Published online Feb 6, 2017. doi: 10.4292/wjgpt.v8.i1.74
Peer-review started: August 2, 2016
First decision: September 2, 2016
Revised: October 11, 2016
Accepted: November 1, 2016
Article in press: November 2, 2016
Published online: February 6, 2017
Processing time: 175 Days and 18.3 Hours
To study the effect of itopride on gastric accommodation, gastric emptying and drinking capacity in functional dyspepsia (FD).
Randomized controlled trial was conducted to check the effect of itopride on gastric accommodation, gastric emptying, capacity of tolerating nutrient liquid and symptoms of FD. We recruited a total of 31 patients having FD on the basis of ROME III criteria. After randomization, itopride was received by 15 patients while 16 patients received placebo. Gastric accommodation was determined using Gastric Scintigraphy. 13C labeled octanoic breadth test was performed to assess gastric emptying. Capacity of tolerating nutrient liquid drink was checked using satiety drinking capacity test. The intervention group comprised of 150 mg itopride. Patients in both arms were followed for 4 wk.
Mean age of the recruited participant 33 years (SD = 7.6) and most of the recruited individuals, i.e., 21 (67.7%) were males. We found that there was no effect of itopride on gastric accommodation as measured at different in volumes in the itopride and control group with the empty stomach (P = 0.14), at 20 min (P = 0.38), 30 min (P = 0.30), 40 min (P = 0.43), 50 min (P = 0.50), 60 min (P = 0.81), 90 min (P = 0.25) and 120 min (P = 0.67). Gastric emptying done on a sub sample (n = 11) showed no significant difference (P = 0.58) between itopride and placebo group. There was no significant improvement in the capacity to tolerate liquid in the itopride group as compared to placebo (P = 0.51). Similarly there was no significant improvement of symptoms as assessed through a composite symptom score (P = 0.74). The change in QT interval in itopride group was not significantly different from placebo (0.10).
Our study found no effect of itopride on gastric accommodation, gastric emptying and maximum tolerated volume in patients with FD.
Core tip: Through this study we wanted to find the effect of itopride on gastric accommodation, gastric emptying and drinking capacity in patients with functional dyspepsia (FD) in Pakistani population. The strength of our study was that we used objective measures, i.e., gastric scintigraphy and 13C labeled octanoic acid breath test to measure gastric accommodation and gastric emptying. Diagnosis of FD was based on ROME III criteria and was done after using extensive investigations to rule out organic cause for the symptoms. We found no effect of itopride on gastric accommodation, gastric emptying and maximum tolerated volume in patients with FD in our study.
- Citation: Abid S, Jafri W, Zaman MU, Bilal R, Awan S, Abbas A. Itopride for gastric volume, gastric emptying and drinking capacity in functional dyspepsia. World J Gastrointest Pharmacol Ther 2017; 8(1): 74-80
- URL: https://www.wjgnet.com/2150-5349/full/v8/i1/74.htm
- DOI: https://dx.doi.org/10.4292/wjgpt.v8.i1.74
Patients presenting with epigastric pain and burning, early satiation and postprandial fullness without any structural, organic or systematic pathology are labeled as having functional dyspepsia (FD)[1,2]. Globally prevalence of FD varies from 1.8% to 57% depending on the geographic location and diagnostic criteria used[3]. There is no published data on community based prevalence of FD from South Asia but experts consider it to be an important problem for our population[3,4]. FD reduces productivity and incurs a considerable cost on health system[5]. Only in 2009 the cost incurred to the health system by this morbidity was $18 billion[6].
Muti-factorial pathogenesis of FD makes it a difficult condition to intervene[7]. These patients have inability of the stomach to change its volume in response to food, decreased stomach compliance and inability of the stomach to empty[7]. Delayed gastric empting is associated with the symptoms of nausea, vomiting and postprandial fullness[8]. These symptoms result in lower productivity of the patients and a compromised quality of life[7,9]. Delayed gastric emptying is found in one third of the patients with FD[8,10]. These patients showed slower gastric emptying as compare to the normal individuals[11]. This is because of sub optimal gastric myoelectric activities[12,13].
Different drug therapies used for FD that include eradication of Helicobacter pylori (H. pylori), use of proton pump inhibitors (PPIs) and anti-depressants failed to demonstrate a convincing effect[5,14,15]. Guidelines recommend eradication of H. pylori but this treatment alone depends on the type of the FD being treated[15]. Evidence in favor of efficacy for using PPIs for all the patients with FD is not clear[16]. It’s argued that PPIs may only be effective in patients having co-morbid reflux symptoms[16]. Though data suggest that anti-depressants like mirtazapine might be beneficial for some sub-groups of FD but still more studies are needed to recommend its usage for all patients with FD[17]. Symptoms of FD are improved by prokinetic agents[18]. Metoclopramide can cause extra pyramidal movement disorders[19]. Use of domperidone can result in rise in prolactin level leading to gynaecomastia[20]. Cisapride can result in prolonged QT interval and arrhythmias[21]. Itopride is a dopamine (D2) antagonist with peripheral action. It doesn’t cause severe elevation of prolactin and or pathological changes on electrocardiogram (ECG)[22]. A recent meta-analysis concluded that itopride improves the symptoms of early satiety and postprandial fullness[23]. Through this study we wanted to find the effect of itopride on gastric accommodation, gastric emptying and capacity of tolerating nutrient liquid drink in patients with FD in Pakistani population.
We conducted a randomized controlled trial to see the effect of itopride on gastric emptying. This study was conducted after approval from Aga Khan University Ethical Review Committee (Clinical trial registration number: NCT01226134). Subject for this study were enrolled after written informed consent that was made on the basis of declaration of Helsinki.
Total of 31 patients were recruited for the purpose of this study from the gastroenterology clinics of Aga Khan University Hospital. Eligibility criteria used for recruiting these patients was; age equal to or greater than 18 years, diagnosed as FD on the base of Rome III criteria, negative for H. pylori on gastric biopsy and Urea Breath Test, negative duodenal biopsy for giardiasis or celiac disease or any other established organic pathology, and normal upper abdominal ultrasound. We excluded; pregnant women, patients taking other medications that alter gastric motility like macrolide and anti-emetics and antibiotics.
Before undergoing randomization patients were assessed for symptoms that included epigastric discomfort, heart burn, acid regurgitation, upper abdominal pain, belching, nausea, early satiety, and postprandial fullness. Blood samples of these patients were taken to check for serum hemoglobin level, white blood cell count, platelet count, serum alanine aminotransferese (SGPT) and prolactin level. Electrocardiogram of the patient was performed to find out the QT interval at the baseline. Single photon emission tomography and satiety drinking test was performed to measure gastric accommodation at baseline. After completing the baseline investigations, out of all patients that were recruited 15 were randomly allocated to the intervention group while 16 were randomly allocated to the placebo group. Patients in the intervention group received 150 mg of itopride for 4 wk. Patients were instructed to take antacids as and when required.
Gastric accommodation: Gastric accommodation was determined by estimating the change in gastric volumes using Gastric Scintigraphy and by the help of computer software which convert the gastric images into 3D images and calculate the estimated gastric volumes[24]. Gastric volumes were determined before giving itopride or placebo agent and after completion of intervention period. We injected 99mTc pertechnetate followed by the use of Analyze software for reconstruction of tomographic images. These images were acquired after an overnight fast among all the participants and then after giving 300 mL of nutrient drink at an interval of 20, 30, 40, 50, 60, 90 and 120 min. Analyze PC 2.5 software system was used to find out stomach volume measurements[24].
Gastric emptying: After an overnight fast, 13C labeled octanoic breadth test was performed to assess gastric emptying[25,26]. A test meal containing 13C was given to patient which is supposed to be completed in 10 min. Breath sample was taken before test meal (150 mL of water with a sandwich of scrambled egg containing 13C octanoic acid and 250 mL of orange juice) and at an interval of every 15 min for 4 h and then half-hourly for another two hours. During the measurement time the subject remained sedentary while reading or watching television. If necessary limited movements between the breaths collections were permitted.
Satiety drinking capacity test: Subjects after an overnight fast were told to come at 8:30 AM in the morning. They were asked to grade their satiety from 0 to 5 (5 being the maximum satiety). A drink containing 6.5 g fat/100 mL, 1.1 g carbohydrate and 5 g of protein (nutridrink kcl 150/100 mL) which tasted of vanilla was taken by the participants at room temperature. Subjects drank at the rate of 15 mL/min. Symptoms were scored at every five minutes interval. Test was ceased once a score of 5 is achieved[27].
Symptoms of FD: Dyspeptic symptoms which included epigastric pain, epigastric discomfort, heart burn, acid regurgitation, upper abdominal pain, belching, nausea, early satiety and postprandial fullness were assessed at baseline and at 4 wk with validated 7-point global overall symptom scale[28].
Change in gastric volumes between baseline and postprandial (accommodation) was the primary endpoint for this study. To detect 16% difference in the Itopride and placebo with power of 80% and 5% level of significance a sample size of 15 subjects was needed in each group. This effect size of 16% [100 × (difference in group means divided by overall mean of the two groups)] corresponds to the difference in the two groups that was relevant clinically. The Analysis of coefficient of variance (ANCOVA) was done for this analysis.
For the purpose of this study, mean and standard deviation were reported for quantitative variables. Means and standard errors adjusted for covariates were reported using ANCOVA. The difference in the change in volume between itporide and the placebo group was compared using Man Whitney U test.
A total of thirty-one individuals were recruited for the purpose of this study. Mean age of these individuals was 33 years. Most of the recruited individuals, 21 (67.7%) were males. After randomization into Itopride and placebo groups, the groups were similar on variables like age, gender, serum haemoglobin, white blood cells, platelet count, serum creatinine, SGPT, prolactin level and QT interval on ECG (Table 1). There was no lost to follow up.
| Variable | Itopride | Placebo | P value |
| Age mean (SD) | 34.2 (6.4) | 31.9 (8.5) | 0.40 |
| Gender n (%) | |||
| Male | 10 (66.7) | 11 (68.8) | 1.001 |
| Female | 5 (33.3) | 5 (31.3) | |
| Hb (g/dL) mean (SD) | 13.6 (2.3) | 14.1 (1.7) | 0.50 |
| WBC (× 10 Eq/L) mean (SD) | 8.1 (2.0) | 7.7 (1.9) | 0.52 |
| Platelet count (× 10 Eq/L) mean (SD) | 257.3 (59.5) | 250.5 (57.2) | 0.75 |
| Creatinine (mg/mL) median (IQR) | 0.9 (0.5) | 0.8 (0.3) | 0.05 |
| SGPT (IU/L) median (IQR) | 20 (9.0) | 25.5 (17.0) | 0.09 |
| Prolactin level (mg/mL) (IQR) | 7.3 (3.6) | 5.7 (2.7) | 0.29 |
| QT interval mean (SD) | 394.1 (21.6) | 399.2 (22.9) | 0.53 |
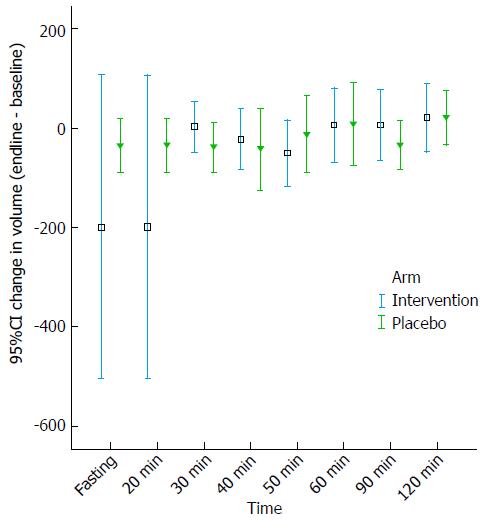
Gastric accommodation was checked using gastric scintigraphy by computing the change in gastric volume at empty stomach, 20, 30, 40, 50, 60, 90 and 120 min. We found that there was no statistically significant difference in the itopride and placebo group on gastric accommodation as measured with the difference in volume in the itopride and control group with the empty stomach (P = 0.14), at 20 min (P = 0.38), 30 min (P = 0.30), 40 min (P = 0.43), 50 min (P =0.50), 60 min (P = 0.81), 90 min (P = 0.25) and 120 min (P = 0.67) (Figure 1). Mean volumes to measure gastric accommodation by scintigraphy using ANCOVA adjusted for age and gender were computed (Table 2).
| Mean (± SE) | Itopride | Placebo |
| Change in volume (post-pre) using scintigraphy | ||
| Fasting | -22.2 (± 15.4) | 7.5 (± 14.9) |
| 20 min | -201.7 (± 102.4) | -31.5 (± 99.1) |
| 30 min | 0.14 (± 22.8) | -36.4 (± 22.1) |
| 40 min | -27.6 (± 32.2) | -37.4 (± 31.2) |
| 50 min | -60.0 (± 31.2) | -3.0 (± 30.2) |
| 60 min | -3.1 (± 36.5) | 15.0 (± 35.4) |
| 90 min | 3.8 (± 29.8) | -31.8 (± 28.8) |
| 120 min | 16.7 (± 28.9) | 23.3 (± 28.0) |
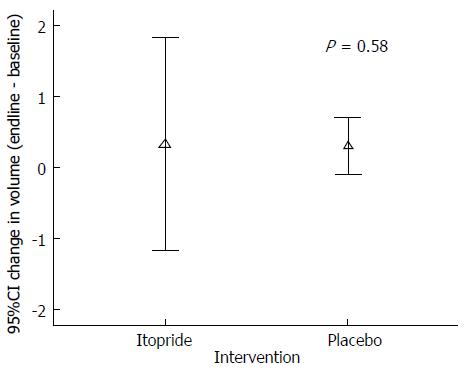
| Effect on gastric emptying on 13C labeled octanoic acid breath test (post-pre) | 0.4 (± 0.4) | 0.2 (± 0.4) |
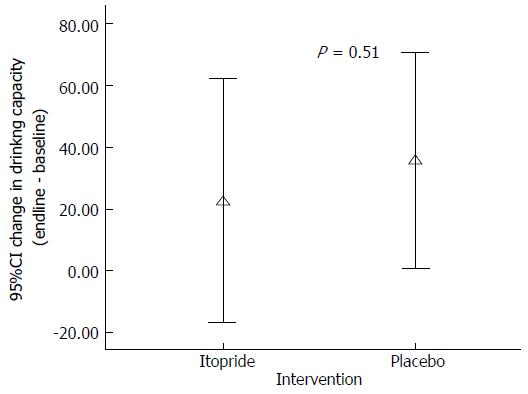
| Effect on drinking capacity (post-pre) | 22.5 (± 18.1) | 36.0 (17.5) |
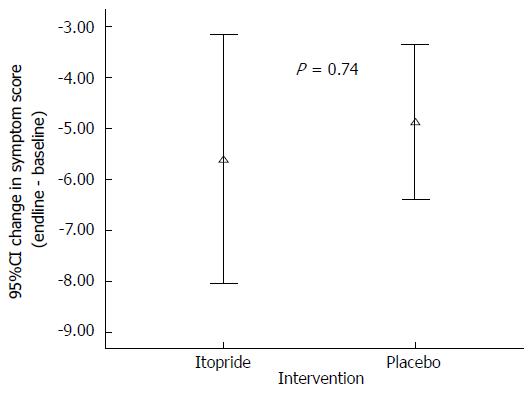
| Change in Symptom score (post-pre) | -5.8 (1.0) | -4.7 (0.9) |
Gastric emptying was done by doing breath tests on a sub sample (n = 11). There was no statistically significant difference (P = 0.58) between intervention and control group in gastric emptying (Figure 2).
At the end of the intervention (Itopride or placebo) there was no significant improvement in the capacity to tolerate liquid in the itopride group as compared to placebo (P = 0.51) (Figure 3).
There was no significant improvement of symptoms as assessed through a composite symptom score (P = 0.74) in the intervention group as compared to placebo (Figure 4). Similarly we didn’t find any significant improvement in the individual symptoms that included epigastric pain (P = 0.83), epigastric discomfort (P = 0.22), heart burn (P = 0.74), upper abdominal pain (P = 0.51), nausea (P = 0.08), early satiety (P = 0.34) and postprandial fullness (P = 0.25) (Table 3).
| Symptoms | Baseline | End of four weeks | ||||
| Itopride | Placebo | P value | Itopride | Placebo | P value | |
| Epigastric pain | 3.0 (1.0) | 4.0 (2.0) | 0.18 | 2.0 (1.0) | 2.0 (1.0) | 0.83 |
| Epigastric discomfort | 2.0 (1.0) | 1.0 (1.0) | 0.03 | 1.0 (1.0) | 1.0 (1.0) | 0.22 |
| Heart burn | 1.0 (3.0) | 1.0 (1.8) | 0.37 | 1.0 (1.0) | 1.0 (1.0) | 0.74 |
| Upper abdominal pain | 3.0 (2.0) | 1.5 (2.5) | 0.32 | 2.0 (1.0) | 1.5 (1.0) | 0.51 |
| Belching | 1.0 (1.0) | 1.0 (0.0) | 0.10 | 1.0 (1.0) | 1.0 (0.0) | 0.02 |
| Nausea | 2.0 (1.0) | 1.0 (0.0) | 0.04 | 1.0 (1.0) | 1.0 (0.0) | 0.08 |
| Early satiety | 2.0 (2.0) | 2.0 (3.0) | 0.56 | 1.0 (1.0) | 1.0 (0.0) | 0.34 |
| Postprandial Fullness | 2.0 (3.0) | 2.0 (2.8) | 0.82 | 1.0 (1.0) | 1.0 (1.0) | 0.25 |
| Total Symptom score | 19.0 (9.0) | 16.5 (5.0) | 0.41 | 12.0 (3.0) | 11.5 (4.5) | 0.71 |
The change in QT interval as a result of itopride group was not statistically different from placebo (0.10). Similarly itopride didn’t alter the serum prolactin level in the intervention group as compare to the placebo group.
In this study we tested the effect of itopride on some of the pathophysiological mechanisms attributed to the causation of symptoms in FD patients. Impaired accommodation is implied as one of the important factors considered to be associated with symptoms in FD patients. A primary objective of our study was to check whether itopride has any effect on gastric accommodation. We didn’t find any effect of itopride on gastric accommodation when assessed through gastric scintigraphy as compared to placebo. This finding is in disagreement with a similar study which showed that itopride worsens the gastric accommodation[29].
We also found that itopride didn’t effect gastric emptying as assessed through 13C labeled octanoic acid breath test. A study done in Japan showed that itopride improves gastric emptying among dyspeptic patients[30]. On the other hand a cross over study that was also done in Japan showed that itopride does not improve gastric emptying[31]. Our study also found that itopride did not improve the drinking capacity as assessed through satiety drinking capacity test as compare to the placebo. This finding is in line with the similar findings in another study where it was found that itopride failed to improve the nutrient drink test induced symptoms[29].
Though in previous studies it was demonstrated that itopride improves symptoms related to FD but we found that it is not true for our set of patients. Itopride failed to show improvement in the overall symptom score as well as effect on individual symptoms including early satiety and postprandial fullness as opposed to the conclusion of a recent meta-analysis[23]. Through previous studies we know that to achieve a considerable improvement in the symptoms of one patient, we need to treat six patients of FD[32]. Itopride was efficacious in reducing the symptom score in Chinese patients having FD[33]. Small sample size might be the reason which resulted in our inability to detect an improvement in individual symptoms as a result of itopride usage as compared to placebo. Our sample included younger individuals and therefore could not study the effect of itopride in older patients with FD. Lack of variability in the age might have affected the results.
Itopride is advocated for the treatment of FD as it is safer drug as compare to other prokinetic agents. In our study we found that itopride didn’t prolong the QT interval compared to placebo. Similarly itopride did not raise the prolactin level compared placebo. Therefore we can say that though itopride did not demonstrate any effect it is safer prokinetic.
Inability of itopride to effect gastric accommodation and gastric emptying might be because of the genetic variability in the dopamine-D2 receptor subtype. TaqIA polymorphism is one example where dopamine-D2 receptor is not fully expressed resulting in compromised functionality of this receptor[34]. Mechanistic studies can identify the genetic factors like dopamine-D2 receptor variability which are anticipated to effect the efficacy of itopride among FD patients in our setting.
The strength of our study was that we used objective measures, i.e., gastric scintigraphy and 13C labeled octanoic acid breath test to measure gastric accommodation and gastric emptying. Diagnosis of FD was based on ROME III criteria and was done after using extensive investigations to rule out organic cause for the symptoms. The study was conducted at one center and therefore we couldn’t capture a broad spectrum of patients suffering from FD. We only checked the effect of 150 mg of itopride on gastric functions and symptoms. We didn’t use an objective measure to find out the absorbed amount of drug in the body.
We found no effect of itopride on gastric accommodation, gastric emptying and maximum tolerated volume in patients with FD in our study.
Higher Education Commission Islamabad for providing grant to conduct this study.
Functional dyspepsia (FD) is defined as the presence of symptoms thought to originate in the gastro-duodenal region in the absence of any organic, systemic, or metabolic disease that is likely to explain the symptoms. Pharmacological treatments for patients with FD remain unsatisfactory. Itopride is a dopamine (D2) antagonist with acetylcholinesterase inhibitory actions. This agent is currently indicated for patients with various upper gastrointestinal (GI) symptoms. The anti-dopaminergic effects of itopride are truly “peripheral”. There is a need to determine the effect of itopride on gastric function and to elaborate further the understanding on the basis of potential therapeutic benefit of this agent in FD patients. Through this study the authors wanted to find the effect of itopride on gastric accommodation, gastric emptying and drinking capacity in patients with FD in Pakistani population.
Data related to the treatment of FD in Pakistani population is lacking. This study focused effect of itopride on gastric functions among patients with FD in their population.
Through this study the authors found out that there is no effect of itopride on gastric functions among patients of FD.
Itopride might not be a suitable medicine for treating patients with FD.
This is an original study investigating itopride in FD and showing no effect of it on physiological and clinical parameters.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Pakistan
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Dumitrascu DL, Savarino EV S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Holtmann G, Talley NJ. Functional dyspepsia. Curr Opin Gastroenterol. 2015;31:492-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 2. | Talley NJ, Ford AC. Functional Dyspepsia. N Engl J Med. 2015;373:1853-1863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 315] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 3. | Ford AC, Marwaha A, Sood R, Moayyedi P. Global prevalence of, and risk factors for, uninvestigated dyspepsia: a meta-analysis. Gut. 2015;64:1049-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 321] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 4. | Mukhtar M, Zubair M, Naz R, Tabassum S, Achackzai M. Functional Dyspepsia: An Unresolved Issue. Intern Med. 2015;5:2. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 5. | Brook RA, Kleinman NL, Choung RS, Melkonian AK, Smeeding JE, Talley NJ. Functional dyspepsia impacts absenteeism and direct and indirect costs. Clin Gastroenterol Hepatol. 2010;8:498-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 142] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 6. | Lacy BE, Weiser KT, Kennedy AT, Crowell MD, Talley NJ. Functional dyspepsia: the economic impact to patients. Aliment Pharmacol Ther. 2013;38:170-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 225] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 7. | Delgado-Aros S, Camilleri M, Cremonini F, Ferber I, Stephens D, Burton DD. Contributions of gastric volumes and gastric emptying to meal size and postmeal symptoms in functional dyspepsia. Gastroenterology. 2004;127:1685-1694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 150] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 8. | Scott AM, Kellow JE, Shuter B, Cowan H, Corbett AM, Riley JW, Lunzer MR, Eckstein RP, Höschl R, Lam SK. Intragastric distribution and gastric emptying of solids and liquids in functional dyspepsia. Lack of influence of symptom subgroups and H. pylori-associated gastritis. Dig Dis Sci. 1993;38:2247-2254. [PubMed] |
| 9. | Vengher I, Dumitrascu D. The relationship between alexithymia, depression and quality of life in patients with functional dyspepsia. J Psychosom Res. 2015;78:629. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 10. | Stanghellini V, De Giorgio R, Barbara G, Cogliandro R, Tosetti C, De Ponti F, Corinaldesi R. Delayed Gastric Emptying in Functional Dyspepsia. Curr Treat Options Gastroenterol. 2004;7:259-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Quartero AO, de Wit NJ, Lodder AC, Numans ME, Smout AJ, Hoes AW. Disturbed solid-phase gastric emptying in functional dyspepsia: a meta-analysis. Dig Dis Sci. 1998;43:2028-2033. [PubMed] |
| 12. | Lin Z, Eaker EY, Sarosiek I, McCallum RW. Gastric myoelectrical activity and gastric emptying in patients with functional dyspepsia. Am J Gastroenterol. 1999;94:2384-2389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 83] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 13. | Pfaffenbach B, Adamek RJ, Bartholomäus C, Wegener M. Gastric dysrhythmias and delayed gastric emptying in patients with functional dyspepsia. Dig Dis Sci. 1997;42:2094-2099. [PubMed] |
| 14. | Tack J, Talley NJ, Camilleri M, Holtmann G, Hu P, Malagelada JR, Stanghellini V. Functional gastroduodenal disorders. Gastroenterology. 2006;130:1466-1479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1243] [Cited by in RCA: 1195] [Article Influence: 62.9] [Reference Citation Analysis (0)] |
| 15. | Du LJ, Chen BR, Kim JJ, Kim S, Shen JH, Dai N. Helicobacter pylori eradication therapy for functional dyspepsia: Systematic review and meta-analysis. World J Gastroenterol. 2016;22:3486-3495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 112] [Cited by in RCA: 93] [Article Influence: 10.3] [Reference Citation Analysis (1)] |
| 16. | Pinto-Sanchez MI, Yuan Y, Bercik P, Moayyedi P. Proton pump inhibitors for functional dyspepsia. Cochrane Database of Systematic Reviews. 2014;7:CD011194. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Moayyedi P, Anglin R. Antidepressants for Functional Dyspepsia: New Indications for Old Therapies? Clin Gastroenterol Hepatol. 2016;14:393-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 18. | Hiyama T, Yoshihara M, Matsuo K, Kusunoki H, Kamada T, Ito M, Tanaka S, Nishi N, Chayama K, Haruma K. Meta-analysis of the effects of prokinetic agents in patients with functional dyspepsia. J Gastroenterol Hepatol. 2007;22:304-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 86] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 19. | Ganzini L, Casey DE, Hoffman WF, McCall AL. The prevalence of metoclopramide-induced tardive dyskinesia and acute extrapyramidal movement disorders. Arch Intern Med. 1993;153:1469-1475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 122] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 20. | Bowman JD, Kim H, Bustamante JJ. Drug-induced gynecomastia. Pharmacotherapy. 2012;32:1123-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 21. | Wysowski DK, Corken A, Gallo-Torres H, Talarico L, Rodriguez EM. Postmarketing reports of QT prolongation and ventricular arrhythmia in association with cisapride and Food and Drug Administration regulatory actions. Am J Gastroenterol. 2001;96:1698-1703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 165] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 22. | Talley NJ, Tack J, Ptak T, Gupta R, Giguère M. Itopride in functional dyspepsia: results of two phase III multicentre, randomised, double-blind, placebo-controlled trials. Gut. 2008;57:740-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 131] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 23. | Huang X, Lv B, Zhang S, Fan YH, Meng LN. Itopride therapy for functional dyspepsia: a meta-analysis. World J Gastroenterol. 2012;18:7371-7377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 59] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (1)] |
| 24. | Bennink RJ, van den Elzen BD, Kuiken SD, Boeckxstaens GE. Noninvasive measurement of gastric accommodation by means of pertechnetate SPECT: limiting radiation dose without losing image quality. J Nucl Med. 2004;45:147-152. [PubMed] |
| 25. | Jackson SJ, Bluck LJ, Coward WA. Use of isotopically labelled octanoic acid to assess the effect of meal size on gastric emptying. Rapid Commun Mass Spectrom. 2004;18:1003-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Ghoos YF, Maes BD, Geypens BJ, Mys G, Hiele MI, Rutgeerts PJ, Vantrappen G. Measurement of gastric emptying rate of solids by means of a carbon-labeled octanoic acid breath test. Gastroenterology. 1993;104:1640-1647. [PubMed] |
| 27. | Tack J, Caenepeel P, Piessevaux H, Cuomo R, Janssens J. Assessment of meal induced gastric accommodation by a satiety drinking test in health and in severe functional dyspepsia. Gut. 2003;52:1271-1277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 192] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 28. | Veldhuyzen van Zanten SJ, Chiba N, Armstrong D, Barkun AN, Thomson AB, Mann V, Escobedo S, Chakraborty B, Nevin K. Validation of a 7-point Global Overall Symptom scale to measure the severity of dyspepsia symptoms in clinical trials. Aliment Pharmacol Ther. 2006;23:521-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 62] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 29. | Choung RS, Talley NJ, Peterson J, Camilleri M, Burton D, Harmsen WS, Zinsmeister AR. A double-blind, randomized, placebo-controlled trial of itopride (100 and 200 mg three times daily) on gastric motor and sensory function in healthy volunteers. Neurogastroenterol Motil. 2007;19:180-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 30. | Harasawa S. Effect of itopride hydrochloride on gastric emptying in chronic gastritis patients (in Japanese). Jpn Pharmacol Ther. 1993;21:303-309. |
| 31. | Nonaka T, Kessoku T, Ogawa Y, Yanagisawa S, Shiba T, Sahaguchi T, Atsukawa K, Takahashi H, Sekino Y, Iida H. Does postprandial itopride intake affect the rate of gastric emptying? A crossover study using the continuous real time 13C breath test (BreathID system). Hepatogastroenterology. 2011;58:224-228. [PubMed] |
| 32. | Holtmann G, Talley NJ, Liebregts T, Adam B, Parow C. A placebo-controlled trial of itopride in functional dyspepsia. N Engl J Med. 2006;354:832-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 180] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 33. | Sun J, Yuan YZ, Holtmann G. Itopride in the treatment of functional dyspepsia in Chinese patients: a prospective, multicentre, post-marketing observational study. Clin Drug Investig. 2011;31:865-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 34. | Richter A, Richter S, Barman A, Soch J, Klein M, Assmann A, Libeau C, Behnisch G, Wüstenberg T, Seidenbecher CI. Motivational salience and genetic variability of dopamine D2 receptor expression interact in the modulation of interference processing. Front Hum Neurosci. 2013;7:250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |