Published online Nov 6, 2016. doi: 10.4292/wjgpt.v7.i4.572
Peer-review started: June 28, 2016
First decision: August 10, 2016
Revised: August 16, 2016
Accepted: October 17, 2016
Article in press: October 19, 2016
Published online: November 6, 2016
Processing time: 125 Days and 10 Hours
To evaluate the efficacy of 5 mL simethicone solution in decreasing gastric foam if given at least 30 min before gastroscopy.
This was a randomized, placebo controlled, endoscopist blinded study performed at Changi General Hospital. Patients were at least 21 years old, had no prior surgical resection of the upper gastrointestinal tract, and scheduled for elective diagnostic gastroscopies. The primary outcome was the total mucosal visibility score (TMVS) which was evaluated using McNally score. The sample size was calculated to be 24 per group (SD 2.4, 80% power, P < 0.05, 2-sample t test).
Fifty-four patients were randomised to receive either simethicone [1 mL liquid simethicone (100 mg) in 5 mL of water] or placebo (5 mL of water) at least 30 min before their gastroscopy. Six accredited consultants conducted the gastroscopy, and the interobserver agreement of scoring TMVS was good with a Kappa statistic of 0.73. The simethicone group had significantly better mean TMVS compared to placebo (5.78 ± SD 1.65 vs 8.89 ± SD 1.97, P < 0.001). The improvement was statistically significant for the duodenum and the gastric antrum, angularis, body, and fundus. Percent 51.9 of patients in the simethicone group had a TMVS of 4 (no bubbles at all) to 5 (only 1 area with minimal bubbles), while in the placebo group 3.7% of patients had TMVS of 4 or 5. The number needed to treat was 2.1 to avoid a TMVS of 6 and more. The simethicone group also had a significantly shorter procedure time with less volume of additional flushes required during gastroscopy to clear away obscuring gastric foam.
With a premedication time of at least 30 min, 5 mL simethicone can significantly decrease gastric foam, decrease the volume of additional flushes, and shorten gastroscopy time.
Core tip: This is the first study to evaluate the efficacy of a low volume (5 mL) simethicone solution compared to a placebo using the McNally score to calculate the total mucosal visibility for gastroscopy. Our study showed that although earlier studies had favored higher volumes (typically 100 mL), a low volume is still effective as long as adequate premedication time of at least 30 min is allowed. Such a small volume is more suitable for patients with swallowing difficulties and the formulation had excellent patient compliance with no adverse effects.
- Citation: Song M, Kwek ABE, Law NM, Ong JPL, Tan JYL, Harichander Thurairajah P, Ang DSW, Ang TL. Efficacy of small-volume simethicone given at least 30 min before gastroscopy. World J Gastrointest Pharmacol Ther 2016; 7(4): 572-578
- URL: https://www.wjgnet.com/2150-5349/full/v7/i4/572.htm
- DOI: https://dx.doi.org/10.4292/wjgpt.v7.i4.572
Image-enhanced gastroscopy, such as narrow-band imaging and magnifying endoscopy, can detect subtle early gastric cancer or precancerous lesions and this technology is widely available in Singapore[1]. However, the presence of foam, bubbles and mucus can preclude the benefits of enhanced endoscopy, as subtle mucosal lesions could be covered. In the latest Singapore Cancer Registry, more than 50% of gastric cancers were diagnosed at stage IV of disease[2]. This suggests that improvement is required for endoscopic detection of early gastric cancer.
Many studies have proven that premedication before gastroscopy will improve the total mucosal visibility scores. However, there is significant heterogeneity between these studies in terms of premedication time, mucosal scoring systems, primary outcome measurements and the type of medications used. Pronase, N-acetylcysteine, dimethicone, dimethylpolysiloxane and simethicone have all been demonstrated by multiple studies to be effective mucolytic and anti-foaming agents[3-11]. All the studies suggested that the best premedication regime is a combination of premedication (a mucolytic and an anti-foaming agent) delivered at large volumes (typically with 100 mL of water). Only one study had a treatment arm looking at small volume premedication (100 mg simethicone in 5 mL water) but this was not compared against a placebo group and the study used a unique 3-grade scoring system[11].
In Singapore, pronase, dimethicone and dimethylpolysiloxane are not available. N-acetylcysteine is a prescription drug and simethicone is an over-the-counter drug for infant colic used off-label for flushes during endoscopy. Premedication before gastroscopy is not routinely given to patients at all endoscopy centers due to several reasons; the perception that premedication may slow down the endoscopy schedule; the worry of adverse reactions from the medications (such as allergic reactions to N-acetylcysteine); and the worry of aspiration from drinking premedication shortly before gastroscopy. As Singapore has an aging population[12], it is common now to perform endoscopy on elderly patients with swallowing dysfunction. However 100 mL premedication solution before gastroscopy puts these patients at risk for aspiration, especially in the setting of moderate sedation during the procedure[13].
We hypothesized that if the premedication time is extended to at least 30 min, 100 mg of simethicone added to 5 mL of water will be able to mix with gastric secretions and swallowed saliva to coat a larger surface area of the gastric mucosa, and significantly improve mucosal visibility compared to placebo.
This study was conducted in Changi General Hospital in Singapore, from 14th August 2015 to 19th November 2015, at the outpatient gastroenterology clinics. All patients who were planned for gastroscopy as part of their management plan were asked by their respective clinic attending if they would permit a research coordinator to speak to them. If they agreed, the research coordinator would find the patient at the endoscopy listing room to obtain informed consent from the patient to participate in the study. Patients who were at least 21 years old, mentally competent to give informed consent, and scheduled for outpatient elective diagnostic gastroscopy were enrolled. Patients who were incarcerated; had prior history of surgical resection of the esophagus, stomach, or duodenum; had known hypersensitivity to simethicone; or required gastroscopy for urgent indications such as suspected gastrointestinal bleeding were all excluded from the study.
This was a randomized, placebo-controlled, endoscopist-blinded study which was approved by the SingHealth Centralized Institutional Review Board (Ref: 2015/2519) and registered under clinicaltrials.gov (NCT02555228). The randomisation sequence (in blocks of 6) was computer generated by a statistician at Changi General Hospital’s Clinical Trials and Research Unit (CTRU). The allocation sequence was written on separate cards as number codes and each card was placed inside a sealed opaque envelope. After a study participant registered for the elective gastroscopy, the research coordinator would open an opaque envelope outside the endoscopy suites and the patient would be allocated to either the simethicone group (100 mg of liquid simethicone added to 5 mL of water) or the placebo group (5 mL of water) based on the number written on a card.
Study participants underwent gastroscopy by 1 of the 6 accredited consultant endoscopists who were blinded to the premedication as well as the premedication time. The premedication was prepared by a research coordinator at a separate location, out of sight from the endoscopist or the endoscopy nurses inside the procedure suites. The patient was informed not to disclose the nature of the premedication to the endoscopy suite nurses or endoscopists. The research coordinator worked together with the scheduling nurse at the endoscopy center to ensure that the premedication was taken at least 30 min before the gastroscopy commenced. To confirm that the patient did not tell the endoscopist about the premedication’s nature, the research coordinator followed the patient into the endoscopy suite and stood beside the patient until the procedure was over and the data collection form had been completed by the endoscopist. Premedication time was defined from the administration of the solution to the insertion of the tip of the gastroscope into the patient’s mouth. All patients received topical analgesic xylocaine 10% spray to the back of their throat and intravenous midazolam with fentanyl to achieve moderate sedation during gastroscopy.
During the procedure, the endoscopist was allowed to flush additional diluted simethicone solution (1 to 3 drops of simethicone added to about 100 mL of water) down the gastroscope channel if there was obscuring gastric foam preventing a satisfactory view. The total volume of additional flushes was recorded by the endoscopy nurse assisting the procedure. After the endoscopist completed an adequate inspection of the mucosal surfaces, the endoscopist withdrew the tip of the gastroscopy up to the gastroesophageal junction and the research coordinator noted the time. The procedure time was defined as the period of time from insertion of the gastroscope to the withdrawal of the gastroscopy back to the gastroesophageal junction. After this, the endoscopist advanced the gastroscope back into the stomach and proceeded to do any interventions deemed necessary such as biopsies of detected lesions. This ensured that the procedure time measured was standardized and not confounded by the number of additional endoscopic interventions due to detection of more lesions.
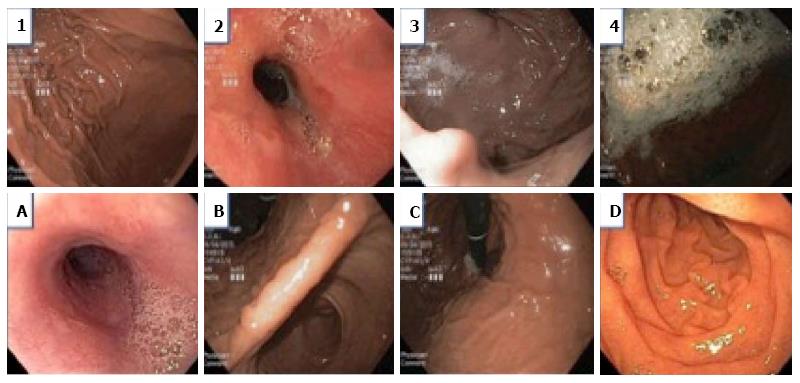
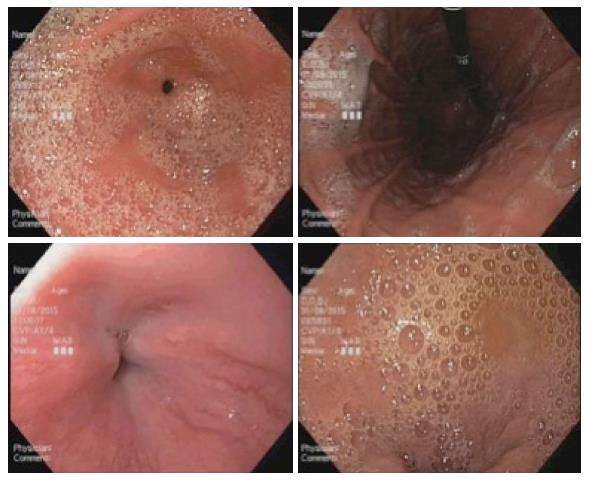
Before initiation of the study, all the endoscopists were instructed on the endoscopic scoring system which was based on the McNally scoring system (Figure 1)[14]. Prior to any additional flushes with diluted simethicone solution, the endoscopists evaluated and noted the McNally score for the esophagus, the gastric fundus and body, the gastric antrum and angularis, and the duodenum. The scoring per area was from the range of 1 to 4; 1 if there was no bubble at all, 2 if there were minimal bubbles which the endoscopist had to actively look out for, 3 if the bubbles were obvious but not totally obscuring the view and 4 if the bubbles were so severe that vision is obscured. The total mucosal visibility score (TMVS) was calculated by the sum of scores in all the areas and ranged from 4 to 16.
The primary end-point measured was the mean TMVS in the simethicone premedication group and the placebo group respectively. The secondary end-points measured were the mean visibility scores per area, the mean procedure time per group, the mean volume of additional flushes required per group, adverse events reported by the patient or monitoring endoscopy nurses, and the number of gastric lesions reported by the endoscopist.
We felt that a difference of 2 points in mean TMVS between the two groups would be clinically significant. An earlier study conducted in Thailand had used a similar scoring system and we adopted the standard deviation in their study results for our estimation[15]. The calculated sample size for each group was 24 patients (SD 2.4, 2 sample t test, P < 0.05, 80% power). Assuming that the drop-out rate could be around 10%, we aimed to recruit 27 patients per group.
All categorical variables were analysed with Pearson Chi-square test and all continuous variables were analysed with 2-sample t test, using SPSS V.19.0 software for Windows (SPSS Inc, Chicago, Illinois, United States). P < 0.05 was deemed statistically significant.
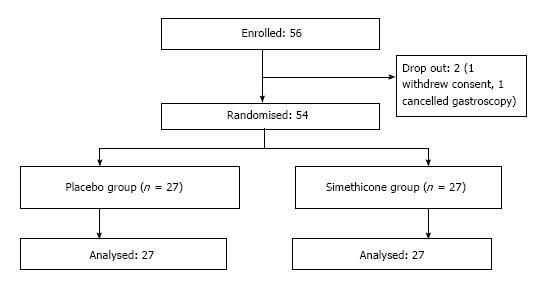
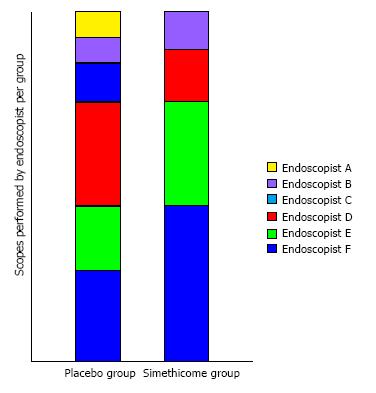
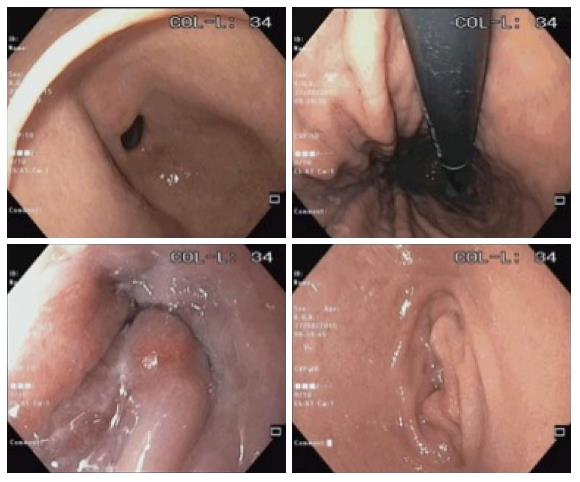
A total of 56 patients were enrolled in the study. One patient withdrew consent on the day of the gastroscopy. Another patient subsequently called to cancel the gastroscopy. The remaining 54 patients completed the study with no adverse events (Figure 2) Baseline characteristics of the patients in the 2 groups were similar in terms of mean age, gender, and premedication time (Table 1). Six experienced endoscopists conducted the gastroscopy for all the patients, 4 of the endoscopists were involved in both groups (Figure 3). Before the study, all endoscopists separately scored the TMVS for the endoscopic images of four anonymous participants. They were blinded to the treatment allocation and patient particulars. The scores were tallied by an independent investigator to determine the interobserver agreement which was found satisfactory with a kappa statistic of 0.73.
| Placebo group(n = 27) | Simethicone group(n = 27) | P vaule | |
| Mean age (yr ± SD) | 52.9 ± 15.5 | 57.7 ± 12.5 | 0.215 |
| Male gender (%) | 9 (33.3) | 15 (55.6) | 0.1 |
| Mean premedication time (min:s ± SD) | 41:08 ± 9:56 | 44:46 ± 12:36 | 0.245 |
| Indication for gastroscopy | |||
| Dyspepsia | 19 (70.4) | 10 (37.0) | 0.014 |
| Reflux symptoms | 3 (11.1) | 6 (22.2) | 0.467 |
| Positive H. pylori serology | 0 | 1 (3.7) | 1 |
| Variceal screen | 1 (3.7) | 2 (7.4) | 1 |
| Anemia | 4 (14.8) | 3 (11.1) | 1 |
| Dysphagia | 0 | 1 (3.7) | 1 |
| Intestinal metaplasia surveillance | 0 | 3 (11.1) | 0.236 |
| Cancer surveillance after endoscopic mucosal resection | 0 | 1 | 1 |
The mean TMVS was significantly lower in the simethicone group compared to the placebo group (5.78 ± 1.65 for simethicone group, 8.89 ± 1.97 for placebo group, P < 0.001). This improvement in mucosal visibility score was significant for the areas of the stomach (body, fundus, antrum, and angularis) and the duodenum. However, simethicone premedication did not significantly improve mucosal visibility score of the esophagus. The simethicone group also had a shorter mean procedure time (P = 0.049) as well as lower mean volume of additional flushes required during gastroscopy (P < 0.001) (Table 2).
| Placebo group | Simethicone group | P value | |
| TMVS ± SD | 8.89 ± 1.97 | 5.78 ± 1.65 | < 0.001 |
| Mean esophagus score ± SD | 1.59 ± 0.57 | 1.48 ± 0.57 | 0.482 |
| Mean duodenum score ± SD | 2.26 ± 0.81 | 1.26 ± 0.53 | < 0.001 |
| Mean antrum and angularis score ± SD | 2.56 ± 1.05 | 1.30 ± 0.54 | < 0.001 |
| Mean body and fundus score ± SD | 2.44 ± 0.97 | 1.74 ± 0.81 | 0.006 |
| Mean volume of additional water flushes required ± SD (mL) | 84.81 ± 110.18 | 3.89 ± 11.46 | < 0.001 |
| Procedure time ± SD (s) | 193.67 ± 87.04 | 154.85 ± 49.07 | 0.049 |
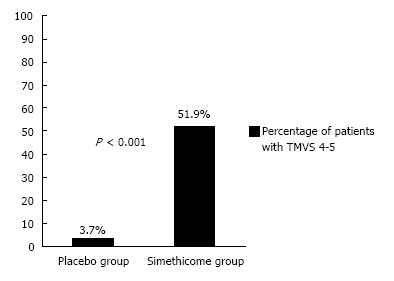
In the simethicone group, 51.9% of the patients had TMVS of 4 (no bubbles in all areas inspected) to 5 (one area had minimal bubbles), whereas in the placebo group only 3.7% of the patients achieved this (P < 0.001) (Figure 4). The number needed to treat (NNT) was 2.1 to avoid a TMVS of 6 or more.
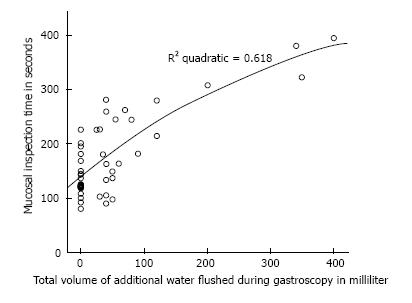
To our knowledge, this is the first study conducted which evaluated the benefit of a very low volume of simethicone monotherapy for gastroscopy preparation, which was compared against a placebo using the McNally scoring method. Prior studies had shown that larger volumes produced better results and a 100 mL solution was generally accepted as the best. Bertoni et al[10] showed in their study that 90 mL solutions were superior to 30 mL solutions when ingested 5 min before the start of gastroscopy. Chang et al[6] showed in their recent study that when ingested within 30 min before gastroscopy, their 100 mL solution consisting of mucolytic and anti-foaming agent resulted in the best mucosal visibility scores. In this study, the premedication time was increased significantly (mean premedication times were 41:08 ± 9:56 min for placebo group and 44:46 ± 12:36 min for simethicone group), which allowed mixing of the simethicone with gastric secretion and swallowed saliva to coat the mucosal surface. This resulted in significant improvement of TMVS compared to placebo (Figures 5 and 6). Although the improvement in mucosal visibility scores was not significant for the esophageal area, the mean scores for the esophageal area were already very low to begin with (1.48 ± 0.57 in the simethicone group and 1.59 ± 0.57 in the placebo group). We postulated that this was because of the tubular structure of the esophagus as well as the peristaltic movements of the esophagus allowing mucus and secretions to flow down into the stomach. In additional, our study population is made up of healthy patients who were predominantly undergoing gastroscopy for dyspepsia; only 1 patient had dysphagia and 9 patients had reflux symptoms. This may result in the study population having a better mucosal visibility score in the esophageal area at baseline and explain why low volume simethicone solution did not make much of a difference. There was also significantly lower volume of additional flushes required during gastroscopy if simethicone was given. This, in turn, resulted in a significantly shorter procedure time for mucosal inspection (Figure 7). The ideal TMVS was 4 with absolutely no bubbles in all areas. In our study, the NNT to achieve a TMVS of 4 to 5 during gastroscopy was just 2.1.
Our study had two main limitations. Firstly, the study was not powered to investigate for the improvement of gastric lesion detection with enhanced endoscopy techniques. Our study participants were all recruited from the clinics for elective gastroscopies and the findings were predominantly benign functional dyspepsia or non-erosive gastritis which did not require further enhanced imaging as determined by the endoscopist. In order to investigate this aspect, we would have required a much larger study population which will likely require a multi-center collaboration. Secondly, we did not investigate the additional benefit of adding mucolytics to the regime which many other studies had done. This is because N-acetylcysteine is not readily available (it requires prior prescription and collection from the pharmacy by the patient) whereas simethicone is stored at all endoscopy suites and we wanted to find a convenient premedication regime that our endoscopists will be comfortable using.
None of the patients had any adverse event. As the volume of premedication required is only 5 to 6 mL in total, this is likely suitable for use in patients with swallowing dysfunction as such volumes are routinely used as modified water swallowing tests swallowing test[13].
Ms Nway Nway Aye, department research coordinator.
Numerous studies have used various formulations before gastroscopy to decrease gastric foam or gastric mucus, with the overall conclusion that a larger volume with combined therapy is more effective.
The potential benefit of extending premedication time has been evaluated in earlier studies with varying results.
This is the first randomized controlled study to evaluate the efficacy of a small volume simethicone solution (as compared to placebo with water) in the context of a premedication time of at least 30 min. It shows that despite earlier studies favoring combination therapy with larger volumes, a longer premedication time with a small volume monotherapy can significantly improve mucosal visibility scores. The study sample was calculated using the standard deviation from the results of a study with the same McNally scoring system. The study results offer a convenient and effective premedication that requires no additional prescription in Singapore and will likely be tolerated by patients with swallowing dysfunction.
This study’s finding has resulted in the standardised use of simethicone premedication at Changi General Hospital endoscopy center prior to elective gastroscopies in the low volume formulation.
It is a good practical idea. They think the importance of search could be more applicable if the study done for enteroscopy not upper endoscopy. The paper is well written, well organised.
Manuscript source: Invited manuscript
Specialty Type: Gastroenterology and Hepatology
Country of Origin: Singapore
Peer-Review Report Classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: De Silva AP, Shehata MMM, Yeoh SW, Zuo XL S- Editor: Qi Y L- Editor: A E- Editor: Lu YJ
| 1. | Song M, Ang TL. Early detection of early gastric cancer using image-enhanced endoscopy: Current trends. Gastrointest Inter. 2014;3:1-7. [RCA] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (1)] |
| 2. | Singapore Cancer Registry 2015. Cancer Survival in Singapore 1973-2012. USA: National Registry of Diseases Office 2015; . |
| 3. | Cha JM, Won KY, Chung IK, Kim GH, Lee SY, Cho YK. Effect of pronase premedication on narrow-band imaging endoscopy in patients with precancerous conditions of stomach. Dig Dis Sci. 2014;59:2735-2741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Kuo CH, Sheu BS, Kao AW, Wu CH, Chuang CH. A defoaming agent should be used with pronase premedication to improve visibility in upper gastrointestinal endoscopy. Endoscopy. 2002;34:531-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Lee GJ, Park SJ, Kim SJ, Kim HH, Park MI, Moon W. Effectiveness of Premedication with Pronase for Visualization of the Mucosa during Endoscopy: A Randomized, Controlled Trial. Clin Endosc. 2012;45:161-164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Chang CC, Chen SH, Lin CP, Hsieh CR, Lou HY, Suk FM, Pan S, Wu MS, Chen JN, Chen YF. Premedication with pronase or N-acetylcysteine improves visibility during gastroendoscopy: an endoscopist-blinded, prospective, randomized study. World J Gastroenterol. 2007;13:444-447. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 47] [Cited by in RCA: 56] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 7. | Neale JR, James S, Callaghan J, Patel P. Premedication with N-acetylcysteine and simethicone improves mucosal visualization during gastroscopy: a randomized, controlled, endoscopist-blinded study. Eur J Gastroenterol Hepatol. 2013;25:778-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Chen MJ, Wang HY, Chang CW, Hu KC, Hung CY, Chen CJ, Shih SC. The add-on N-acetylcysteine is more effective than dimethicone alone to eliminate mucus during narrow-band imaging endoscopy: a double-blind, randomized controlled trial. Scand J Gastroenterol. 2013;48:241-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Asl SM, Sivandzadeh GR. Efficacy of premedication with activated Dimethicone or N-acetylcysteine in improving visibility during upper endoscopy. World J Gastroenterol. 2011;17:4213-4217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Bertoni G, Gumina C, Conigliaro R, Ricci E, Staffetti J, Mortilla MG, Pacchione D. Randomized placebo-controlled trial of oral liquid simethicone prior to upper gastrointestinal endoscopy. Endoscopy. 1992;24:268-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Chang WK, Yeh MK, Hsu HC, Chen HW, Hu MK. Efficacy of simethicone and N-acetylcysteine as premedication in improving visibility during upper endoscopy. J Gastroenterol Hepatol. 2014;29:769-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 12. | National Population and Talent Division, Prime Minister’s Office, Singapore Department of Statistics, Ministry of Home Affairs, Immigration and Checkpoints Authority. Population in Brief 2015. Singapore: National Population and Talent Division, Prime Minister’s Office, Singapore Department of Statistics, Ministry of Home Affairs, Immigration and Checkpoints Authority 2015; . |
| 13. | Osawa A, Maeshima S, Tanahashi N. Water-swallowing test: screening for aspiration in stroke patients. Cerebrovasc Dis. 2013;35:276-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 14. | McNally PR, Maydonovitch CL, Wong RK. The effectiveness of simethicone in improving visibility during colonoscopy: a double-blind randomized study. Gastrointest Endosc. 1988;34:255-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Keeratichananont S, Sobhonslidsuk A, Kitiyakara T, Achalanan N, Soonthornpun S. The role of liquid simethicone in enhancing endoscopic visibility prior to esophagogastroduodenoscopy (EGD): A prospective, randomized, double-blinded, placebo-controlled trial. J Med Assoc Thai. 2010;93:892-897. [PubMed] |