INTRODUCTION
Traditionally, bilirubin, a product of heme metabolism was thought to be a cytotoxic waste product, toxic to neurons[1]. However, it was later observed to possess other properties: Vasodilatory, anti-oxidant, anti-inflammatory, anti-mutagenic, immune-modulatory, antiproliferative and anti-apoptotic on vascular cells[2,3]. It has also been suggested to have a lipid lowering effect by reducing plasma and low-density lipid peroxidation[3]. By virtue of these properties, bilirubin was hypothesized to have a protective effect in coronary artery disease (CAD)[4]. Vitek et al[5] studied this relation in patients with Gilbert syndrome (a hereditary disorder leading to unconjugated hyperbilirubinemia with normal liver chemistries) and reported a 2% prevalence of ischemic CAD with Gilbert syndrome (n = 50) compared to 12.1% in the general population (n = 2296, P < 0.05)[5].
BILIRUBIN METABOLISM
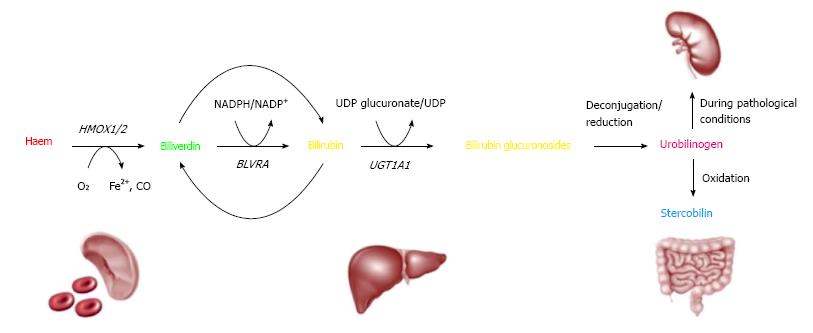
Hemoglobin is released from the senescent red blood cells and non-erythroid hemoproteins. Hemoglobin is broken down into heme pigment and globin chains. Heme pigment is oxidatively metabolized by heme oxygenase into biliverdin, carbon monoxide and free iron. Biliverdin is further degraded by biliverdin reductase into unconjugated bilirubin. Following its uptake by the hepatocytes, unconjugated bilirubin is converted to conjugated bilirubin by the action of uridine-diphosphoglucuronate glucuronosyltransferase (UDP-GT). The gene that codes for UDP-GT is UGT1A1 gene and a genetic variation in the promoter region of UGT1A1 gene is associated with Gilbert syndrome. This genetic variation involves insertion of an additional thymine-adenine base pair in the TATA box of the UGT1A1 gene instead of the normal 6 pairs[4,6] (Figure 1). This leads to deficiency of the enzyme that leads to accumulation of unconjugated bilirubin within the blood[4]. Patients with Gilbert have otherwise normal serum liver chemistries[6]. However, another condition called Crigler-Najjar syndrome with unconjugated hyperbilirubinemia stems from complete or near complete loss of UGT1A1 activity. Type 1 Crigler Najjar is a rare and lethal recessive disorder compared to type 2 Crigler Najjar where the UGT1A1 activity is still maintained, albeit at a minimal level[7]. Patients with Criggler Najjar Type 1 develop severe neurological impairment and carry a high early mortality unless they receive liver transplantation.
Figure 1 Bilirubin metabolism (from heme to bilirubin).
Hemoglobin is cleaved to yield globin and heme (red). Heme is enzymatically converted to biliverdin (green) by liberating iron, via oxidation with loss of a carbon atom (CO). This, in turn, yields bilirubin (orange) after enzymatic reduction of biliverdin. In the liver, bilirubin is conjugated to enable excretion, requiring the enzyme UGT1A1[4].
Conjugated bilirubin is excreted into the bile canaliculus by the canalicular membrane transporter multidrug resistance related protein 2 (MRP2). Mutations in the gene that affects this transport protein leads to conjugated hyperbilirubinemia. This condition is called Dubin-Johnson syndrome[8]. Another autosomal recessive disorder, in which patients have multiple defects in hepatocyte uptake and excretion of bilirubin, leads to increase in conjugated bilirubin and is called Rotor syndrome.
PROTECTIVE PROPERTIES OF BILIRUBIN
Several mechanisms have been proposed highlighting the protective effects of bilirubin: (1) Bilirubin has anti-oxidant properties independent of whether it is free or albumin bound, conjugated or unconjugated. Bilirubin increases in response to the oxidative stress and acts as a scavenger of the reactive oxygen species[9,10]. Bilirubin sub-fractions (Bu and Bc) have demonstrated inhibition of low-density lipoproteins oxidation, which in turn retards the peroxidation of lipids, hence could potentially restrict the progression of atherosclerosis[10]. Of note, unconjugated bilirubin in concentrations as low as 10 nmol/L has been reported to protect neuronal cultures from the oxidative stress generated by 10000 times higher concentrations of hydrogen peroxide[11]. This anti-oxidative effect of bilirubin is amplified by the recycling of bilirubin to biliverdin and so forth via redox reactions (Figure 1)[3]. This recycling of bilirubin amplifies its anti-oxidant potential up to 10000 times; (2) Bilirubin has been shown to be inversely associated with increased arterial stiffness[12,13]. Pre-clinical studies have observed this effect to be mediated by preservation of vascular nitric oxide, which mediates endothelial relaxation[2,13]. Decreased levels of nitric oxide impair the ability of the coronary vessels to dilate during exercise or stress, thus, provoking myocardial ischemia in patients with CAD[14]. Besides vaso-relaxation, nitric oxide also inhibits leukocyte adhesion to endothelium, vascular smooth muscle cell migration and proliferation, platelet aggregation and neointimal formation[2,15]. Thus, preservation of nitric oxide offers a significant protection against atherosclerosis[12]; (3) Bilirubin has also been seen to reduce platelet aggregation. Kundur et al[3] showed that when unconjugated bilirubin is added to platelet rich plasma at concentrations seen in Gilbert syndrome (0.99-5.85 mg/dL), it inhibits both - collagen induced and adenosine diphosphate induced platelet aggregation, in a dose dependent fashion for the latter. P and E-selectin are markers of platelet activation and released from activated platelets and endothelium. They predominantly mediate adhesion of platelets and inflammatory cells to endothelium and facilitate the formation of large stable platelet-leukocyte aggregates that can lead to thrombus formation. Bilirubin, biliverdin and inducible heme oxygenase have an inhibitory effect on P- and E-selectin[16]. This is supported by studies showing reduced levels of circulating inflammatory biomarkers, P selectin and CD 40 ligand in patients with Gilbert syndrome[3]; (4) Bilirubin and heme oxygenase exhibit anti-inflammatory properties and prevent oxidant induced microvascular leukocyte adhesion. Heme oxygenase (the rate-limiting enzyme in bilirubin production) also functions as a vasodilatory and anti- proliferative agent during vascular injury[15]. An inverse association has been demonstrated between total bilirubin levels and the markers of inflammation, namely, C-reactive protein (direct marker); neutrophil to lymphocyte ratio and red cell distribution width (indirect markers)[17]. In animal models, bilirubin has also been seen to exhibit anti-complement effect in vitro, thus, conferring protection against increased thrombogenicity and clot formation[3,17-19]. This anti-inflammatory effect has been hypothesized to antagonize the process of atherosclerosis, which is a low-grade chronic inflammatory state; (5) preclinical studies on mice have demonstrated a protective effect of bilirubin on angiotensin-II induced hypertension. Angiotensin-II is known to cause superoxide production, which is attenuated by induction of heme oxygenase via redox reactions. This reaction leads to production of bilirubin and carbon monoxide; hence an increased bilirubin level is associated with increased attenuation of superoxide production[20]. This protective effect, in conjunction with carbon monoxide, by inhibition of angiotensin-II has also been suggested and extrapolated in the cardiomyocytes preventing left ventricular hypertrophy[21-23]; and (6) Bilirubin has also been described to solubilize cholesterol and promotes its clearance through the bile[24,25]. This finding is also supported by the evidence of reduced levels of total cholesterol, low-density lipoprotein (LDL) and apolipoprotein B/apolipoprotein-A1 ratio and elevated high density lipoprotein (HDL) to LDL ratio in patients with Gilbert syndrome[3].
EVIDENCE SUPPORTING THE PROTECTIVE EFFECT OF BILIRUBIN ON CAD
Several studies have suggested a cardioprotective role of bilirubin. Schwertner et al[26] was the first to report this protective effect of high level of bilirubin in CAD. They compared 619 subjects in training set (complete data on all risk factors considered was available) vs 258 subjects in test set (some risk factor data was not available). They observed a statistically significant inverse association between bilirubin and CAD. Fifty percent reduction in total bilirubin was associated with 47% increased odds of CAD, both univariate and multivariate after adjustment for other risk factors[26]. In 1996, Hopkins et al[27] evaluated 161 patients with early familial CAD and compared them to 155 control subjects. Patients with familial CAD had significantly lower bilirubin concentration as compared to controls (8.9 ± 6.1 micromol/L vs 12.4 ± 8.1 micromol/L, P = 0.0001). After adjustment for other risk factors, bilirubin was found to be an independent protective factor with an odds ratio of 0.25 (P = 0.001) for an increase of 1 mg/dL. The benefits of elevated bilirubin were seen to be comparable to those of HDL. In a meta-analysis published in 2003 by Novotny et al[28] a significant inverse relationship was shown between serum bilirubin levels and the severity of atherosclerosis. Eleven relevant studies were used for analysis and the subjects involved were males. The relation between serum bilirubin levels and severity of atherosclerosis had a spearman rank coefficient of r = -0.31 (P < 0.0001).
Subsequently, in another study by Erdogan et al[29] in 2012, 179 patients undergoing angiography were analyzed to evaluate for CAD. Out of them, 110 patients had good collateral formation and 69 had poor collateral development. Higher serum bilirubin levels were associated with good collateral development as compared to poor collateral development (0.80 ± 0.27 mg/dL vs 0.53 ± 0.19 mg/dL, P < 0.001). These findings suggest a possible protective effect of elevated serum bilirubin levels against myocardial ischemia in patients with chronic total coronary occlusion with collaterals limiting infarct size and providing additional blood flow to the ischemic area[29]. Wei et al[30] also showed similar results with a significant inverse correlation between CAD and total bilirubin (n = 1260) in patients who underwent coronary angiography.
In 2013, Stojanov et al[31] reported cardioprotective effects of increased levels of bilirubin in 628 healthy subjects. The subjects were 442 men and 186 women aged 18 to 22 years. They divided the subjects into 2 groups based on levels of bilirubin. Subjects with level below the upper limit of reference were classified as “low bilirubin” (≤ 0.95 mg/dL in women and ≤ 1.4 mg/dL in men) and those with value above the upper limit of reference were classified as “high bilirubin”. Men with high bilirubin concentration (> 1.4 mg/dL) had higher concentration of albumin and uric acid (P < 0.001) and lower level of oxidized LDL (P < 0.05). In females, high bilirubin (> 0.95 mg/dL) was associated with significantly higher albumin (P < 0.05) and lower thiobarbituric acid-reacting substances (TBARS) (P < 0.05). These findings support the evidence of an anti-oxidant effect of bilirubin secondary to inverse association with ox-LDL and anti-inflammatory effect secondary to direct correlation with albumin, which is a negative acute phase reactant in inflammatory response[31]. Shortly after that study, Canpolat et al[18] used computed tomographic angiography (CTA) to evaluate the relationship between bilirubin levels and nature of coronary plaques. The study included 1115 subjects who underwent CTA for evaluation of CAD. Patients were divided into 4 quartiles depending on the total bilirubin level. Patients with any coronary plaque were observed to have statistically significant lower levels of serum bilirubin (P = 0.002). Patients with critical stenosis (> 50% obstruction) had lower bilirubin levels compared to non-critical stenosis (0.57 ± 0.18 mg/dL vs 0.70 ± 0.24 mg/dL, P < 0.001). The authors concluded that lower serum levels of bilirubin were significantly associated with the presence, severity and the noncalcified morphology of atherosclerotic plaques.
Later, Song et al[32] designed a prospective cohort study with 8593 subjects followed over a period of 4 years. Low bilirubin levels (< 0.32 mg/dL) were observed to be an independent risk factor associated with an increased risk of CAD development (n = 80, 0.9% of total subjects) with adjusted hazard ratio (HR) of 1.890 (95%CI: 1.088-3.284, P = 0.024). Low bilirubin levels were shown to further increase the risk of CAD development six fold in patients with metabolic syndrome with HR of 2.016 (95%CI: 1.069-3.800, P = 0.030). The authors concluded by suggesting addition of bilirubin level to the risk assessment tool for assessing CAD in patients. Similar results showing an inverse association of bilirubin levels with coronary artery calcification were reported by Mahabadi et al[33]. However, they attributed the cardioprotective effects from bilirubin to a more favorable cardiovascular risk profile observed in their patients with CAD and elevated bilirubin levels in their study[33].
Akboga et al[17] conducted another study evaluating anti-inflammatory properties of bilirubin. In a retrospective cross-sectional study, they included 1501 patients who underwent coronary angiography. They divided them into 3 groups based on Gensini scores: No CAD (control group, n = 380), mild CAD (n = 497) and severe CAD (n = 624), with the objective of establishing anti-inflammatory effects of bilirubin in addition to its anti-oxidant effects. A significant inverse correlation between total bilirubin and C-reactive protein (r = -0.112, P < 0.001), neutrophil to lymphocyte ratio (r = -0.070, P = 0.026) and red cell distribution width (r = -0.074, P = 0.027) was observed. These findings helped establish anti-inflammatory properties of bilirubin in addition to their anti-oxidant effects. They also re-confirmed the inverse association of bilirubin with CAD severity [spearman’s rank correlation coefficient (r) = -0.173, P < 0.001].
GENETIC POLYMORPHISMS OF UGT1A1*28 AND THEIR RELATION WITH CAD
Polymorphisms in the UGT1A1 gene (also known as UGT1A1*28) leads to unconjugated hyperbilirubinemia due to deficiency or decreased activity of UGT1A1[34]. Thus, patients with UGT1A1*28 allele have shown to have higher levels of bilirubin[35]. Whereas, patients with wild type allele, i.e., normal genotype have normal levels of bilirubin. To understand the true role of bilirubin it is prudent to look into the association between UGT1A1*28 and CAD. Establishing an inverse association between the two would strengthen the hypothesis of bilirubin being protective in CAD.
In 2003, the Rotterdam study (case control study of 114 patients) hypothesized that since individuals homozygous for UGT1A1*28 have higher serum bilirubin, they would have a lower risk of CAD. They found that the relative risk of myocardial infarction (MI) for heterozygous genotype was 0.9 (95%CI: 0.7-1.3) and with homozygous UGT1A1*28 was 1.3 (95%CI: 0.8-2.2). After adjusting for factors like age, gender, smoking, body mass index, diabetes mellitus, systolic blood pressure, total cholesterol, and HDL-cholesterol, the risk estimate was 1.0 (0.7-1.4) for heterozygotes and 1.2 (0.7-2.1) for homozygotes. Authors argued that the protective effect could have been missed because of the lack of power to detect such an effect. However, no association was seen between low serum bilirubin and the risk for CAD[36].
The Framingham offspring study (a prospective cohort study) in 2006 observed 1780 participants over 24 years and found that subjects homozygous for the gene UGT1A1*28 had approximately one-third the risk for CAD compared to individuals homozygous for the wild type allele. However, the association between the incidence of myocardial infarction (MI) and gene polymorphism was not significant although the trend was similar. It was concluded that the carriers homozygous for UGT1A1*28 allele with higher bilirubin concentrations exhibited a strong association with lower risk of cardiovascular disease[34].
Lingenhel et al[37] studied two polymorphisms in the promoter region of UGT1A1 gene to analyze whether UGTA1A gene polymorphisms or bilirubin levels are independently associated with risk of CAD development. This case-control study enlisted 477 patients with premature, familial CAD and 619 controls that were matched for age and gender. Bilirubin levels were found to be significantly lower in the familial CAD group as compared to the controls (P = 1.2 × 10-10 in men and 1.9 × 10-9 in women). The low bilirubin levels were found to be significantly associated to CAD whereas UGTA1A polymorphisms were not, with odds ratio of 0.9 (CI: 0.86-0.94, P = 2.6 × 10-6) for men and 0.77 (CI: 0.68-0.87, P = 3.2 × 10-5) for women respectively for each 0.1mg/dl increase in bilirubin levels[37]. Hence, indicating that increased bilirubin levels and not genetic polymorphisms are associated with reduced risk of CAD.
Hsieh et al[38] sought to explore the association of UGTA1A polymorphisms with risk of CAD development. A case-control design was set up (n = 135; cases = 61, controls = 74) and although bilirubin levels in the control group were found to be significantly higher than CAD group, no significant differences were observed in the polymorphism of UGTA1A between the two groups.
Rantner et al[39] in their prospective case control study, investigated plasma bilirubin concentration and UGT1A1 promoter TA repeat polymorphism in a cohort of patients with peripheral arterial disease and age and diabetes matched control group. They observed significantly lower bilirubin concentrations in patients than in controls. UGT1A1 polymorphism was strongly associated with bilirubin concentration in both patients and controls. However, UGT1A1 polymorphism was not associated with peripheral arterial disease.
EVIDENCE NOT SUPPORTING THE PROTECTIVE EFFECT OF BILIRUBIN ON CAD
Contrary to the evidence presented above, several studies have negated the protective effect of bilirubin on CAD. British Regional Health Study (BRHS) was a prospective study designed to examine the relationship between the level of bilirubin and risk of ischemic CAD. Subjects (n = 7685) were followed up for a mean of 11.5 years, out of which 737 individuals were seen to develop major ischemic CAD. A U-shaped relationship was observed between serum bilirubin and risk of ischemic CAD with increased risk at bilirubin concentrations < 0.4 mg/dL and at > 0.7 mg/dL (RR = 0.99, CI: 0.73-1.34)[40]. A similar U-shaped relationship between serum bilirubin level and risk of developing CAD was observed in the Prospective Epidemiological Study of Myocardial Infarction (PRIME) study. In the PRIME study, 216 individuals who had developed CAD at 5-year follow up were designated as cases, and 434 individuals as matched controls. Individuals with bilirubin levels < 0.33 mg/dL and > 0.8 mg/dL were seen to have higher incidence of development of CAD, even after adjustment of other risk factors. However, the association between bilirubin levels > 0.8 mg/dL and the incidence of CAD was not statistically significant (OR = 0.68, CI: 0.34-1.39, P = 0.29). This U-shaped association was suggested as being due to reduced endogenous antioxidants with insufficient dietary intake[41]. The clinical significance of this U-shaped relation remains unclear.
Acet et al[42] investigated patients (n = 360) undergoing percutaneous coronary intervention (PCI) within 12 h of symptom onset with the aim to establish a relation between bilirubin levels and infarct-related artery patency in the setting of ST-segment elevation myocardial infarction (STEMI). The group with elevated total bilirubin was seen to have higher impaired flow (defined as pre-PCI TIMI ≤ 2 flow) than normal flow (Pre-PCI TIMI > 3) (P < 0.001). Furthermore, the in-hospital mortality and major adverse cardiac events were significantly higher in the high total bilirubin group (P = 0.002, P < 0.001 respectively). However, an important point to note is that the study did not exclude patients with elevated markers of hepatocellular injury thus making the true relationship between isolated elevated bilirubin levels and CAD difficult to interpret.
Ayaz et al[43] reported an independent positive association between bilirubin and left ventricular mass/hypertrophy in a population with untreated hypertension (n = 114). After performing a linear and logistical regression, total bilirubin (P = 0.011) was shown to be an independent risk factor for CAD. The authors attribute this to the suppression of reactive oxygen species[44]. A similar effect has been seen in pre-clinical studies in rats, which showed a protective effect of elevated bilirubin on left ventricular hypertrophy in spontaneously hypertensive rats. They hypothesized the role of liver growth factor inhibition by bilirubin. However, this relationship still needs to be better elucidated.
In another study, 221 patients who were evaluated for CAD by coronary angiography showed a moderate but significant positive correlation between direct bilirubin levels and the Gensini score (r = 0.158, P = 0.019). However, no such significant correlation was demonstrated between total bilirubin and the Gensini score. This study was limited by its small sample size (n = 221)[5]. Authors interpreted that the relationship between bilirubin and CAD was unlikely causal. However, the cardiovascular risk factors in CAD have shown to be additive and in presence of several risk factors the beneficial effects of bilirubin might get masked. Thus, despite the results of this study, elevated serum bilirubin levels might confer protective effects in patients with favorable risk profile for CAD[5].
EXPERT COMMENTARY
While the available evidence regarding the effects of bilirubin are not definitive and different studies provide contradictory findings, some useful conclusions can be drawn.
First, it is possible that the protective effects seen with higher bilirubin levels are possibly mediated through heme oxygenase or by other substrates involved in the pathway of bilirubin production, namely, biliverdin and carbon monoxide. Although few studies have reported an inverse association between bilirubin and the risk of CAD, no such association was seen with UGT1A1 gene polymorphism and the risk of CAD. Thus, a conclusion can be safely inferred that if at all bilirubin is protective in CAD, it is likely that bilirubin production (by induction of heme oxygenase and accompanied by production of carbon monoxide) and not just its excretion indirectly confers the protective effect observed with CAD. This would reflect as bilirubin having a protective effect on CAD whereas, in reality it is only a mediator or a marker.
Second, low bilirubin levels can be indicative of decreased heme oxygenase activity (a powerful anti-oxidant) or could be indicative of high oxidative stress in patients leading to consumption of the natural anti-oxidants including bilirubin. Hence, lower levels of bilirubin are perhaps not the causal factor for CAD but may indicate patients at an increased risk of developing CAD[45].
Third, bilirubin requires vitamin E as the co-oxidant, hence patients with a high bilirubin and deficiency of vitamin E, have less atheroprotective effect that weakens the inverse association between elevated bilirubin levels and the risk of CAD[46].
Also, Grosser et al[47-49] have reported induction of heme oxygenase with statin and aspirin therapy. Induction of heme oxygenase increases the bilirubin production. Individuals with UGT1A1 gene variants have a lower capability of exclusion of bilirubin; hence, bilirubin accumulation is to be expected in individuals on statins and aspirin. Hence as per this hypothesis, patients with CAD or at an increased risk of CAD with UGT1A1 gene polymorphism, on aspirin and statin should have increased levels of bilirubin. In that case, bilirubin might be looked upon as a marker of the drug activity.
Mendelian randomization is done to establish a causal relationship[50]. As mentioned above, a lack of significant association between the gene polymorphisms of UGT1A1 and risk for CAD goes in favor of bilirubin being a marker than a primary mediator for the cardioprotective effects observed with CAD. Moreover, it also points out towards incomplete penetrance of the UGT1A1 gene. Inconsistent results further support the need for further exploration of the underlying mechanisms and a prospective study with a high power to establish a definite causal relationship between bilirubin levels and CAD.
Manuscript source: Invited manuscript
Specialty Type: Gastroenterology and Hepatology
Country of Origin: United States
Peer-Review Report Classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Canpolat U, Jorgensen ME, Zhou T S- Editor: Qi Y L- Editor: A E- Editor: Lu YJ