Published online Nov 6, 2016. doi: 10.4292/wjgpt.v7.i4.503
Peer-review started: April 28, 2016
First decision: July 4, 2016
Revised: August 4, 2016
Accepted: August 15, 2016
Article in press: August 17, 2016
Published online: November 6, 2016
Processing time: 187 Days and 2 Hours
Despite little evidence for the therapeutic benefits of a high-fiber diet for diverticulitis, it is commonly recommended as part of the clinical management. The ongoing uncertainty of the cause(s) of diverticulitis confounds attempts to determine the validity of this therapy. However, the features of a high-fiber diet represent a logical contradiction for colon diverticulitis. Considering that Bernoulli’s principle, by which enlarged diameter of the lumen leads to increased pressure and decreased fluid velocity, might contribute to development of the diverticulum. Thus, theoretically, prevention of high pressure in the colon would be important and adoption of a low FODMAP diet (consisting of fermentable oligosaccharides, disaccharides, monosaccharides, and polyols) may help prevent recurrence of diverticulitis.
Core tip: The ongoing uncertainty of the cause(s) of diverticulitis confounds attempts to determine the validity of this therapy; however, the features of a high-fiber diet represent a logical contradiction for colon diverticulitis. Prevention of high pressure in the colon may help to avoid or correct diverticulitis, and this may be achieved by adoption of a low FODMAP diet (restriction of fermentable oligosaccharides, disaccharides, monosaccharides, and polyols).
- Citation: Uno Y, van Velkinburgh JC. Logical hypothesis: Low FODMAP diet to prevent diverticulitis. World J Gastrointest Pharmacol Ther 2016; 7(4): 503-512
- URL: https://www.wjgnet.com/2150-5349/full/v7/i4/503.htm
- DOI: https://dx.doi.org/10.4292/wjgpt.v7.i4.503
The theory of colon diverticulitis and diet association was expanded upon by Painter[1], first in 1969 when he reported that diverticular disease (DD) occurred in people who ate a low residue diet with refined flour and sugar, then again in 1970 when he stated there was no DD in Africa[2]. Painter went on to explain that Denis Parsons Burkitt, the famed United Kingdom surgeon and dietary fiber proponent, provided a personal communication of his observation of short oro-anal transit time in Africans. This information helped to inspire Painter to theorize that a low residue intake related to, what he described as, the “civilized diet” would lead to a viscous stool that passes through the colon more slowly, and that this difference in fecal consistency would explain the incidence of DD in the civilized nations. Moreover, Painter recommended a diet with high residue intake, such as that consisting of wholemeal bread, unprocessed bran, porridge and fruit, replace the traditional low-residue diet of the civilized nations to reduce risk of DD. In 1971, Painter and Burkitt[3] jointly published their “fiber hypothesis” for DD, suggesting that a diet based on unrefined, natural foods with adequate fiber may prevent DD.
Subsequent studies found that intake of a higher fiber diet led to increased volume and less viscous feces accompanied by a shorter transit time[4-6], thereby preventing the rise of internal pressure in the large intestine. Advocates of the fiber diet suggested that it would help to spread the lumen of the large intestine, thereby suppressing the excessive contraction that would otherwise be caused by large amounts of compacted feces. These findings have led to the widely accepted theory that DD is strongly related to constipation[7].
The most important data published so far in support of the fiber hypothesis is that showing a correlation between amount of feces and transit time. In particular, the relationship between stool volume and transit time is not inverse, but is exponential [i.e., log(time) = 2.81633 - 0.56057log(weight)][4]. It is not feasible to shorten transit time for stools over 300 g; therefore, theoretically, the effectiveness of high-fiber diet is limited. Methanogenesis has been linked to the presence of diverticulosis[8], and cellulose, which is contained in dietary fibers, is fermented by methane-producing bacteria[9]. A very recent study used a gas-sensing capsule to measure gas produced by diets of various fiber content found that the high-fiber diet produced more gas in the large intestine than the low-fiber diet[10]. Therefore, ingestion of excess dietary fiber may exacerbate the conditions that support accumulation of feces and gas in the intestine.
Although the mechanism underlying diverticula generation remains unknown, the fiber hypothesis has been widely adopted as an appropriate intervention. Indeed, over the past 45 years, the fiber hypothesis itself has become the basis for dietary advice of DD. The most current patient guide published by the American Gastroenterology Association (AGA) formally recommends a daily fiber intake of at least 25 g[11].
Yet, findings from several recent studies have cast doubt on the validity of the high-fiber hypothesis[12-16]. A subsequent study by Peery et al[17] found no association between a low-fiber diet and DD. More concerning, however, were their results from the cross-sectional study of 2104 participants between the ages of 30 and 80 years old, and including 878 cases of DD and 1226 controls without DD, which indicated that high total fiber intake was associated with an increased prevalence of multiple diverticula. The particular fiber subtypes that showed significant association with the occurrence of multiple diverticula were grains, insoluble fiber, and soluble fiber. Another study by the same group a year later found no increased risk of diverticulosis in the descending or sigmoid colon associated with either less frequent bowel movements or symptoms of constipation, and no association between dietary fiber intake and diverticulosis[18]. A subsequent study by Braunschmid et al[19] found that colonic diverticular disease did not correlate with constipation symptoms.
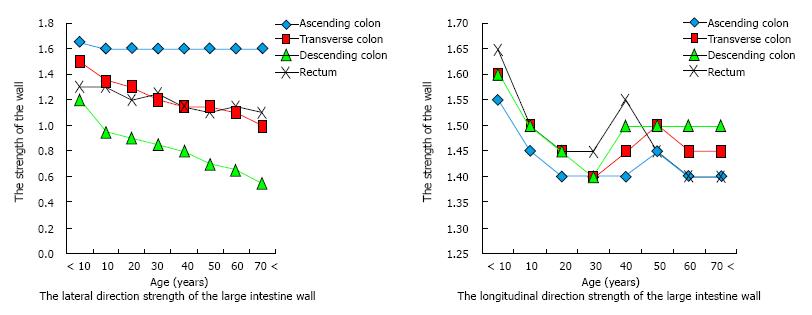
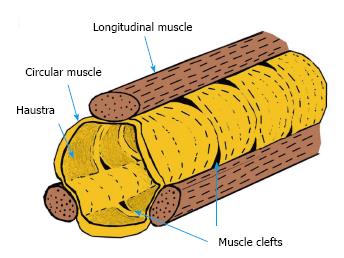
Theoretically, formation of a mucosal hernia requires intraluminal high pressure and a pre-existing defect in the involved muscle layer (i.e., weakened integrity). Normally, the muscle layer of the human colon is composed of circular muscle and longitudinal muscle, the latter of which is bound as three separate formations (i.e., the taenia) that run from the cecum to the distal portion of the sigmoid colon[20]. When surgical specimens of diverticulitis in sigmoid colon were examined using electron microscopy, the taenia showed up-regulated elastin, with concentration levels greater than 200% compared to those in controls. Increased elastin in the region of the colonic tissue afflicted by diverticulitis may cause unequal elasticity and strength compared to the adjacent areas of unafflicted tissues[21]. Moreover, several conditions that cause the colon tissue to thicken, such as inflammatory or infectious conditions, affect elasticity, as increased thickness leads to reduced tensile strength. Finally, prevalence of colonic DD has been correlated with advancing age[22], presumably due to the large intestine wall becoming brittle. Intriguingly, studies of both European, Asians and African populations have shown similar findings of sigmoid colon strength decreasing with age[23]. Japanese have shown descending colon (lateral direction) strength decreasing with age (Figure 1)[24].
Investigation of colonic tearing during colonoscopy showed that when the sigmoid colon wall is affected by pressure forces from the inside the muscularis propria ruptures first, followed by the serosa and the mucosa sequentially[25]. As such, the muscle layer appears to be the weakest point in the wall of the colon, even under physiologic conditions. Thus, considering the collective data of factors that affect the structural integrity of the distal colon, it can be hypothesized that development of diverticula in this area may be related to vulnerability of the intestinal wall that increases with age.
In contrast to the descending colon, the ascending colon shows no reduction in strength associated with aging (Figure 1). Hard stool is usually not present in the proximal colon, and this region does not form a closed space. Theoretically, herniation in the proximal colon requires the presence of a natural vulnerability.
Not all animals have taenia of the colon, and those that do are humans and monkeys among the primates, horses, and guinea pigs and rabbits among the rodents[26]. While natural occurrence of DD has been reported in monkeys and horses, the rodents are used for study of diverticula since the condition can be created experimentally and their small size facilitates convenient research investigation[27-30]. These features of diverticula in the animals with taenia have led to suspicion of a causal relationship between the two.
Colonic DD are most frequently located along the side of taenia[31]. It has thus been speculated that these sites represent the weakest points in the colon muscles, possibly explained by the fact that they are where penetrating vessels cross through[7,32-35]. It is theorized that until diverticulum formation is complete, the outpouching process is advanced by ongoing forces of pressure; although, this hypothesis has not yet been proven experimentally. In line with this theory, however, it is believed that DD does not occur between the tenia libera and tenia omentalis of the transverse colon because of the low vasculature at this site.
A report from 1925 by Lineback[36] demonstrates defects on both sides of the taenia. The circular muscle forms a convergence at the site that is in contact with the longitudinal muscle, and the most frequent site of diverticula is the cleft between the bundles of circular muscle (Figure 2).
Furthermore, since circular muscles and longitudinal muscles are connected, these clefts may be widened upon contraction of the muscle. This theory does not contradict the fact that multiple diverticula of the same size are present simultaneously. Moreover, these clefts may contain blood vessels[36], and may be present from birth. In line with this theory is the explanation as to DD not occurring between the tenia libera and tenia omentalis of the transverse colon because of the low influence of gas at this site.
It is widely believed that feces produced by a high-fiber diet increases colon diameter and in turn decreases intraluminal pressure, in accordance with Laplace’s law (wall tension = pressure × radius)[35,37]. This idea is based upon the theory of “segmentation hypothesis” that was first put forth by Painter, in which he described the colon obstructed at both ends as an enclosed space that acts as a series of “little bladders”[1,7]. However, the large intestine is functionally and structurally different from a bladder; indeed, it is a continuous space without complete obstruction, with its outlet at the anus. Besides, in order for this theory to be feasible and valid the colon wall must be flexible enough to adapt the Laplace’s law. In patients who have optimal contractility of the intestines, the high-fiber diet may be an effective prevention or intervention measure. However, in patients who have an intestinal wall that is stiff or has excessive contraction, the intestinal tract will not extend adequately in response to the high-fiber diet, leading to higher intraluminal pressure and consequent increased forces acting on the intestinal wall.
Colon affected by diverticulitis shows thickened wall and signs of post-inflammatory fibrosis[38]. In vivo studies using colon manometry in patients with diverticulitis showed motility of the intestinal tract as being increased in the descending colon and sigmoid colon but no concomitant increase in the rectum[39,40]. Another study using CT colonography (colonoscopy) to assess symptoms of patients with diverticula in the sigmoid colon showed that pain was associated with air pressure[41]. Considering these data, high bulk feces produced by a high-fiber diet would not be expected to prevent the recurrence of diverticulitis. Not only that but, theoretically, this diet might even promote the symptoms of diverticulitis and pose a risk of recurrence.
High pressure in the lumen of the colon cannot be produced by small hard stool alone. Although multiple hard stools can accumulate in the intestinal lumen, they cannot exclusively explain the influences of pressure.
In theory, in order to obtain a pressure increase sufficient to affect the intestinal wall, a substance that can rapidly move in the colon is required; such a substance would be gaseous or liquid in form. In 1964, Painter[7,42] used a fluid (barium) to produce internal pressure in the intestinal tract and showed that pressures > 50 mmHg were reached in the closed sigmoid colon; additionally, the author theorized that the pressure levels may have reached > 90 mmHg, but limitations of the equipment precluded accurate measurement. The higher forces (specifically 56-80 mmHg) were confirmed by other studies using barium enema administration from a height of 3 feet (91 cm) above the examination table, and these pressures were considered safe[43]. However, when the barium enema was administered from a height of 6 feet (1.8 m) above the table the intraluminal colonic pressures reached 140-168 mmHg, which surpass the threshold of safety and can cause perforation[43].
Intestinal perforation caused by air pressure has been the subject of many studies[44-49]. The upper limit of the safety air pressure of the endoscope is 80 mmHg[50]. Brayko et al[51] studied the characteristics of high-pressure perforation in serosa and mucosa and found that the pressures required for rupture (202 mmHg and 226 mmHg respectively) translated to low risk of perforation of the diverticula in normal endoscopy[51]. Another study, however, showed that the lower pressure forces of CT colonography (38-40 mmHg) can induce abdominal pain[41]. Thus, the human colon can feel pain caused by air pressure at ≥ 40 mmHg, but the risk of perforation occurs at ≥ 80 mmHg. While the diverticulum may be induced in the cleft of the muscle layer by a pressure of < 80 mmHg, it is not easily ruptured due to the strength of the mucosa and serosa (which require pressures > 200 mmHg). Considering that most cases of diverticula present as asymptomatic (without abdominal pain), it is likely then that the pressure required for completion of a new diverticulum might be 40 mmHg or less.
Intestinal pressure is affected by the dynamics of liquid as well as air. Compressed gas, according to Boyle’s law, has a higher energy than the uncompressed liquid. Therefore, the presence of liquid will increase air pressure in a confined space such as the intestine. A study using barium contrast showed that the transit time in the proximal sigmoid of patients with DD was twice as fast as that in the non-DD control group, but the total time of gastrointestinal emptying was similar in both groups[52]. These results can be explained by Bernoulli’s principle, which states that if the diameter of the lumen is large, the pressure will be increased and the fluid velocity will be decreased (Figure 3). Considering this law, narrowing of the rectum will be expected to increase the internal pressure of the sigmoid colon, and when the descending colon is contracted, the pressure of the proximal colon will be expected to be increased. There are seven sphincters located along the length of the colon[53]. The hydrodynamics of each sphincter and influence of its contraction (including the Haustral type) may be explained by Bernoulli’s principle (Figure 4). Thus, the difference in frequency of right DD and left DD may be related to differences in pressure at each site.
Japan has a high incidence of DD in the proximal colon[54]. Moreover, study of Japanese cases of DD, but specifically with the condition affecting the right side, showed a high intraluminal pressure (> 20 mmHg) and abnormal motility in the ascending colon[55]. When another group examined the dynamics of gas pressures by scintigraphy they found that gas generated in the right and left colon does not move, as evidenced by observations at 60 min post-injection when gas injected into the jejunum remained in the cecum and the right colon and gas injected into the rectum remained in the recto-sigmoid colon[56]. Thus, the segmental location at which gas is fermented will be impacted by the corresponding pressure.
It has already been established that excessive pressure in the colon is related to the intraluminal concentrations of both gas and water. The next question of interest is then, what is the cause of increased levels of gas and the water in the colon?
Dietary fiber increases gas within the colon[10,57]. The primary dietary fiber contained in vegetable and fruit is inulin, and its intake leads to flatulence[58]. The 2015 AGA guidelines cited at the beginning of this article recommend that dietary fiber be obtained through intake of legumes (as lentils), yogurt, and fresh fruit[11]. However, yogurt and beans contain oligosaccharides, both of which are known to generate gas in the gut by fermentation[59,60]. In the case of individuals with fructose intolerance, eating of fresh fruit can result in abdominal pain, belching, bloating, an uncomfortable feeling of fullness, indigestion, and diarrhea[61]. Therefore, the diet components recommended by the AGA are expected to produce a substantial amount of gas in the intestines.
Recently, there has been increasing interest in developing diet-based therapies for IBS, and the diet consisting of low fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAP) appears a promising candidate. FODMAPs are not digested or absorbed in the small intestine[62,63]. Therefore, their intake causes increased fluid in the ileum due to the corresponding high osmotic pressure; in addition, they lead to a large amount of gas produced by fermentation in the colon. The daily adoption of the low FODMAP diet by patients with IBS has led to significant improvements in symptoms[64,65].
The detrimental impact of a high FODMAP diet on the intestinal tract has been shown by a study in which the participants drank lactulose[66], which has been demonstrated by MRI to increase fluid content in the ileum[67]. Since this study measured the lactulose-induced increase of gas indirectly, with the hydrogen breath test, a more recent study of the FODMAP diet obtained a direct confirmation of the increasing intestinal gas by abdominal X-ray[68]; these findings, thus, support the theory that the FODMAP diet increases the intraluminal volume of gas and fluid.
The breath test, however, remains a valid method of analysis. Jang et al[69] performed a breath test in patients with right DD at 180 min following ingestion of 10 g of lactulose and determined that methane gas was increased but hydrogen gas was decreased in these individuals as compared to controls. It is important to note here, though, that gas volume is known to significantly increase in response to lactulose ingestion at time points greater than the 180 min used in that study, specifically at 240 to 300 min after the ingestion[68]. Therefore, to more accurately investigate the influence of colon gas on clinical symptoms, it is necessary that the study design allow for adequate time for fermentation to occur in the large intestine.
The low FODMAP diet purports avoidance of foods that contain lactose. Lactose not only causes an increase in intestinal water content (via increased osmotic load in the ileum) but is also readily fermented by the colonic microbiota, which leads to production of short-chain fatty acids and gas: Mainly hydrogen (H2), carbon dioxide (CO2), and methane (CH4)[70]. Thus, individuals with lactose intolerance can experience diarrhea and abdominal distension as a result of dietary intake.
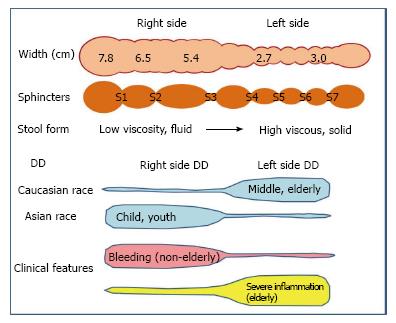
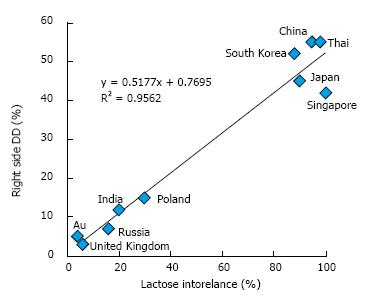
Prevalence of lactose intolerance is high in Asia and Africa, and lower in Caucasian populations. From this point forward, the article will discuss the potential correlation between DD of right colon (RDD) and lactose intolerance. Among the nine countries with reports of RDD and lactose intolerance in the publicly available literature (Table 1)[34,71-84], all show a strong correlation (r2 = 0.9524, Figure 5). Incidence of RDD is lowest in European countries, while the incidence of lactose intolerance is lowest in the United States; however, the Asian countries show high incidences for both RDD and lactose intolerance. These findings support a hypothesis of lactose intolerance and RDD.
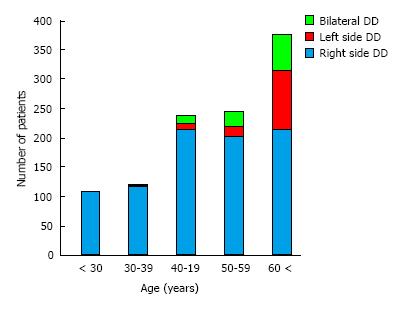
In Japan, DD in young individuals almost exclusively involves the right side of the colon (Figure 6)[77]. LDD risk was found to increase with age, likely due to increased vulnerability of the muscle layer over a person’s lifespan. In adults under 29 years of age, 100% of the DD cases involved the right side. In Japan, pediatric DD between the ages of 7-15 ages is not rare: These cases of pediatric DD occur in cecum and ascending colon[85,86]. This finding cannot be explained merely by age-related vulnerability of the muscle layer and may indicate factors related to childhood. It has been reported that up to 86% of Japanese children develop lactose intolerance by the age of 6 (30% in 3-year-old, 36% in 4-year-old, 58% in 5-year-old)[76]. The time that it takes for gas to increase in the intestines after lactose intake is 1-2 h[87-89], which is shorter than the times required for any of the other constituents of a high-FODMAP diet. Thus, lactose intake may induce a large amount of gas and liquid in the right colon of Japanese children, especially those with lactose intolerance. The physical pressure brought on by the increased gas and fluid will affect the mucous membrane, presumably pushing it outward into the physiological cleft that exists from birth. This may explain why diverticulum in young Japanese tends to be generated only on the right side.
Yet, many Europeans and Americans experience DD of the left colon LDD. This phenomenon may be related to the higher ingestion of wheat[90], compared to Asian societies historically. The fructan component of wheat is a part of the high-FODMAP. The time required for fermentation of fructan in the gut is relatively long, between 2 and 6 h[91,92], so that the high pressure would occur in the left colon. In Japan, however, consumption of wheat has increased since World War II, and this change in dietary pattern - towards one that more closely resembles the European and American diets - has been accompanied by an increase in colonic DD. For example, DD was reportedly 2% in the 1960s[93] but had increased to 20% by the 1980s[94]. Additionally, the cases of LDD have increased in Japan as well[94-97]. This trend is similar to that reported in South Koreans[79].
Besides the change in eating habits, the increased longevity of the Japanese population in recent decades may also have contributed to the observed rise in LDD. In addition, the prevalence of IBS has remarkable increased in Japan; according to the various revised definitions of IBS made by the Rome diagnostic criteria, incidence was 3.6% in 1996 (Rome I)[98], 10.7% in 2006 (Rome II)[99], 14.0% in 2010 (Rome III)[100]. Thus, not only may DD and IBS be correlated but they also may share an etiologic component of diet.
Several reports have addressed the potential correlation between IBS and DD[54,101-103]. Symptoms consistent with IBS are common among patients with DD, and this symptomology has been reported as significantly higher in the DD patients when compared to non-DD controls[103-105]. However, this overlap of symptoms can cause diverticulitis to be misdiagnosed as IBS[104].
IBS and DD are distinct conditions, the former having demonstrated characteristics of inflammation as a distinguishing feature; as such, it may be inappropriate to adapt the diagnostic criteria of IBS to patients with DD. Yet, the two share common symptoms of abdominal bloating accompanied by abdominal pain, which are presumed to be consequent to internal pressure in the digestive tract. Regardless, if the cause of symptoms in either is an excess volume of gas and liquid content in the colon, reduction of either or both might help to prevent the chronic symptoms of diverticulosis.
IBS is classified as a functional disorder, while DD is classified as an organic disease. The most obvious difference between the two is that including a case having homeostatic stenosis after inflammation and/or inflammation among the group of DD. Shape change and inflammation is the result, the cause may be the same. Patient with DD will sustain severe symptoms than IBS without diverticulum. Cuomo et al[105] suggested that these symptoms may be used to differentiate the patients with DD from those with IBS. However, their study design was based upon a patient population presenting with fever and requiring hospitalization and treatment, so that cases of diverticulum without inflammation were not considered.
It is possible that inflammation related to diverticulitis may lead to excessive contraction and support development of IBS[106]. In both conditions, Bernoulli’s principle may be at play, namely production of non-uniform pressure in the intestinal tract caused by any variety of factors. It is also possible that in patients with asymptomatic DD without stenosis, a high-fiber diet with high FODMAPs may be lead to IBS. In such a diet, the inulin and oligosaccharides may produce short-chain fatty acids and gases by fermentation at 6 to 48 h after ingestion, and pH of feces is reduced from 7 to 6[107,108].
It has been demonstrated that IBS patients have reduced colonic intraluminal pH, compared to healthy controls[109,110]; the lower pH is suggestive of higher colonic fermentation. Specifically, these studies used a wireless motility capsule (SmartPill™) to show that IBS patients had a pH of 6.8 in the colon (vs healthy controls who had a pH of 7.3) and showed that colonic low-pH levels were correlated with IBS symptom severity scores and abdominal pain. IBS is characterized by excessive contraction of the descending colon, starting from the sigmoid colon[111]. Interestingly, when another study found low-pH of the cecum in IBS patients, it was correlated with a reduction in right colon contraction[112]. These collective findings indicate that IBS is likely subject to the Bernoulli’s principle, which is also inferred for the pathogenesis of DD.
The high fiber hypothesis represents a logical contradiction. A high-fiber diet is most likely not suitable for long-term management of diverticulitis. The anatomical clefts that are present in the musculature of the large intestine are prone to diverticula caused by gas and fluid-related force pressures following Bernoulli’s principle. RDD, however, may also be related to lactose intolerance. The currently recommended diet of high fiber with high FODMAPs may bring about substantial amounts of gas in the colon and a low pH, which is linked with IBS symptoms. Theoretically, then, a low FODMAP diet will be valid for the prevention of recurrent diverticulitis.
Manuscript source: Unsolicited manuscript
Specialty Type: Gastroenterology and Hepatology
Country of Origin: Japan
Peer-Review Report Classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D, D
Grade E (Poor): 0
P- Reviewer: Horesh N, Montomoli J, Stam MAW S- Editor: Gong ZM L- Editor: A E- Editor: Lu YJ
| 1. | Painter NS. Diverticulosis of the colon and diet. Br Med J. 1969;2:764-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.2] [Reference Citation Analysis (1)] |
| 2. | Painter NS. Pressures in the colon related to diverticular disease. Proc R Soc Med. 1970;63 Suppl:144-145. [PubMed] |
| 3. | Painter NS, Burkitt DP. Diverticular disease of the colon: a deficiency disease of Western civilization. Br Med J. 1971;2:450-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 571] [Cited by in RCA: 538] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 4. | Burkitt DP, Walker AR, Painter NS. Effect of dietary fibre on stools and the transit-times, and its role in the causation of disease. Lancet. 1972;2:1408-1412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 699] [Cited by in RCA: 541] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 5. | Spiller GA, Chernoff MC, Shipley EA, Beigler MA, Briggs GM. Can fecal weight be used to establish a recommended intake of dietary fiber (plantix). Am J Clin Nutr. 1977;30:659-661. [PubMed] |
| 6. | Saito T, Hayakawa T, Nakamura K, Takita T, Suzuki K, Innami S. Fecal output, gastrointestinal transit time, frequency of evacuation and apparent excretion rate of dietary fiber in young men given diets containing different levels of dietary fiber. J Nutr Sci Vitaminol (Tokyo). 1991;37:493-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (1)] |
| 7. | Painter NS. Diverticular disease of the colon: A deficiency disease of western civilization. USA: William Heinemann Medical Books Ltd 1975; . [DOI] [Full Text] |
| 8. | Yazici C, Arslan DC, Abraham R, Cushing K, Keshavarzian A, Mutlu EA. Breath Methane Levels Are Increased Among Patients with Diverticulosis. Dig Dis Sci. 2016;61:2648-2654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Robert C, Bernalier-Donadille A. The cellulolytic microflora of the human colon: evidence of microcrystalline cellulose-degrading bacteria in methane-excreting subjects. FEMS Microbiol Ecol. 2003;46:81-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 118] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 10. | Kalantar-Zadeh K, Yao CK, Berean KJ, Ha N, Ou JZ, Ward SA, Pillai N, Hill J, Cottrell JJ, Dunshea FR. Intestinal Gas Capsules: A Proof-of-Concept Demonstration. Gastroenterology. 2016;150:37-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 11. | American Gastroenterological Association. A Patient Guide: Managing Diverticulitis. Gastroenterology. 2015;149:1977-1978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 12. | Tan KY, Seow-Choen F. Fiber and colorectal diseases: separating fact from fiction. World J Gastroenterol. 2007;13:4161-4167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 37] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Ünlü C, Daniels L, Vrouenraets BC, Boermeester MA. A systematic review of high-fibre dietary therapy in diverticular disease. Int J Colorectal Dis. 2012;27:419-427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 14. | Peery AF, Sandler RS. Diverticular disease: reconsidering conventional wisdom. Clin Gastroenterol Hepatol. 2013;11:1532-1537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Tursi A, Papa A, Danese S. Review article: the pathophysiology and medical management of diverticulosis and diverticular disease of the colon. Aliment Pharmacol Ther. 2015;42:664-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 16. | Elisei W, Tursi A. Recent advances in the treatment of colonic diverticular disease and prevention of acute diverticulitis. Ann Gastroenterol. 2016;29:24-32. [PubMed] |
| 17. | Peery AF, Barrett PR, Park D, Rogers AJ, Galanko JA, Martin CF, Sandler RS. A high-fiber diet does not protect against asymptomatic diverticulosis. Gastroenterology. 2012;142:266-272.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 204] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 18. | Peery AF, Sandler RS, Ahnen DJ, Galanko JA, Holm AN, Shaukat A, Mott LA, Barry EL, Fried DA, Baron JA. Constipation and a low-fiber diet are not associated with diverticulosis. Clin Gastroenterol Hepatol. 2013;11:1622-1627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 104] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 19. | Braunschmid T, Stift A, Mittlböck M, Lord A, Weiser FA, Riss S. Constipation is not associated with diverticular disease - Analysis of 976 patients. Int J Surg. 2015;19:42-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Meyers MA, Volberg F, Katzen B, Abbott G. Haustral anatomy and pathology: a new look. I. Roentgen identification of normal patterns and relationships. Radiology. 1973;108:497-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Whiteway J, Morson BC. Elastosis in diverticular disease of the sigmoid colon. Gut. 1985;26:258-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 114] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 22. | Almerie MQ, Simpson J. Diagnosing and treating diverticular disease. Practitioner. 2015;259:29-33, 3. [PubMed] |
| 23. | Watters DA, Smith AN, Eastwood MA, Anderson KC, Elton RA, Mugerwa JW. Mechanical properties of the colon: comparison of the features of the African and European colon in vitro. Gut. 1985;26:384-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 81] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Iwasaki T. Study on the strength of human intestinal walls. J Kyoto Prefectural Univ Med. 1953;52:673-702. |
| 25. | Uno Y, Morita T. Colonic perforation and serosal tears associated with colonoscopy. Lancet. 1997;349:1888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 26. | Stevens CE, Hume ID. Contributions of microbes in vertebrate gastrointestinal tract to production and conservation of nutrients. Physiol Rev. 1998;78:393-427. [PubMed] |
| 27. | Hodgson J. Animal models in the study of diverticular disease. Part I: aetiology and treatment. Clin Gastroenterol. 1975;4:201-219. [PubMed] |
| 28. | Hare WC. Observations On A Case Of A Diverticulum In The Small Colon Of The Horse. Can J Comp Med Vet Sci. 1959;23:272-273. [PubMed] |
| 29. | Bunton TE, Bacmeister CX. Diverticulosis and colonic leiomyosarcoma in an aged rhesus macaque. Vet Pathol. 1989;26:351-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 30. | Hayama SI, Rika Akamatsu R, Kishimoto M, Suzuki M, Hideo Nigi H. A case of diverticular disease of the colon in a Japanese monkey (Macaca fuscata). Primates. 1988;29: 423-426. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 31. | Meyers MA, Volberg F, Katzen B, Abbott G. Haustral anatomy and pathology: a new look. II. Roentgen interpretation of pathological alterations. Radiology. 1973;108:505-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | Griffiths JD. Extramural and intramural blood-supply of colon. Br Med J. 1961;1:323-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 59] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 33. | Slack WW. The anatomy, pathology, and some clinical features of divericulitis of the colon. Br J Surg. 1962;50:185-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 76] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 34. | Hughes LE. Postmortem survey of diverticular disease of the colon. I. Diverticulosis and diverticulitis. Gut. 1969;10:336-344. [PubMed] |
| 35. | Maykel JA, Opelka FG. Colonic diverticulosis and diverticular hemorrhage. Clin Colon Rectal Surg. 2004;17:195-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 36. | Lineback PE. Studies on the musculature of the human colon, with special reference to the taeniae. Am J Anat. 1925;36:357-383. [RCA] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 37. | Sheth AA, Longo W, Floch MH. Diverticular disease and diverticulitis. Am J Gastroenterol. 2008;103:1550-1556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 115] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 38. | Fenoglio-Preiser CM, Lantz PL, Listrom MB, Davis M, Rike FO. Gastrointestinal pathology: An atlas and text. New York: Raven Press 1989; 632-634. |
| 39. | Bassotti G, Battaglia E, Spinozzi F, Pelli MA, Tonini M. Twenty-four hour recordings of colonic motility in patients with diverticular disease: evidence for abnormal motility and propulsive activity. Dis Colon Rectum. 2001;44:1814-1820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 81] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 40. | Viebig RG, Pontes JF, Michelsohn NH. Electromanometry of the rectosigmoid in colonic diverticulosis. Arq Gastroenterol. 1994;31:135-144. [PubMed] |
| 41. | Sosna J, Bar-Ziv J, Libson E, Eligulashvili M, Blachar A. CT colonography: positioning order and intracolonic pressure. AJR Am J Roentgenol. 2008;191:1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 42. | Painter NS. The aetiology of diverticulosis of the colon with special reference to the action of certain drugs on the behaviour of the colon. Ann R Coll Surg Engl. 1964;34:98-119. [PubMed] |
| 43. | Noveroske RJ. Intracolonic pressures during barium enema examination. Am J Roentgenol Radium Ther Nucl Med. 1964;91:852-863. [PubMed] |
| 44. | Wojtalik RS, Lindenauer SM, Kahn SS. Perforation of the colon associated with adynamic ileus. Am J Surg. 1973;125:601-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 45. | Kozarek RA, Earnest DL, Silverstein ME, Smith RG. Air-pressure-induced colon injury during diagnostic colonoscopy. Gastroenterology. 1980;78:7-14. [PubMed] |
| 46. | Sandhu KS, Cohen H. Cecal perforation following fiberoptic flexible sigmoidoscopy. Am J Med. 1987;82:546-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 47. | Luchette FA, Doerr RJ, Kelly K, Kulaylat M, Stephan RM, Hassett JM. Colonoscopic impaction in left colon strictures resulting in right colon pneumatic perforation. Surg Endosc. 1992;6:273-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 48. | Foliente RL, Chang AC, Youssef AI, Ford LJ, Condon SC, Chen YK. Endoscopic cecal perforation: mechanisms of injury. Am J Gastroenterol. 1996;91:705-708. [PubMed] |
| 49. | Tzelepis GE, Nasiff L, McCool FD, Hammond J. Transmission of pressure within the abdomen. J Appl Physiol (1985). 1996;81:1111-1114. [PubMed] |
| 50. | Woltjen JA. A retrospective analysis of cecal barotrauma caused by colonoscope air flow and pressure. Gastrointest Endosc. 2005;61:37-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 51. | Brayko CM, Kozarek RA, Sanowski RA, Howells T. Diverticular rupture during colonoscopy. Fact or fancy? Dig Dis Sci. 1984;29:427-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 52. | Leandro PA, Cecconello I, Habr-Gama A, de Olivereira e Silva A, Pontes JF. Gastrointestinal motility in normal subjects and patients with diverticulosis of the colon. Arq Gastroenterol. 1984;21:157-163. [PubMed] |
| 53. | Reeders JW, Rodenbush G. Clinical radiology and endoscopy of the colon. New York: Thieme Medical Publishers, Inc 1994; . |
| 54. | Spiller R. Editorial: new thoughts on the association between diverticulosis and irritable bowel syndrome. Am J Gastroenterol. 2014;109:1906-1908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 55. | Sugihara K, Muto T, Morioka Y. Motility study in right sided diverticular disease of the colon. Gut. 1983;24:1130-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 46] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 56. | Harder H, Serra J, Azpiroz F, Passos MC, Aguadé S, Malagelada JR. Intestinal gas distribution determines abdominal symptoms. Gut. 2003;52:1708-1713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 65] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 57. | Marthinsen D, Fleming SE. Excretion of breath and flatus gases by humans consuming high-fiber diets. J Nutr. 1982;112:1133-1143. [PubMed] |
| 58. | Muir JG, Shepherd SJ, Rosella O, Rose R, Barrett JS, Gibson PR. Fructan and free fructose content of common Australian vegetables and fruit. J Agric Food Chem. 2007;55:6619-6627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 175] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 59. | Suarez FL, Springfield J, Furne JK, Lohrmann TT, Kerr PS, Levitt MD. Gas production in human ingesting a soybean flour derived from beans naturally low in oligosaccharides. Am J Clin Nutr. 1999;69:135-139. [PubMed] |
| 60. | Cummings JH, Macfarlane GT, Englyst HN. Prebiotic digestion and fermentation. Am J Clin Nutr. 2001;73:415S-420S. [PubMed] |
| 61. | Choi YK, Kraft N, Zimmerman B, Jackson M, Rao SS. Fructose intolerance in IBS and utility of fructose-restricted diet. J Clin Gastroenterol. 2008;42:233-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 86] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 62. | Gibson PR, Shepherd SJ. Personal view: food for thought--western lifestyle and susceptibility to Crohn’s disease. The FODMAP hypothesis. Aliment Pharmacol Ther. 2005;21:1399-1409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 242] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 63. | Gibson PR, Shepherd SJ. Food choice as a key management strategy for functional gastrointestinal symptoms. Am J Gastroenterol. 2012;107:657-666; quiz 667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 132] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 64. | Halmos EP, Power VA, Shepherd SJ, Gibson PR, Muir JG. A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenterology. 2014;146:67-75.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 810] [Cited by in RCA: 829] [Article Influence: 75.4] [Reference Citation Analysis (0)] |
| 65. | Chumpitazi BP, Cope JL, Hollister EB, Tsai CM, McMeans AR, Luna RA, Versalovic J, Shulman RJ. Randomised clinical trial: gut microbiome biomarkers are associated with clinical response to a low FODMAP diet in children with the irritable bowel syndrome. Aliment Pharmacol Ther. 2015;42:418-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 286] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 66. | Clausen MR, Jørgensen J, Mortensen PB. Comparison of diarrhea induced by ingestion of fructooligosaccharide Idolax and disaccharide lactulose: role of osmolarity versus fermentation of malabsorbed carbohydrate. Dig Dis Sci. 1998;43:2696-2707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 40] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 67. | Undseth R, Berstad A, Kløw NE, Arnljot K, Moi KS, Valeur J. Abnormal accumulation of intestinal fluid following ingestion of an unabsorbable carbohydrate in patients with irritable bowel syndrome: an MRI study. Neurogastroenterol Motil. 2014;26:1686-1693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 68. | Uno Y. Pilot Study on Gas Patterns of Irritable Bowel Syndrome and Small Intestinal Bacterial Overgrowth Following Ingestion of Lactulose. Open J Gastroenterol. 2015;5:155-163. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 69. | Jang SI, Kim JH, Youn YH, Park H, Lee SI, Conklin JL. Relationship between intestinal gas and the development of right colonic diverticula. J Neurogastroenterol Motil. 2010;16:418-423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 70. | Deng Y, Misselwitz B, Dai N, Fox M. Lactose Intolerance in Adults: Biological Mechanism and Dietary Management. Nutrients. 2015;7:8020-8035. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 180] [Cited by in RCA: 183] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 71. | Bolin TD, Davis AE, Seah CS, Chua KL, Yong V, Kho KM, Siak CL, Jacob E. Lactose intolerance in Singapore. Gastroenterology. 1970;59:76-84. [PubMed] |
| 72. | Wong SK, Ho YH, Leong AP, Seow-Choen F. Clinical behavior of complicated right-sided and left-sided diverticulosis. Dis Colon Rectum. 1997;40:344-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 61] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 73. | Silanikove N, Leitner G, Merin U. The Interrelationships between Lactose Intolerance and the Modern Dairy Industry: Global Perspectives in Evolutional and Historical Backgrounds. Nutrients. 2015;7:7312-7331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 157] [Cited by in RCA: 118] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 74. | Rungjiratananon S, Sirinthornpunya S. Prevalence of colonic diverticulosis in irritable bowel syndrome patients compared to other patients in Rajavithi hospital prevalence of colonic diverticulosis. Thai J Gastroenterol. 2009;10:148-151. |
| 75. | Chan CC, Lo KK, Chung EC, Lo SS, Hon TY. Colonic diverticulosis in Hong Kong: distribution pattern and clinical significance. Clin Radiol. 1998;53:842-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 55] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 76. | Nose O, Iida Y, Kai H, Harada T, Ogawa M, Yabuuchi H. Breath hydrogen test for detecting lactose malabsorption in infants and children. Prevalence of lactose malabsorption in Japanese children and adults. Arch Dis Child. 1979;54:436-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 77. | Kubo A, Ishiwata J, Maeda Y, Kida T, Yamabe K, Shimosegawa T. Clinical studies on diverticular disease of the colon. Jpn J Med. 1983;22:185-189. [PubMed] [DOI] [Full Text] |
| 78. | Park SH, Chang YW, Kim SJ, Lee MH, Nam JH, Oh CH, Kim JW, Jang JY, Yang JO, Yoo JA. [Efficacy of Lactose-free Milk in Korean Adults with Lactose Intolerance]. Korean J Gastroenterol. 2016;67:22-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 79. | Oh HK, Han EC, Ha HK, Choe EK, Moon SH, Ryoo SB, Jeong SY, Park KJ. Surgical management of colonic diverticular disease: discrepancy between right- and left-sided diseases. World J Gastroenterol. 2014;20:10115-10120. [PubMed] [DOI] [Full Text] |
| 80. | Pawłowska K, Umławska W, Iwańczak B. Prevalence of Lactose Malabsorption and Lactose Intolerance in Pediatric Patients with Selected Gastrointestinal Diseases. Adv Clin Exp Med. 2015;24:863-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 81. | Blachut K, Paradowski L, Garcarek J. Prevalence and distribution of the colonic diverticulosis. Review of 417 cases from Lower Silesia in Poland. Rom J Gastroenterol. 2004;13:281-285. [PubMed] |
| 82. | Goenka MK, Nagi B, Kochhar R, Bhasin DK, Singh A, Mehta SK. Colonic diverticulosis in India: the changing scene. Indian J Gastroenterol. 1994;13:86-88. [PubMed] |
| 83. | Smith GD, Lawlor DA, Timpson NJ, Baban J, Kiessling M, Day IN, Ebrahim S. Lactase persistence-related genetic variant: population substructure and health outcomes. Eur J Hum Genet. 2009;17:357-367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 84. | Golder M, Ster IC, Babu P, Sharma A, Bayat M, Farah A. Demographic determinants of risk, colon distribution and density scores of diverticular disease. World J Gastroenterol. 2011;17:1009-1017. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 85. | Yoshimoto K, Terakura H, Hiki S, Ogata H. Diverticulitis in children. Stomach and Intestine (Tokyo). 2012;47:1135-1140. [DOI] [Full Text] |
| 86. | Watanuki H, Minota N, Takahara M. Study on diverticulitis of the large intestine in children. J Jpn Red Cross Himeji Hosp. 2015;39:71-74. |
| 87. | Mummah S, Oelrich B, Hope J, Vu Q, Gardner CD. Effect of raw milk on lactose intolerance: a randomized controlled pilot study. Ann Fam Med. 2014;12:134-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 88. | Yang JF, Fox M, Chu H, Zheng X, Long YQ, Pohl D, Fried M, Dai N. Four-sample lactose hydrogen breath test for diagnosis of lactose malabsorption in irritable bowel syndrome patients with diarrhea. World J Gastroenterol. 2015;21:7563-7570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 89. | Schommer K, Reljic D, Bärtsch P, Sauer P. Gastrointestinal complaints in runners are not due to small intestinal bacterial overgrowth. J Negat Results Biomed. 2011;10:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 90. | De Giorgio R, Volta U, Gibson PR. Sensitivity to wheat, gluten and FODMAPs in IBS: facts or fiction? Gut. 2016;65:169-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 127] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 91. | Murray K, Wilkinson-Smith V, Hoad C, Costigan C, Cox E, Lam C, Marciani L, Gowland P, Spiller RC. Differential effects of FODMAPs (fermentable oligo-, di-, mono-saccharides and polyols) on small and large intestinal contents in healthy subjects shown by MRI. Am J Gastroenterol. 2014;109:110-119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 233] [Cited by in RCA: 262] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 92. | Fedewa A, Rao SS. Dietary fructose intolerance, fructan intolerance and FODMAPs. Curr Gastroenterol Rep. 2014;16:370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 89] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 93. | Inoue M. The epidemiologic and clinical features of diverticular disease of the colon. J Jpn Soc Coloproctol. 1992;45:904-913. [RCA] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 94. | Miura S, Kodaira S, Aoki H, Hosoda Y. Bilateral type diverticular disease of the colon. Int J Colorectal Dis. 1996;11:71-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 30] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 95. | Takano M, Yamada K, Sato K. An analysis of the development of colonic diverticulosis in the Japanese. Dis Colon Rectum. 2005;48:2111-2116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 96. | Yamada E, Inamori M, Uchida E, Tanida E, Izumi M, Takeshita K, Fujii T, Komatsu K, Hamanaka J, Maeda S. Association between the location of diverticular disease and the irritable bowel syndrome: a multicenter study in Japan. Am J Gastroenterol. 2014;109:1900-1905. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 97. | Tarao K, Sekino Y, Nonaka T, Iida H, Inamori M, Nakajima A, Maeda S, Natsumeda Y, Ikegami T, Ohshige K. Recent trends in colonic diverticulosis in Yokohama City: a possibility of changing to a more Western profile. Intern Med. 2015;54:2545-2550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 98. | Itasaka T, Takahashi T. Epidemiological study of irritable bowel syndrome using population screening. Ther Res. 1996;17:4059-4063. |
| 99. | Shiotani A, Miyanishi T, Takahashi T. Sex differences in irritable bowel syndrome in Japanese university students. J Gastroenterol. 2006;41:562-568. [PubMed] |
| 100. | Miwa H. Life style in persons with functional gastrointestinal disorders--large-scale internet survey of lifestyle in Japan. Neurogastroenterol Motil. 2012;24:464-471, e217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 101. | Annibale B, Lahner E, Maconi G, Usai P, Marchi S, Bassotti G, Barbara G, Cuomo R. Clinical features of symptomatic uncomplicated diverticular disease: a multicenter Italian survey. Int J Colorectal Dis. 2012;27:1151-1159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 102. | Sirinthornpunya S, Rungjiratananon S. Association of colonic diverticular disease and irritable bowel syndrome in Thai patients. J Med Assoc Thai. 2014;97 Suppl 11:S18-S24. [PubMed] |
| 103. | Jung HK, Choung RS, Locke GR, Schleck CD, Zinsmeister AR, Talley NJ. Diarrhea-predominant irritable bowel syndrome is associated with diverticular disease: a population-based study. Am J Gastroenterol. 2010;105:652-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 99] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 104. | Longstreth GF, Tieu RS. Clinically Diagnosed Acute Diverticulitis in Outpatients: Misdiagnosis in Patients with Irritable Bowel Syndrome. Dig Dis Sci. 2016;61:578-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 105. | Cuomo R, Barbara G, Andreozzi P, Bassotti G, Casetti T, Grassini M, Ierardi E, Maconi G, Marchi S, Sarnelli G. Symptom patterns can distinguish diverticular disease from irritable bowel syndrome. Eur J Clin Invest. 2013;43:1147-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 106. | Cohen E, Fuller G, Bolus R, Modi R, Vu M, Shahedi K, Shah R, Atia M, Kurzbard N, Sheen V. Increased risk for irritable bowel syndrome after acute diverticulitis. Clin Gastroenterol Hepatol. 2013;11:1614-1619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 93] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 107. | Kaur A, Rose DJ, Rumpagaporn P, Patterson JA, Hamaker BR. In vitro batch fecal fermentation comparison of gas and short-chain fatty acid production using “slowly fermentable” dietary fibers. J Food Sci. 2011;76:H137-H142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 125] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 108. | Noack J, Timm D, Hospattankar A, Slavin J. Fermentation profiles of wheat dextrin, inulin and partially hydrolyzed guar gum using an in vitro digestion pretreatment and in vitro batch fermentation system model. Nutrients. 2013;5:1500-1510. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 109. | Choi CH, Ringel-Kulka T, Temas DJ, Kim A, Scott K, Ringel Y. Altered colonic bacterial fermentation is a steadfast pathophysiological factor in irritable bowel syndrome. Gastroenterology. 2014;146:S8. [DOI] [Full Text] |
| 110. | Ringel-Kulka T, Choi CH, Temas D, Kim A, Maier DM, Scott K, Galanko JA, Ringel Y. Altered Colonic Bacterial Fermentation as a Potential Pathophysiological Factor in Irritable Bowel Syndrome. Am J Gastroenterol. 2015;110:1339-1346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 107] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 111. | Clemens CH, Samsom M, Van Berge Henegouwen GP, Smout AJ. Abnormalities of left colonic motility in ambulant nonconstipated patients with irritable bowel syndrome. Dig Dis Sci. 2003;48:74-82. [PubMed] |
| 112. | Farmer AD, Mohammed SD, Dukes GE, Scott SM, Hobson AR. Caecal pH is a biomarker of excessive colonic fermentation. World J Gastroenterol. 2014;20:5000-5007. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 37] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (1)] |