24]. A thorough history and physical is warranted, other diagnostic tools include stools samples to test for C. difficile toxins or C. difficile PCR, CT abdomen pelvis and limited endoscopy with biopsies.
The first step in management include treating underlying conditions, managing electrolyte abnormalities, avoid opiates, early mobilization when feasible and early enteral nutrition.
When this therapy fail after 24-48 h and the risk of rupture is present, defined as cecum diameter > 12 cm[25]. We must proceed with other options that include neostigmine use, endoscopic decompression, percutaneous cecal decompression or surgical management.
Neostigmine is successful in achieving decompression in more than 88% of cases[26]. The drug is used at a dose of 2 mg intravenously given slowly over 5 min with monitoring of vital signs continuously for at least 30 min. Side effects include bradycardia, hypotension, nausea, vomiting and abdominal cramping.
Endoscopic decompression is les commonly used due to the risk of perforation, when performed this should be followed by the placement of a decompression tube since this increases the success rate from 50% to 80%[27]. In patients in whom these therapies fail, the next step according to the American Society of Gastroenterology and Endoscopy guidelines should be either percutaneous cecostomy or surgical management[28].
Diarrhea in the ICU can be defined as > 3 loose stools a day[29]. The incidence is around 20%[30]. Diarrhea in the ICU can be divided as infectious and non-infectious. Due to its incidence and possible serious underlying conditions it should never be dismissed and proper workup should be sought.
Clostridium difficile infection is the most common cause of infectious diarrhea in the ICU been present in 44% of patient with either infectious or non-infectious diarrhea in the ICU[31]. Other enteric pathogens include Salmonella, C. perfringens, S. aureus and P. aeruginosa. Antibiotic use is the most widely recognized risk factor for infectious diarrhea in the ICU; other risk factors include gastric acid suppression[27], advanced age and illness severity. A review of C. difficile infection is beyond the scope of this review article.
The most common causes for non-infectious diarrhea in the ICU include antibiotic associated diarrhea, enteral feeding associated diarrhea and medications. Regarding antibiotic associated diarrhea, when C. difficile is not found the theory behind this condition is the reduction on the concentration of anaerobic organisms in the gut with subsequent reduction of carbohydrate fermentation leading to an osmotic diarrhea[31].
Enteral feeding associated diarrhea is commonly quoted as the cause of diarrhea during ICU rounds. Interestingly a recent meta-analysis comparing total parenteral nutrition vs enteral nutrition did not find a higher incidence of diarrhea in the enteral feeds group[32]. A common sense approach would be to avoid high caloric density formulations due to their osmotic effects when possible. Fiber use to decrease diarrhea has been proven effective in the non-icu population, but this effects have not been reproduced in the ICU population. Probiotics also did not change its incidence[33].
GI dysmotility is a common but often overlooked occurrence in the critically ill patients. By itself it may be the reflection of end organ damage and deterioration as well as a sign of a serious underlying disorder. The clinician should pay close attention to it and initiate the appropriate work up as soon as possible to prevent grim outcomes.
Manuscript source: Invited manuscript
Specialty Type: Gastroenterology and hepatology
Country of Origin: United States
Peer-Review Report Classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Kuribayashi S S- Editor: Gong ZM L- Editor: A E- Editor: Lu YJ
| 1. | Binder HJ. Organization of the Gastrointestinal System. Medical Physiology. Philadelphia, PA: Elsevier 2017; 852-862. |
| 2. | Reintam A, Parm P, Redlich U, Tooding LM, Starkopf J, Köhler F, Spies C, Kern H. Gastrointestinal failure in intensive care: a retrospective clinical study in three different intensive care units in Germany and Estonia. BMC Gastroenterol. 2006;6:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 72] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 3. | Taylor RW. Gut Motility Issues in Critical Illness. Crit Care Clin. 2016;32:191-201. [PubMed] [DOI] [Full Text] |
| 4. | Khayyam U, Sachdeva P, Gomez J, Ramzan Z, Smith MS, Maurer AH, Fisher RS, Parkman HP. Assessment of symptoms during gastric emptying scintigraphy to correlate symptoms to delayed gastric emptying. Neurogastroenterol Motil. 2010;22:539-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Mutlu GM, Mutlu EA, Factor P. GI complications in patients receiving mechanical ventilation. Chest. 2001;119:1222-1241. [PubMed] |
| 6. | Kar P, Jones KL, Horowitz M, Chapman MJ, Deane AM. Measurement of gastric emptying in the critically ill. Clin Nutr. 2015;34:557-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (1)] |
| 7. | DeLegge MH. Managing gastric residual volumes in the critically ill patient: an update. Curr Opin Clin Nutr Metab Care. 2011;14:193-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Hurt RT, McClave SA. Gastric residual volumes in critical illness: what do they really mean? Crit Care Clin. 2010;26:481-490, viii-viix. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Landzinski J, Kiser TH, Fish DN, Wischmeyer PE, MacLaren R. Gastric motility function in critically ill patients tolerant vs intolerant to gastric nutrition. JPEN J Parenter Enteral Nutr. 2008;32:45-50. [PubMed] |
| 10. | Goetze O, Nikodem AB, Wiezcorek J, Banasch M, Przuntek H, Mueller T, Schmidt WE, Woitalla D. Predictors of gastric emptying in Parkinson’s disease. Neurogastroenterol Motil. 2006;18:369-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 107] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 11. | Poulard F, Dimet J, Martin-Lefevre L, Bontemps F, Fiancette M, Clementi E, Lebert C, Renard B, Reignier J. Impact of not measuring residual gastric volume in mechanically ventilated patients receiving early enteral feeding: a prospective before-after study. JPEN J Parenter Enteral Nutr. 2010;34:125-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 126] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 12. | Nguyen NQ, Bryant LK, Burgstad CM, Chapman M, Deane A, Bellon M, Lange K, Bartholomeuz D, Horowitz M, Holloway RH. Gastric emptying measurement of liquid nutrients using the (13)C-octanoate breath test in critically ill patients: a comparison with scintigraphy. Intensive Care Med. 2013;39:1238-1246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Siddiqui I, Ahmed S, Abid S. Update on diagnostic value of breath test in gastrointestinal and liver diseases. World J Gastrointest Pathophysiol. 2016;7:256-265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Bonten MJ, Gaillard CA, van der Hulst R, de Leeuw PW, van der Geest S, Stobberingh EE, Soeters PB. Intermittent enteral feeding: the influence on respiratory and digestive tract colonization in mechanically ventilated intensive-care-unit patients. Am J Respir Crit Care Med. 1996;154:394-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 78] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | Ciocon JO, Galindo-Ciocon DJ, Tiessen C, Galindo D. Continuous compared with intermittent tube feeding in the elderly. JPEN J Parenter Enteral Nutr. 1992;16:525-528. [PubMed] |
| 16. | McClave SA, Taylor BE, Martindale RG, Warren MM, Johnson DR, Braunschweig C, McCarthy MS, Davanos E, Rice TW, Cresci GA. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J Parenter Enteral Nutr. 2016;40:159-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1695] [Cited by in RCA: 1859] [Article Influence: 206.6] [Reference Citation Analysis (3)] |
| 17. | Alkhawaja S, Martin C, Butler RJ, Gwadry-Sridhar F. Post-pyloric versus gastric tube feeding for preventing pneumonia and improving nutritional outcomes in critically ill adults. Cochrane Database Syst Rev. 2015;CD008875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 18. | Caddell KA, Martindale R, McClave SA, Miller K. Can the intestinal dysmotility of critical illness be differentiated from postoperative ileus? Curr Gastroenterol Rep. 2011;13:358-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Nguyen T, Frenette AJ, Johanson C, Maclean RD, Patel R, Simpson A, Singh A, Balchin KS, Fergusson D, Kanji S. Impaired gastrointestinal transit and its associated morbidity in the intensive care unit. J Crit Care. 2013;28:537.e11-537.e17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 20. | Adike A, Quigley EM. Gastrointestinal motility problems in critical care: a clinical perspective. J Dig Dis. 2014;15:335-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 21. | van der Spoel JI, Oudemans-van Straaten HM, Kuiper MA, van Roon EN, Zandstra DF, van der Voort PH. Laxation of critically ill patients with lactulose or polyethylene glycol: a two-center randomized, double-blind, placebo-controlled trial. Crit Care Med. 2007;35:2726-2731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Traut U, Brügger L, Kunz R, Pauli-Magnus C, Haug K, Bucher HC, Koller MT. Systemic prokinetic pharmacologic treatment for postoperative adynamic ileus following abdominal surgery in adults. Cochrane Database Syst Rev. 2008;CD004930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 73] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 23. | Ogilvie WH. William Heneage Ogilvie 1887-1971. Large-intestine colic due to sympathetic deprivation. A new clinical syndrome. Dis Colon Rectum. 1987;30:984-987. [PubMed] |
| 24. | Lessa FC, Mu Y, Bamberg WM, Beldavs ZG, Dumyati GK, Dunn JR, Farley MM, Holzbauer SM, Meek JI, Phipps EC. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372:825-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2023] [Cited by in RCA: 1972] [Article Influence: 197.2] [Reference Citation Analysis (1)] |
| 25. | Johnson CD, Rice RP, Kelvin FM, Foster WL, Williford ME. The radiologic evaluation of gross cecal distension: emphasis on cecal ileus. AJR Am J Roentgenol. 1985;145:1211-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 68] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Ponec RJ, Saunders MD, Kimmey MB. Neostigmine for the treatment of acute colonic pseudo-obstruction. N Engl J Med. 1999;341:137-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 27. | Geller A, Petersen BT, Gostout CJ. Endoscopic decompression for acute colonic pseudo-obstruction. Gastrointest Endosc. 1996;44:144-150. [PubMed] |
| 28. | Eisen GM, Baron TH, Dominitz JA, Faigel DO, Goldstein JL, Johanson JF, Mallery JS, Raddawi HM, Vargo JJ, Waring JP. Acute colonic pseudo-obstruction. Gastrointest Endosc. 2002;56:789-792. [PubMed] |
| 29. | Manatsathit S, Dupont HL, Farthing M, Kositchaiwat C, Leelakusolvong S, Ramakrishna BS, Sabra A, Speelman P, Surangsrirat S; Working Party of the Program Committ of the Bangkok World Congress of Gastroenterology 2002. Guideline for the management of acute diarrhea in adults. J Gastroenterol Hepatol. 2002;17 Suppl:S54-S71. [PubMed] |
| 30. | Marcon AP, Gamba MA, Vianna LA. Nosocomial diarrhea in the intensive care unit. Braz J Infect Dis. 2006;10:384-389. [PubMed] |
| 31. | Bartlett JG. Clinical practice. Antibiotic-associated diarrhea. N Engl J Med. 2002;346:334-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 863] [Cited by in RCA: 763] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 32. | Gramlich L, Kichian K, Pinilla J, Rodych NJ, Dhaliwal R, Heyland DK. Does enteral nutrition compared to parenteral nutrition result in better outcomes in critically ill adult patients? A systematic review of the literature. Nutrition. 2004;20:843-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 422] [Cited by in RCA: 365] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 33. | Kamarul Zaman M, Chin KF, Rai V, Majid HA. Fiber and prebiotic supplementation in enteral nutrition: A systematic review and meta-analysis. World J Gastroenterol. 2015;21:5372-5381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 60] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |