Published online May 6, 2017. doi: 10.4292/wjgpt.v8.i2.90
Peer-review started: October 19, 2016
First decision: November 14, 2016
Revised: February 25, 2017
Accepted: March 12, 2017
Article in press: March 14, 2017
Published online: May 6, 2017
Processing time: 200 Days and 18.4 Hours
Ingestion of caustic substances and its long-term effect on the gastrointestinal system maintain its place as an important public health issue in spite of the multiple efforts to educate the public and contain its growing number. This is due to the ready availability of caustic agents and the loose regulatory control on its production. Substances with extremes of pH are very corrosive and can create severe injury in the upper gastrointestinal tract. The severity of injury depends on several aspects: Concentration of the substance, amount ingested, length of time of tissue contact, and pH of the agent. Solid materials easily adhere to the mouth and pharynx, causing greatest damage to these regions while liquids pass through the mouth and pharynx more quickly consequently producing its maximum damage in the esophagus and stomach. Esophagogastroduodenoscopy is therefore a highly recommended diagnostic tool in the evaluation of caustic injury. It is considered the cornerstone not only in the diagnosis but also in the prognostication and guide to management of caustic ingestions. The degree of esophageal injury at endoscopy is a predictor of systemic complication and death with a 9-fold increase in morbidity and mortality for every increased injury grade. Because of this high rate of complication, prompt evaluation cannot be overemphasized in order to halt development and prevent progression of complications.
Core tip: Caustic ingestion maintains its place as an important public health issue in spite of the multiple efforts to educate the public. This is due to the ready availability of caustic agents and the loose regulatory control on its production. Substances with extremes of pH are very corrosive and can create severe injury in the upper gastrointestinal tract. Locations most seriously affected are in the esophagus and stomach and may lead to chronic complications like stricture formation, gastric outlet obstruction, and malignant transformation. Prompt evaluation is therefore emphasized in order to halt development and prevent progression of these complications.
- Citation: De Lusong MAA, Timbol ABG, Tuazon DJS. Management of esophageal caustic injury. World J Gastrointest Pharmacol Ther 2017; 8(2): 90-98
- URL: https://www.wjgnet.com/2150-5349/full/v8/i2/90.htm
- DOI: https://dx.doi.org/10.4292/wjgpt.v8.i2.90
Ingestion of caustic substances and its long-term effect on the gastrointestinal system maintain its place as an important public health issue in spite of the multiple efforts to educate the public and contain its growing number. This is due to the ready availability of these caustic agents as items of household use and loose regulatory control on its production. According to the American Association of Poison Control (AAPCC), there were approximately two hundred thousand cases of cleaning substance exposure since 2000[1]. Data from developing countries, however, are sparse given that cases are largely underreported.
The age of occurrence presents in a bimodal fashion. The first peak is in the 1 to 5-year-old age group. Compared to adults, children are more likely to ingest caustic substances either accidentally or out of curiosity. Their higher exposure rate, however, is usually offset by a lower overall rate of complicated caustic injury because children often spit out the corrosive material immediately. The second peak is in the adolescent and young adult (21 years and older) age group. Majority of ingestions at this age group are intentional suicide attempts resulting in a greater and more extensive injury[2,3].
Caustic agents can be acidic or alkaline in nature. Common alkali-containing caustic agents are household bleaches, drain openers, toilet bowel cleaners, dishwashing agents and detergents. Acid-containing agents implicated in caustic ingestion include toilet bowl cleaners, anti-rust compound, swimming pool cleaners, vinegar, formic acid used in the rubber tanning industry and other similar acids[3,4]. The type of caustic agent most commonly implicated in poisonings varies from country to country. In the annual report of the AAPCC in 2008, the most commonly implicated caustic agent was the alkali-sodium hypochlorite, which was found in bleaches, toilet bowl cleaners, drain cleaners and household disinfectants. Local experiences from Denmark, Israel, United Kingdom, Peru, Spain, Australia, Saudi Arabia and Turkey also showed that alkaline agents were more commonly involved in caustic injury[4]. Most caustic substances were ingested in the liquid form and events commonly occurred at home[4]. Indian data, on the other hand, showed that majority of ingestions in their country were due to acids since these were cheaper and more readily available[3,4].
Substances with extremes of pH (less than 2 or greater than 12) are very corrosive and can create severe injury and burns in the upper gastrointestinal tract. Locations most seriously affected are in the esophagus and stomach since the corrosive material often remains in these areas for a longer period of time. However, injuries can also occur in any area in contact with the caustic agents such as the oral mucosa, pharyngeal area, upper airways, and duodenum[5,6].
Acids and alkali agents have contrasting characteristics and differ in how they cause tissue damage. Alkaline agents are usually colorless, relatively tasteless, more viscous, and have a less marked odor. Hence, the amount ingested tends to be more[4]. Once ingested, alkaline substances react with proteins and fats and are transformed into proteinases and soaps, resulting in liquefactive necrosis. This leads to deeper penetration into tissues with a greater likelihood of transmural injury[6]. Acids, on the other hand, have a pungent odor and an unpleasant taste. It tends to be consumed in smaller amounts and are swallowed rapidly after ingestion[4]. Once it reacts with tissue proteins, these substances are converted to acid proteins. The mode of tissue injury is coagulation necrosis[6]. The coagulum prevents the corrosive agent from spreading transmurally, hence reducing the incidence of full thickness injury[4]. This distinction, however, is not always the case. In the setting of strong acid or strong base ingestion, both these substances easily penetrate the esophageal or gastric mucosa and cause full-thickness injury[7].
The traditional opinion is that acids preferentially damage the stomach. Its lower surface tension and the formation of protective esophageal eschar allow acids to bypass the esophagus rapidly without much damage while affecting the stomach more severely. Conversely, alkalis cause more injury to the esophagus. The higher surface tension of alkalis that permit a longer contact time with esophageal tissues and the acidic contents in the stomach that act to neutralize gastric injury explain the more severe damage to the esophagus.
Mucosal injury begins within minutes of caustic ingestion. It is characterized by necrosis and hemorrhagic congestion secondary to the formation of thrombosis in the small vessels. These events continue in the next several days until approximately 4 to 7 d later when mucosal sloughing, bacterial invasion, granulation tissue and collagen deposition occur. The healing process typically begins three weeks after ingestion. It is during this time (first 3 wk) that the tensile strength of esophageal and/or gastric tissues is the lowest. If the ulcerations extend well beyond the muscularis layer, the wall becomes vulnerable to perforation[3,6]. It is for this reason that authorities advocate avoiding endoscopy between the 5th and the 15th day after caustic ingestion[3,6]. By the 3rd week, scar retraction occurs and may continue for a few more months until stricture formation occurs. The lower esophageal sphincter pressure becomes also impaired in the process causing an increased frequency and severity of acid reflux that further aggravates existing mucosal injury and accelerates the stricture formation[7].
The severity of injury depends on several aspects: Concentration of the substance, amount ingested, length of time of tissue contact, and pH of the agent. Solid materials easily adhere to the mouth and pharynx, causing greatest damage to these regions. Liquids, on the other hand, pass through the mouth and pharynx more quickly consequently producing its maximum damage in the esophagus and stomach[7,8].
The clinical presentation of caustic ingestion is diverse and do not always correlate with the degree of injury. Symptoms mainly depend on the location of damage. Hoarseness and stridor are signs that are highly suggestive of an upper respiratory tract involvement, particularly the epiglottis and larynx. Presence of these findings may signal a potentially life-threatening respiratory event[7]. The upper gastrointestinal tract, on the other hand, may present as dysphagia or odynophagia for esophageal injury and hematemesis or epigastric pain for gastric involvement[7,8].
Short-term complications include perforation and death. Perforation of the esophagus or stomach can occur at any time during the first 2 to 3 wk of ingestion. A sudden worsening of symptoms or an acute deterioration of a previously stable condition should warrant a thorough investigation to rule out the possibility of a perforated viscus[7,8].
Chronic complications of caustic ingestion include stricture formation, gastric outlet obstruction and malignant transformation. Patients with esophageal strictures usually complain of dysphagia and substernal pressure, and may become symptomatic 3 wk or later after ingestion. Symptoms of early satiety, post-prandial nausea or vomiting, and extreme weight loss suggest gastric obstruction. The latter commonly occurs in the first 5 to 6 wk of ingestion[6].
Carcinoma of the esophagus is a well-recognized consequence of caustic ingestion - partly due to the chronic inflammation from the initial burn, the trauma induced by repeated dilation, and the continuous tissue reaction from food stasis. Patients with a history of caustic ingestion often have a 1000-3000-fold increase in the incidence of esophageal carcinoma. Conversely, up to 3% of patients with carcinoma of the esophagus may have a history of caustic ingestion[7,8]. For alkaline ingestion in particular, subsequent development of squamous cell carcinoma has been reported to occur approximately 40 years after injury. This is mainly because of the liquefactive necrosis caused by alkali agents, which causes a deeper penetration of injury compared to the less severe and often limited mucosal injury of acidic substances. Periodic endoscopic evaluation is therefore suggested starting 20 years after the caustic ingestion with an interval of 1 to 3 years.
Laboratories were not found to directly correlate with the severity or the outcome of the injury. One study showed that age, an elevated white blood cell count (> 20000 cells/mm), and the presence of gastric deep ulcer or gastric necrosis are independent predictors of death[9]. Basically, laboratory work-ups play a more important role in guiding patient management than in predicting morbidity or mortality[7,8].
Plain chest radiography may show gas shadow in the mediastinum or below the diaphragm suggesting esophageal or gastric perforation, respectively. If perforation is suspected, an upper gastrointestinal series using a water-soluble agent can be performed.
Endoscopic ultrasound can also be used to evaluate the esophageal wall. Though in comparison to the conventional endoscopy, no difference was achieved in predicting early complications. Reports show that destruction of the muscularis layer on EUS could be a reliable sign of stricture formation and a marker for decreased response to balloon dilatation. However, further studies are needed to establish the role of EUS in caustic injury[7,8].
In assessing the extent and boundary of injury, computed tomography (CT) scan has a slightly higher diagnostic contribution than upper endoscopy. It can show the depth of necrosis and even the presence of transmural damage, thereby allowing clinicians to assess threatened or established perforations[7,8]. And because of its non-invasive quality, CT scan may prove to be a promising diagnostic in the early evaluation of caustic injury[7].
Magnetic resonance imaging (MRI), in general, provides little advantage over CT scan in the assessment of caustic injury. Besides its obvious benefit of processing images without the use of ionizing radiation, it does not reliably distinguish the different layers of the esophageal wall, which is crucial for the initial assessment of the extent of mucosal involvement. In addition, some patients, particularly the acutely ill, may not be able to tolerate the slower throughput of MRI and may not be able to cooperate during the procedure resulting in movement artifacts.
Esophagogastroduodenoscopy is an important and highly recommended diagnostic tool in the evaluation of caustic injury especially during the first 12 to 48 h of caustic ingestion, though several reports indicate that it can be safely performed up to 96 h post-ingestion. Gentle and cautious insufflation during the procedure cannot be sufficiently emphasized. Endoscopy is generally not advised 5 to 15 d after caustic ingestion due to tissue softening and friability during the healing stage. With findings of extensive damage and necrosis, aborting the procedure is not mandatory[7,8]. However, endoscopy is usually contraindicated in several situations; such as hemodynamic instability, severe respiratory compromise, and suspected perforations[8].
In the absence of symptoms and in the presence of accidental ingestions (especially those of less corrosive substances), significant lesions are usually not observed on upper endoscopy. As such, it is not required in some reports to perform endoscopy for asymptomatic patients with ingestion of low potency materials. This, however, is not applicable to patients with intentional ingestions since the substances they commonly consume are more potent. Therefore, emergent endoscopy among these patients is generally recommended[7,8].
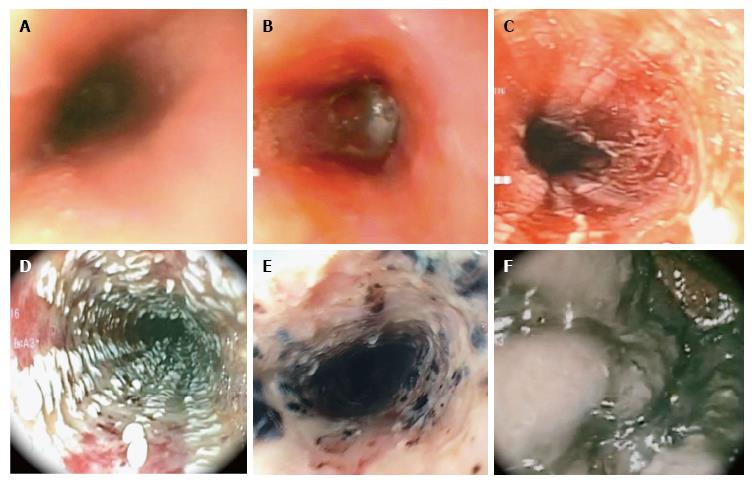
Ultimately, endoscopy is considered the cornerstone in the diagnosis, prognostication, and guide to management of caustic ingestions. Various endoscopic grading is available and Zargar’s classification is one of the most commonly used (Table 1 and Figure 1). In his study, Zargar et al[10] found that early major complications and death were confined to patients with grade III injuries. All patients with grade 0, I and IIA burns recovered without sequelae. Majority of grade IIB and all survivors with grade III injury developed eventual esophageal or gastric cicatrization[10]. In general, the degree of esophageal injury at endoscopy is a predictor of systemic complication and death with a 9-fold increase in morbidity and mortality for every increased injury grade[10].
| Zargar classification | Description |
| Grade 0 | Normal mucosa |
| Grade I | Edema and erythema of the mucosa |
| Grade IIA | Hemorrhage, erosions, blisters, superficial ulcers |
| Grade IIB | Circumferential lesions |
| Grade IIIA | Focal deep gray or brownish-black ulcers |
| Grade IIIB | Extensive deep gray or brownish-black ulcers |
| Grade IV | Perforation |
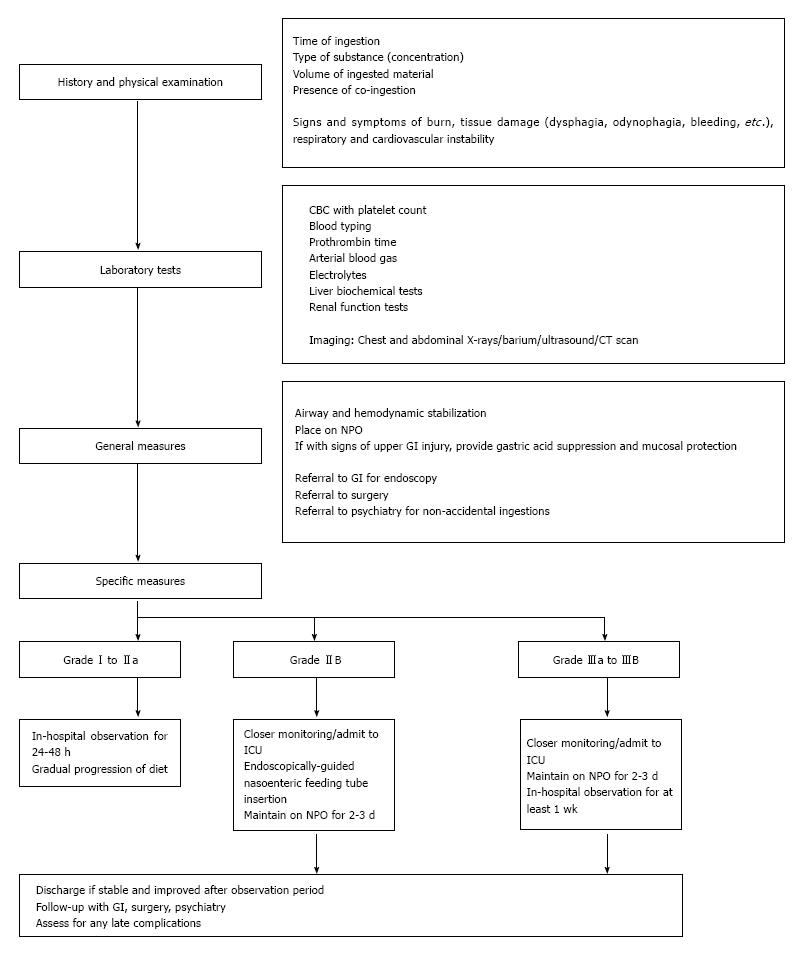
Management of caustic injury includes immediate resuscitation and evaluation of extent of damage. In general, correlation between symptomatology and endoscopic post-corrosive severity is still unproven. The patient’s initial signs and symptoms are oftentimes unreliable to gauge the extent of involvement since 20% of caustic ingestions may not present with oropharyngeal injury[11,12]. Nevertheless, for patients with a clear history of accidental ingestion of a low-volume, low-concentration caustic substance and with no signs and symptoms of oropharyngeal injury, endoscopy may be deferred. These patients may then be discharged after a 48-h observation period[11]. For those with large volume of ingestions and with significant findings on endoscopy (at least grade IIB), in-patient observation for any immediate complications in the intensive care unit is generally advised[13,14].
The cornerstone of all caustic ingestions is airway and hemodynamic stabilization. Since direct exposure of the upper respiratory tract by the corrosive substance may occur, patients should be evaluated for the need to do immediate intubation or tracheostomy. Intubation with direct visualization under fiberoptic laryngoscopy is most appropriate to avoid the risk of bleeding and further airway injury from “blind” airway access[10,15,16]. If the epiglottis and larynx are edematous, tracheostomy should be performed.
In previous protocols, neutralizing agents (weakly acidic or basic substances) for caustic ingestion was viewed as one of the first steps for treating caustic intoxications[11]. However, it has now been emphasized that these substances should not be administered due to the additional thermal injury and chemical destruction of tissues these reactions produce[14,17].
Routine nasogastric intubation for the purpose of evacuating any remaining caustic material is no longer warranted prior to endoscopic assessment of mucosal injury. This is due to the possibility of inducing retching or vomiting leading to further esophageal exposure by reflux of the remaining intragastric caustic material. Moreover, insertion of a foreign body in the acute setting may act as a nidus for infection, which may subsequently delay mucosal healing[16].
A preliminary survey of expert opinion from members of the world society of emergency surgery showed that 93% opted to use nasogastric tubes in patients with evidence of oropharyngeal injury while 7% avoided placement in any scenario. Among the 93%, more than two thirds opted to insert a nasogastric tube endoscopically. The theoretical advantage is said to provide a patent route for enteral feeding while serving as a stent to maintain luminal integrity and to decrease stricture formation[18].
Upon admission, the patient should be kept fasting. Gastric acid suppression with H2 blockers or intravenous proton pump inhibitors are often initiated to allow faster mucosal healing and to prevent stress ulcers. Efficacy of these agents for caustic ingestion has not yet been proven, although a small study done in 2013 has shown endoscopic healing after omeprazole infusion[7,16,19].
Sucralfate is now a common adjunct in the management of acute ulcers. It achieves its therapeutic effect by maintaining mucosal vascular integrity and blood flow. In the setting of caustic ingestion, sucralfate is said to hasten mucosal healing by providing a physical barrier between the harmful effects of the corrosive substance and the gastroesophageal mucosa[20-22]. Several small randomized controlled studies have assessed the efficacy of sucralfate in corrosive esophagitis. Results from these studies showed that sucralfate may decrease the frequency of stricture formation with advanced corrosive esophagitis. However, further research with a larger sample size is required to support its recommended use in this setting[20,23].
To date, evidence is still conflicting with regard the use of antibiotics. A study in 1992 analyzed the utility of antibiotic together with systemic steroid administration in caustic ingestion. It was concluded that antibiotics with steroids may be useful in preventing strictures in patients with extensive burns[24]. But since it was not possible to separate the effect of the antibiotic from that of the possible effect of the steroid in this study, it may be difficult to support the use of antibiotic in preventing stricture formation with such limited data. Hence, the consensus maintains that patients treated with steroids should also be treated with antibiotics[16].
Initial studies on corticosteroid administration to prevent stricture formation in caustic ingestion were mainly on children and results were conflicting. Methylprednisolone at a dose of 1 g/1.73 m2 per day for 3 d showed benefit in reducing stricture development[25]. Likewise, dexamethasone (1 mg/kg per day) was shown to be better than prednisolone (2 mg/kg per day) in preventing stricture formation (38.9% vs 66.7%) and severe stricture development (27.8% vs 55.6%)[26].
However, another study showed that prednisolone at a dose of 2 mg/kg intravenous did not provide any benefit in preventing stricture development[27]. A systematic pooled analysis of caustic ingestion supported this finding as it failed to show additional benefit with the use of steroid in patients with grade II esophageal burns[28]. Based on the above evidence, it seems prudent to avoid systemic corticosteroids in caustic ingestion until further research confirms its efficacy.
Intralesional steroid such as triamcinolone (40-100 mg/session) has long been known to augment the dilatation of caustic-induced esophageal strictures although results from most studies are still conflicting[29,30].
Recently, mitomycin C has been shown to decrease the rate of caustic stricture formation in animals due to its antifibroblastic properties[31]. It has been used as an adjunct[32-34] after dilatation of caustic strictures in humans (including those with long strictures) by applying mitomycin-C topically at a dose of 0.4 mg/mL[34,35]. In a study of 16 patients treated with endoscopic topical application of mitomycin-C, a decrease in the number of dilatations and apparent relief of dysphagia were achieved compared to triamcinolone[35].
Endoscopy is important not only in the diagnosis of corrosive ingestion but also in determining subsequent management. In general, patients with normal looking mucosa or those with very mild injury may be discharged. For those with Zargar grade I or IIA, in-hospital observation is advised and gradual progression of diet from liquids is done in the next 24 to 48 h. Patients with at least grade IIB are monitored more closely. An endoscopically-guided nasoenteric feeding tube may be placed with caution, bypassing the areas of necrosis, to facilitate feeding while initiating trial of per orem feeding. For grade III injuries, the patient’s response to treatment and feeding is usually observed for at least a week[14]. Prophylactic esophageal stenting in the acute setting is generally not recommended[36] due to a high perforation rate.
Esophageal stricture is one of the most common sequelae of caustic injury. Up to 70% of patients with grade IIB and more than 90% of patients with grade III injury are likely to develop esophageal stricture[37].
Peak development of strictures commonly starts on the 8th week post-ingestion, although it has been reported to occur as early as 3 wk[7,37,38]. The timing of management is crucial in achieving long-term functional effects.
The primary non-surgical treatment of caustic esophageal stricture is endoscopic dilatation. This can be achieved with Bougies or balloon dilators. For tight and fibrotic strictures, bougies dilators are often more reliable than balloon dilators[37]. A prospective study published in 2015 assessed a rigorous weekly schedule of bougie dilatation (Savary-Gilliard) along with intralesional triamcinolone in patients with refractory esophageal corrosive strictures. It was noted that this intervention was safe and effective in improving dysphagia, achieving clinically significant dilatation, reducing dilatation frequency, maintaining luminal patency of ≥ 14 mm[14,39].
Using balloon dilators, a lower dilatation force should be used initially to avoid perforation[40]. This may need to be repeated and advanced slowly to achieve effective and safe dilatation. The interval between dilatations varies from 1-3 wk among different studies[16] but usually an interval of 3-4 wk is recommended.
For either technique, the goal is to achieve relief of symptoms (particularly dysphagia) and maintain efficient luminal diameter of up to to 15 mm[41].
Though endoscopic dilatation with balloon has been the standard of treatment for benign esophageal strictures, the recurrence rate still reaches 30%-40%. Approximately 10% of these patients fail to achieve clinical improvement and remain refractory to repeated dilatations. In such patients a good option is stent insertion. Recently, 3 types of stents are now available: Self expanding metal stents (SEMS), plastic sent, and biodegradable (BD) stent - each with its own advantage and disadvantage.
SEMS are often discouraged in benign esophageal stenosis due to its high rate of necrosis and ulceration, tissue hyperplasia, new stricture or fistula formation, and the tendency for the metal portion to embed within the esophageal wall. Plastic stents are said to have lesser tissue hyperplasia but with higher rate of stent migration and lower tendency to sustain significant radial force. Both of these stents require repeated endoscopic intervention for stent retrieval. Recently, BD have been introduced in the hopes of avoiding the above complications and the need for re-intervention for stent extraction[42].
A study in 2012 compared these 3 stents in patients with refractory benign esophageal stenoses. In this study, long-term resolution of dysphagia was highest in the metal stents group (40%) compared to BD stents (30%) and plastic stents (10%). Tissue migration was highest in the plastic stent group and lowest in the BD stent group[43]. To date, there is still no ideal stent recommended for universal use among patients with benign esophageal strictures, the choice for each patient should be individualized[44].
Corrective surgery for esophageal strictures from caustic injury is done only in severe cases where endoscopic therapy fails or is deemed harmful. Surgical options include partial or total esophagectomy with gastric pull up or, preferably colonic interposition[38]. Gastric pull-up in general, is quicker and requires only one anastomosis. However, the long-term functional outcome may decrease with development of complications such as recurrence of stricture, bothersome reflux, and subsequent metaplasia over the anastomotic site[7,16,45-52]. On the other hand, colon interposition is a more complex procedure requiring 3 anastomoses, albeit with a more stable long-term functional outcome. It is often associated with a lower incidence of stricture formation than gastric pull-up hence its preferential use in the setting of a relatively spared and healthy stomach[16]. Mortality rates of late reconstructive surgery depend on local surgical expertise.
Manuscript source: Invited manuscript
Specialty Type: Gastroenterology and hepatology
Country of Origin: Philippines
Peer-Review Report Classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Hashimoto N, Hoff DAL, Imaeda H S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Bronstein AC, Spyker DA, Cantilena LR, Green J, Rumack BH, Heard SE. 2006 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS). Clin Toxicol (Phila). 2007;45:815-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 213] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 2. | Lupa M, Magne J, Guarisco JL, Amedee R. Update on the diagnosis and treatment of caustic ingestion. Ochsner J. 2009;9:54-59. [PubMed] |
| 3. | Satar S, Topal M, Kozaci N. Ingestion of caustic substances by adults. Am J Ther. 2004;11:258-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Lakshmi CP, Vijayahari R, Kate V, Ananthakrishnan N. A hospital-based epidemiological study of corrosive alimentary injuries with particular reference to the Indian experience. Natl Med J India. 2013;26:31-36. [PubMed] |
| 5. | Kardon E. Caustic ingestion.Emergency Medicine Toxicology. Available from: http://www.medicine.medscape.com. |
| 6. | Chibishev A, Simonovska N, Shikole A. Post-corrosive injuries of upper gastrointestinal tract. Prilozi. 2010;31:297-316. [PubMed] |
| 7. | Contini S, Scarpignato C. Caustic injury of the upper gastrointestinal tract: a comprehensive review. World J Gastroenterol. 2013;19:3918-3930. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 273] [Cited by in RCA: 208] [Article Influence: 17.3] [Reference Citation Analysis (7)] |
| 8. | Park KS. Evaluation and management of caustic injuries from ingestion of Acid or alkaline substances. Clin Endosc. 2014;47:301-307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 9. | Rigo GP, Camellini L, Azzolini F, Guazzetti S, Bedogni G, Merighi A, Bellis L, Scarcelli A, Manenti F. What is the utility of selected clinical and endoscopic parameters in predicting the risk of death after caustic ingestion? Endoscopy. 2002;34:304-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Zargar SA, Kochhar R, Mehta S, Mehta SK. The role of fiberoptic endoscopy in the management of corrosive ingestion and modified endoscopic classification of burns. Gastrointest Endosc. 1991;37:165-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 278] [Article Influence: 8.2] [Reference Citation Analysis (2)] |
| 11. | Chibishev A, Pereska Z, Chibisheva V, Simonovska N. Ingestion of caustic substances in adults: a review article. IJT. 2013;6:723-734. |
| 12. | Cheng HT, Cheng CL, Lin CH, Tang JH, Chu YY, Liu NJ, Chen PC. Caustic ingestion in adults: the role of endoscopic classification in predicting outcome. BMC Gastroenterol. 2008;8:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 117] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 13. | Keh SM, Onyekwelu N, McManus K, McGuigan J. Corrosive injury to upper gastrointestinal tract: Still a major surgical dilemma. World J Gastroenterol. 2006;12:5223-5228. [PubMed] |
| 14. | Triadafilopoulos G. Caustic esophageal injury in adults. UpToDate. Topic 2267 Version 13.0. [accessed 2016 Aug 10]. Available from: https://www.uptodate.com/contents/caustic-esophageal-injury-in-adults?source=search_result&search=caustic esophageal injury&selectedTitle=1~23. |
| 15. | Arévalo-Silva C, Eliashar R, Wohlgelernter J, Elidan J, Gross M. Ingestion of caustic substances: a 15-year experience. Laryngoscope. 2006;116:1422-1426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 106] [Article Influence: 5.6] [Reference Citation Analysis (1)] |
| 16. | Rathnaswami A, Ashwin R. Corrosive Injury of the upper gastrointestinal tract: a review. Arch Clin Gastroenterol. 2016;2:56-62. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Penner GE. Acid ingestion: toxicology and treatment. Ann Emerg Med. 1980;9:374-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Kluger Y, Ishay OB, Sartelli M, Katz A, Ansaloni L, Gomez CA, Biffl W, Catena F, Fraga GP, Di Saverio S. Caustic ingestion management: world society of emergency surgery preliminary survey of expert opinion. World J Emerg Surg. 2015;10:48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 19. | Cakal B, Akbal E, Köklü S, Babalı A, Koçak E, Taş A. Acute therapy with intravenous omeprazole on caustic esophageal injury: a prospective case series. Dis Esophagus. 2013;26:22-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Gümürdülü Y, Karakoç E, Kara B, Taşdoğan BE, Parsak CK, Sakman G. The efficiency of sucralfate in corrosive esophagitis: a randomized, prospective study. Turk J Gastroenterol. 2010;21:7-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Tytgat GN, Hameeteman W, van Olffen GH. Sucralfate, bismuth compounds, substituted benzimidazoles, trimipramine and pirenzepine in the short- and long-term treatment of duodenal ulcer. Clin Gastroenterol. 1984;13:543-568. [PubMed] |
| 22. | Akman M, Akbal H, Emir H, Oztürk R, Erdogan E, Yeker D. The effects of sucralfate and selective intestinal decontamination on bacterial translocation. Pediatr Surg Int. 2000;16:91-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 23. | Coronel G, De Lusong M. Sucralfate for the prevention of esophageal stricture formation in corrosive esophagitis: an open label, randomized controlled trial. Endoscopy. 2011;43:A42. |
| 24. | Howell JM, Dalsey WC, Hartsell FW, Butzin CA. Steroids for the treatment of corrosive esophageal injury: a statistical analysis of past studies. Am J Emerg Med. 1992;10:421-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 49] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Usta M, Erkan T, Cokugras FC, Urganci N, Onal Z, Gulcan M, Kutlu T. High doses of methylprednisolone in the management of caustic esophageal burns. Pediatrics. 2014;133:E1518-E1524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 26. | Bautista A, Varela R, Villanueva A, Estevez E, Tojo R, Cadranel S. Effects of prednisolone and dexamethasone in children with alkali burns of the oesophagus. Eur J Pediatr Surg. 1996;6:198-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Anderson KD, Rouse TM, Randolph JG. A controlled trial of corticosteroids in children with corrosive injury of the esophagus. N Engl J Med. 1990;323:637-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 167] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 28. | Fulton JA, Hoffman RS. Steroids in second degree caustic burns of the esophagus: a systematic pooled analysis of fifty years of human data: 1956-2006. Clin Toxicol (Phila). 2007;45:402-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 29. | Kochhar R, Poornachandra KS. Intralesional steroid injection therapy in the management of resistant gastrointestinal strictures. World J Gastrointest Endosc. 2010;2:61-68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 54] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (1)] |
| 30. | Kochhar R, Ray JD, Sriram PV, Kumar S, Singh K. Intralesional steroids augment the effects of endoscopic dilation in corrosive esophageal strictures. Gastrointest Endosc. 1999;49:509-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 95] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 31. | Türkyilmaz Z, Sönmez K, Karabulut R, Gülbahar O, Poyraz A, Sancak B, Başaklar AC. Mitomycin C decreases the rate of stricture formation in caustic esophageal burns in rats. Surgery. 2009;145:219-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 32. | Uhlen S, Fayoux P, Vachin F, Guimber D, Gottrand F, Turck D, Michaud L. Mitomycin C: an alternative conservative treatment for refractory esophageal stricture in children? Endoscopy. 2006;38:404-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 60] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 33. | Rosseneu S, Afzal N, Yerushalmi B, Ibarguen-Secchia E, Lewindon P, Cameron D, Mahler T, Schwagten K, Köhler H, Lindley KJ. Topical application of mitomycin-C in oesophageal strictures. J Pediatr Gastroenterol Nutr. 2007;44:336-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 64] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 34. | El-Asmar KM, Hassan MA, Abdelkader HM, Hamza AF. Topical mitomycin C can effectively alleviate dysphagia in children with long-segment caustic esophageal strictures. Dis Esophagus. 2015;28:422-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 35. | Méndez-Nieto CM, Zarate-Mondragón F, Ramírez-Mayans J, Flores-Flores M. Topical mitomycin C versus intralesional triamcinolone in the management of esophageal stricture due to caustic ingestion. Rev Gastroenterol Mex. 2015;80:248-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 36. | Mills LJ, Estrera AS, Platt MR. Avoidance of esophageal stricture following severe caustic burns by the use of an intraluminal stent. Ann Thorac Surg. 1979;28:60-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 37. | Katz A, Kluger Y. Caustic material ingestion injuries-paradigm shift in diagnosis and treatment. Health Care Current Reviews. 2015;3:1-4. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 38. | Gupta V, Wig JD, Kochhar R, Sinha SK, Nagi B, Doley RP, Gupta R, Yadav TD. Surgical management of gastric cicatrisation resulting from corrosive ingestion. Int J Surg. 2009;7:257-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 39. | Nijhawan S, Udawat HP, Nagar P. Aggressive bougie dilatation and intralesional steroids is effective in refractory benign esophageal strictures secondary to corrosive ingestion. Dis Esophagus. 2016;29:1027-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 40. | Nishikawa Y, Higuchi H, Kikuchi O, Ezoe Y, Aoyama I, Yamada A, Kanki M, Nomura S, Nomura M, Horimatsu T. Factors affecting dilation force in balloon dilation of severe esophageal strictures: an experiment using an artificial stricture model. Surg Endosc. 2016;30:4315-4320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 41. | Broor SL, Raju GS, Bose PP, Lahoti D, Ramesh GN, Kumar A, Sood GK. Long term results of endoscopic dilatation for corrosive oesophageal strictures. Gut. 1993;34:1498-1501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 61] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 42. | Alvarez O, Llano R, Restrepo D. The current state of biodegradable self-expanding stents in interventional gastrointestinal and pancreatobiliary endoscopy. Rev Col Gastroenterol. 2015;30:172-179. |
| 43. | Canena JM, Liberato MJ, Rio-Tinto RA, Pinto-Marques PM, Romão CM, Coutinho AV, Neves BA, Santos-Silva MF. A comparison of the temporary placement of 3 different self-expanding stents for the treatment of refractory benign esophageal strictures: a prospective multicentre study. BMC Gastroenterol. 2012;12:70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 44. | Ham YH, Kim GH. Plastic and biodegradable stents for complex and refractory benign esophageal strictures. Clin Endosc. 2014;47:295-300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 45. | Javed A, Pal S, Krishnan EK, Sahni P, Chattopadhyay TK. Surgical management and outcomes of severe gastrointestinal injuries due to corrosive ingestion. World J Gastrointest Surg. 2012;4:121-125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 46. | Ramasamy K, Gumaste VV. Corrosive ingestion in adults. J Clin Gastroenterol. 2003;37:119-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 129] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 47. | Panieri E, Rode H, Millar AJ, Cywes S. Oesophageal replacement in the management of corrosive strictures: when is surgery indicated? Pediatr Surg Int. 1998;13:336-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 40] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 48. | Gupta NM, Gupta R. Transhiatal esophageal resection for corrosive injury. Ann Surg. 2004;239:359-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 46] [Article Influence: 2.2] [Reference Citation Analysis (1)] |
| 49. | Knezević JD, Radovanović NS, Simić AP, Kotarac MM, Skrobić OM, Konstantinović VD, Pesko PM. Colon interposition in the treatment of esophageal caustic strictures: 40 years of experience. Dis Esophagus. 2007;20:530-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 77] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 50. | Gerzic ZB, Knezevic JB, Milicevic MN, Jovanovic BK. Esophagocoloplasty in the management of postcorrosive strictures of the esophagus. Ann Surg. 1990;211:329-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 45] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 51. | Chirica M, Veyrie N, Munoz-Bongrand N, Zohar S, Halimi B, Celerier M, Cattan P, Sarfati E. Late morbidity after colon interposition for corrosive esophageal injury: risk factors, management, and outcome. A 20-years experience. Ann Surg. 2010;252:271-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 52. | Javed A, Pal S, Dash NR, Sahni P, Chattopadhyay TK. Outcome following surgical management of corrosive strictures of the esophagus. Ann Surg. 2011;254:62-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |