Published online Aug 6, 2016. doi: 10.4292/wjgpt.v7.i3.428
Peer-review started: February 11, 2016
First decision: March 14, 2016
Revised: April 12, 2016
Accepted: May 7, 2016
Article in press: May 9, 2016
Published online: August 6, 2016
Processing time: 173 Days and 16.8 Hours
AIM: To investigate the clinical characteristics, treatment, medication use, and treatment response in patients with ulcerative colitis (UC) across ethnic groups.
METHODS: This study retrospectively analyzed medical records of all 268465 patients who visited the Bumrungrad International Digestive Disease Center during 2005-2010. The demographics, clinical characteristics, medication use, results of investigations, and medical and surgical management for patients with UC were evaluated. Evaluation included sigmoidoscopy and colonoscopy performed in compliance with the American Society of Gastrointestinal Endoscopy practice guidelines. Patient ethnicities were categorized into seven groups: Thai, Oriental, South Asian (SA), Middle Eastern (ME), Caucasian, African, and Hispanic. UC pathological severity was classified into inactive, mild, moderate, and severe. Associations between categorical variables were analyzed using the χ2 or Fischer’s exact test. Associations between categorical and interval variables were analyzed using Student’s t-test and/or analysis of covariance.
RESULTS: UC was diagnosed in 371 of the 268465 patients: male 56.33%; ME 42%, Caucasian 23%, and Thai 19%. Annual incidence of UC was 82 cases per 100000 with wide ethnic variation, ranging from 29 to 206 cases per 100000 in Oriental and ME patients, respectively. Of the patients with UC, 16.71% had severe UC with highest incidence among the patients from ME (20.39%) and lowest among the Caucasian population (11.90%). ME had highest proportion of pancolitis (52.90%), followed by Caucasian (45.35%) and Asian (34.40%). Only 20.93% of Caucasian patients received steroid, compared with 26.40% and 27.10% of Asian and Middle Eastern, respectively (P = 0.732). Overall, 13.72% of UC patients did not respond to steroid therapy, with non-significantly higher proportions of non-responders among Asian and Middle Eastern patients (15.22% and 15.04%, respectively) (P = 0.781). On average, 5.93% underwent surgical management with ethnic variation, ranging from 0% in African to 18% in SA. Cancer was found in three (Thai, ME, and African) cases (0.82 institution-specific incidence).
CONCLUSION: Incidence, symptom duration, pathological severity, clinical manifestations, medication use, treatment response, need for surgical consultation, and cancer incidence of patients with UC potentially vary by ethnicity.
Core tip: Incidence and prevalence of ulcerative colitis have been shown to vary across geographical areas and ethnic groups. Patients from different ethnic origins and/or healthcare systems have been managed using the same guidelines for diagnosis and treatment of ulcerative colitis. In this study, comparative analysis of symptom duration, pathological severity, extra-intestinal manifestations, surgical consultation need, medication use, and cancer incidence across ethnic groups were presented. Understanding how these attributes vary by ethnicity is useful for service delivery design, especially in this facility that is responsible for the care of patients from diverse backgrounds.
- Citation: Permpoon V, Pongpirul K, Anuras S. Ethnic variations in ulcerative colitis: Experience of an international hospital in Thailand. World J Gastrointest Pharmacol Ther 2016; 7(3): 428-433
- URL: https://www.wjgnet.com/2150-5349/full/v7/i3/428.htm
- DOI: https://dx.doi.org/10.4292/wjgpt.v7.i3.428
Ulcerative colitis (UC) is a chronic inflammatory condition of the colon in genetically susceptible individuals exposed to environmental risk factors[1]; it is also an emerging global disease[2]. Ethnicity has long been hypothesized as one of the determinants for developing UC based on the varying incidence and prevalence across geographical boundaries[2-5]. In general, Asian and Middle Eastern populations have a lower incidence of UC than Caucasian individuals (6.3 vs 24.3 per 100000 person-years)[2]. As patients in low-income countries have been diagnosed less frequently than those in richer countries, UC has been believed to be associated with industrialization of nations[2].
Health care systems in each country may play significant roles in the diagnosis and management of UC. Although existing epidemiological data are useful for design of health service delivery within a country, these data might not be adequate for a healthcare institution that provides medical services in one setting to patients from various origins. Although developed countries have a higher incidence of UC, the effective health care systems have better clinical outcomes than that of less developed ones. In contrast, the epidemiologic findings are more likely to be affected by the genetics of the population than by the health care system.
Better understanding of the clinical course of UC can help to guide clinical decisions. Patients are symptomatic for varying lengths of time before the definitive diagnosis is made and their symptoms vary in severity at presentation. To accurately diagnose the disease properly and avoid disease progression due to delay in diagnosis, the patient should be evaluated by endoscopy with biopsy. Distribution of the lesions could guide medication choices and routes. A patient suffering pancolitis for longer period of time (i.e., more than 10 years) has an increased risk of cancer and therefore should be re-assessed with colonoscopy at optimal time intervals. Recommended indications for surgical management have recently been updated but still rely on clinical judgment[6].
As a large private hospital for medical tourism in Asia, Bumrungrad International Hospital (BIH) serves more than one million patients from at least 190 countries annually. This allows comparative analyses across ethnic groups. This study compared clinical characteristics including incidence and severity, medication use, treatment response, surgical consultation need, and cancer incidence across ethnic groups.
This retrospective study analyzed demographics, clinical characteristics, results of investigations, as well as medical and surgical management information in medical records of all patients who visited the Digestive Disease Center (BIDDC) during 2005-2010. Colonoscopy and sigmoidoscopy were performed in compliance with the American Society of Gastrointestinal Endoscopy (ASGE) practice guidelines. Ulcerative colitis was diagnosed based on clinical grounds and supported by the appropriate findings on total colonoscopy, biopsy, and by negative stool examination for infectious causes. All patients received total colonoscopy to confirm the distribution of the colitis. The Montreal classification was used to classify severity of the disease.
Patient ethnicities were arbitrarily categorized into seven groups: Thai, Oriental, South Asian, Middle Eastern, Caucasian, African, and Hispanic. With sample size limitation, some analyses were done using the patients in three major ethnic groups: Asian (Thai, Oriental, South Asian), Middle Eastern, and Caucasian. UC severity was classified based on pathological findings into inactive, mild, moderate, and severe using standard, well-accepted published criteria. The need for surgical consultation was based on the content of relevant operative note in medical record; only colon-related surgeries (i.e., partial colectomy and total procto-colectomy, colostomy, and ileo-anal pouch) were included. A patient with clinical response to high-dose glucocorticoids (prednisone 40 to 60 mg/d or equivalent) within 30 d for oral therapy or 7 to 10 d for intravenous therapy was classified as steroid responsive. Steroid dependence was defined if glucocorticoids cannot be tapered to less than 10 mg/d within three months of starting steroids, without recurrent disease, or if relapse occurs within 3 mo of stopping glucocorticoids. A patient without a meaningful clinical response to glucocorticoids up to doses of prednisone 40 to 60 mg/d (or equivalent) within 30 d for oral therapy or 7 to 10 d for intravenous therapy was classified as steroid refractory.
Descriptive statistics were used where appropriate. Association between categorical variables was analyzed using χ2 test or Fischer’s exact test. Association between categorical and interval variables was analyzed using Student’s t-test and/or analysis of covariance where appropriate. The statistical analysis of this study was performed by the corresponding author who had formal biostatistics training as part of his doctoral education. This study was approved by Bumrungrad International Institutional Review Board (BI/IRB No.146-09-11).
Of 268465 individuals who visited BIDDC during 2005-2010, half were Thai (49%) (Table 1). The distribution of ethnicity of patients visiting the BIDDC was slightly different from hospital patient ethnic profiles (Thai:Non-Thai = 60:40). UC was diagnosed in 371 patients (Male 56.33%), 42% of which were Middle Eastern, 23% Caucasian and 19% Thai. Based on 2008-2010 data, overall annual facility-specific incidence of UC was estimated to be 82 cases per 100000 with wide ethnic variation, ranging from 29 to 206 cases per 100000 in Oriental and Middle Eastern patients, respectively.
| Ethnicity | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | Total | %Total | UC | UC | Annual Incidence |
| Thai | 18820 | 20093 | 20256 | 23423 | 24224 | 23456 | 130272 | 49% | 69 | 19% | 32 |
| Oriental | 5333 | 6737 | 7060 | 8237 | 8614 | 9614 | 45595 | 17% | 23 | 6% | 29 |
| South Asian | 2329 | 2206 | 2003 | 2344 | 2566 | 2935 | 14383 | 5% | 33 | 9% | 140 |
| Middle Eastern | 4567 | 5831 | 6418 | 7828 | 7726 | 9483 | 41853 | 16% | 155 | 42% | 206 |
| Caucasian | 4088 | 4712 | 5264 | 5209 | 5658 | 5572 | 30503 | 11% | 86 | 23% | 174 |
| African | 488 | 598 | 836 | 1050 | 1287 | 1193 | 5452 | 2% | 5 | 1% | 47 |
| Hispanic | 44 | 86 | 60 | 73 | 67 | 77 | 407 | 0% | 0 | 0% | 0 |
| Total | 35669 | 40263 | 41897 | 48164 | 50142 | 52330 | 268465 | 371 | 821 |
Eighty-one percent of the patients presented with no more than one year of symptoms. Patients experienced UC symptoms for a mean of 13.06 mo (95%CI: 9.05-17.07) before their first visit to BIDDC (Table 2). Thai and Caucasian patients presented with a mean of 6.34 and 6.74 mo of UC symptoms, respectively. Middle Eastern patients had symptoms for more than 18 mo on average at presentation.
| Ethnicity | Duration (mo) | 95%CI | Inactive | Mild | Moderate | Severe | %Severe | Extra-intestinal | Surgery |
| Thai | 6.34 | 2.67-10.00 | 2 | 41 | 16 | 10 | 14.49% | 2.90% | 5.80% |
| Oriental | 13.44 | -10.42-37.31 | 0 | 9 | 10 | 4 | 17.39% | 0.00% | 8.70% |
| South Asian | 14.04 | 3.45-24.63 | 2 | 16 | 8 | 6 | 18.75% | 15.15% | 12.12% |
| Middle Eastern | 18.46 | 10.63-26.29 | 8 | 55 | 58 | 31 | 20.39% | 9.68% | 3.87% |
| Caucasian | 6.74 | 0.08-13.41 | 6 | 35 | 33 | 10 | 11.90% | 9.30% | 6.98% |
| African | 34.67 | -29.98-99.32 | 0 | 3 | 2 | 0 | 0.00% | 40.00% | 0.00% |
| Overall | 13.06 | 9.05-17.07 | 18 | 159 | 127 | 61 | 16.71% | 8.63% | 5.93% |
Overall, 16.71% of patients had severe UC with highest incidence among Middle Eastern patients (20.39%) and lowest among Caucasian (11.90%). Extra-intestinal manifestations were found in 8.63% of the patients with great ethnic variation (40% African vs 0% Oriental Non-Thai) (Tables 2 and 3). On average, 5.93% of the patients underwent surgical management with ethnic variation, ranging from 0% in African to 18% in South Asian patients (Table 2).
| Organs | Manifestations (No.) |
| Skin | Psoriasis (4) |
| Erythema nodosum (1) | |
| Pyoderma gangrenosum (1) | |
| Dermatitis (1) | |
| Musculoskeletal | Arthritis (4) |
| Sacroilitis (1) | |
| Osteoporosis (4) | |
| Liver | Sclerosing cholangitis (4) |
| Miscellaneous | Oropharyngeal ulcer (2) |
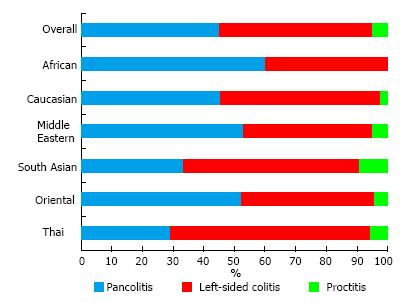
Pancolitis, left-sided colitis, and proctitis were identified in 45.02%, 50.13%, and 4.85%, respectively (Figure 1). Thai patients had significantly higher proportion of left-sided colitis (65.22%) than the other ethnic origins (P = 0.005). When compared across major ethic groups, Middle Eastern patients had highest prevalence of pancolitis (52.90%), followed by Caucasian (45.35%) and Asian (34.40%) patients (P = 0.021).
Only 20.93% of Caucasian patients received steroid, compared with 26.40% and 27.10% of Asian and Middle Eastern, respectively (P = 0.732). Overall, 13.72% of UC patients did not respond to steroid therapy, with non-significantly higher proportions of non-responders among Asian and Middle Eastern patients (15.22% and 15.04%, respectively) (P = 0.781). Of 277 cases that received thiopurine, 8 patients were non-responders (19.05%), including 5 Middle Eastern patients. Caucasians were relatively the best responders to both steroids and thiopurine.
Cancer was found in three individuals (one Thai, one Middle Eastern, and one African) out of 366 cases, resulting in 0.82 institution-specific incidence. Low and high grade dysplasia was found in 2 and 1 cases, respectively.
Although existing incidence and prevalence of UC have been showed to vary across geographical areas[2,4] and ethnic groups[5], evidence from our study provided more data on the Middle Eastern patients and also suggested the potential variation of many other aspects (duration of UC symptoms, severity, distribution, and response to medications) across patients of differing ethnic origins. These attributes are useful for service delivery design, especially in this facility that is responsible for the care of patients from diverse backgrounds.
Based on the clinical findings, we hypothesized that there is potential association between symptom duration and disease severity at presentation. UC has been a disease that progresses over time; hence, earlier and more aggressive management might be needed[7]. As Middle Eastern patients had longer duration of symptoms and more severity at presentation than Thai and Caucasian, we observed that the “progressive” nature might be different across ethnicities and the degree of clinical management should therefore be different. Further study is still required to prove this concept, however.
Different initial anatomic locations of inflammation present with various clinical patterns that have different prognoses and different rates of complications which could lead to the need for surgery[8]. The variation of anatomic distribution of the UC across ethnic groups suggested an association between anatomic location and patient ethnicity. We therefore propose that the association between anatomic location and clinical outcomes of UC could be confounded by patient ethnicity[9].
Assuming comparably high socio-economic status of our patients, the Thai population had two times lower incidence of pancolitis than Oriental. This finding is useful for both clinicians and patients to choose “optimal” investigation when expense, invasiveness, and yield are of concern. That is, a Thai patient who had mild-to-moderate left-sided colitis from initial colonoscopy and prefers gentle procedure and/or has cost concern might be more likely to get sigmoidoscopy than a Japanese patient with similar conditions for follow-up visits. This is supported by our findings on different anatomic locations of UC across patient ethnic groups presented above. The dynamics of the clinical decision-making process would become more personalized, especially when a unified international standard of care for the procedure is not available.
Our institutional data revealed that Middle Eastern patients had almost twice the incidence of UC as that of Caucasian patients. Based on our informal customer interview, majority of the Middle Eastern patients either could not find a specialized center for inflammatory bowel disease or preferred to travel for care outside their countries. Our clinical practice has taken this into account by tailoring the initial investigations to meet the different needs. For example, although a Thai patient who presents with chronic diarrhea would receive stool examination and culture, a Middle Eastern patient with the exact same condition would also be tested for fecal calprotectin[10]. Ideally, tailored clinical services to patients from different origins should be based on the standard guidelines of the countries of origin. In reality, however, clinical practice guidelines are not readily available for all countries and the service delivery design, therefore, must be based on our institutional data.
Findings from our study might also be beneficial for modification of current international standard guidelines[11,12]. Standard guidelines have been developed based on evidence from studies in specific populations therefore limited generalizability. We propose that each of the components in a guideline can be modified for optimal care for patients from each of the ethnic groups. For example, based on our data, 20.39% and 3.87% of Middle Eastern patients were severe UC and underwent surgical management, respectively. If a patient from this ethnic origin, were diagnosed as having severe UC and asked about his/her probability of surgical need, we would be able to calculate the conditional probability of 18%.
Some limitations of our study should be noted. First, generalization of our institutional incidence data was limited by potential selection bias. However, the main purpose of our study was to customize our medical services to meet relatively different needs of patients from various origins rather than to conclude about UC incidence of an ethnic origin. Second, anatomical change over time could be present in some patients but was not adjusted for in our analysis presented here. Some study limitations should be noted. First, the uses of institutional data may either under- or over-estimate the incidence. Although annual incidence of 1.2 to 20.3 cases per 100000 persons have been reported[4], our institution-specific annual incidence of 82 per 100000 populations was much higher with great variation across ethnic origins. The main objective of this study was not to present population-based epidemiological data; the incidence data presented here therefore do not represent an ethnic group as a whole. Current literature on this topic has been dominated by Caucasian data whereas other ethnicities are less well represented. Our institution is a rare setting that serves patients from many geographic origins with significant ethnic variation. Although wide variation of the patients’ country of origin existed, we do not have differential selection of patients.
The authors would like to thank the Knowledge Angels of Bumrungrad Research Center for their kind help with data collection and analysis and Professor Dr. James S Miser for language edits.
Ulcerative colitis (UC) is a chronic inflammatory condition of the colon in genetically susceptible individuals exposed to environmental risk factors. Incidence and prevalence of ulcerative colitis vary across geographical areas and ethnic groups. In an era of globalization and medical tourism, a healthcare institution is more likely to provide care to patients of differing ethnic origins.
In general, Asian and Middle Eastern populations have a lower incidence of UC than Caucasian individuals. Patients from different ethnic origins and/or healthcare systems have been managed using the same guidelines for diagnosis and treatment of UC.
In this study, comparative analysis of symptom duration, pathological severity, extra-intestinal manifestations, surgical consultation need, medication use, and cancer incidence across ethnic groups were presented.
Understanding how these attributes vary by ethnicity is useful for service delivery design, especially in this facility that is responsible for the care of patients from diverse backgrounds.
In this paper, the authors conducted a retrospective single-center study to investigate the clinical characteristics, treatment, medication use, and treatment response of patients with UC in Thailand. It is interesting that the results showed ethnic differences in severity, distribution, and response to treatments for UC.
Manuscript Source: Unsolicited manuscript
Specialty Type: Gastroenterology and Hepatology
Country of Origin: Thailand
Peer-Review Report Classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Devendra CD, Inoue T S- Editor: Qi Y L- Editor: A E- Editor: Lu YJ
| 1. | Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2693] [Cited by in RCA: 2747] [Article Influence: 119.4] [Reference Citation Analysis (2)] |
| 2. | Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46-54.e42; quiz e30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3789] [Cited by in RCA: 3527] [Article Influence: 271.3] [Reference Citation Analysis (5)] |
| 3. | Ahuja V, Tandon RK. Inflammatory bowel disease in the Asia-Pacific area: a comparison with developed countries and regional differences. J Dig Dis. 2010;11:134-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 152] [Article Influence: 10.1] [Reference Citation Analysis (1)] |
| 4. | Loftus EV. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504-1517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Hou JK, El-Serag H, Thirumurthi S. Distribution and manifestations of inflammatory bowel disease in Asians, Hispanics, and African Americans: a systematic review. Am J Gastroenterol. 2009;104:2100-2109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 154] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 6. | Ross H, Steele SR, Varma M, Dykes S, Cima R, Buie WD, Rafferty J. Practice parameters for the surgical treatment of ulcerative colitis. Dis Colon Rectum. 2014;57:5-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 123] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 7. | Torres J, Billioud V, Sachar DB, Peyrin-Biroulet L, Colombel JF. Ulcerative colitis as a progressive disease: the forgotten evidence. Inflamm Bowel Dis. 2012;18:1356-1363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 211] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 8. | Farmer RG, Easley KA, Rankin GB. Clinical patterns, natural history, and progression of ulcerative colitis. A long-term follow-up of 1116 patients. Dig Dis Sci. 1993;38:1137-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 235] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 9. | VanderWeele TJ, Shpitser I. On the definition of a confounder. Ann Stat. 2013;41:196-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 139] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 10. | Stragier E, Van Assche G. The use of fecal calprotectin and lactoferrin in patients with IBD. Review. Acta Gastroenterol Belg. 2013;76:322-328. [PubMed] |
| 11. | Kornbluth A, Sachar DB. Ulcerative colitis practice guidelines in adults: American College Of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2010;105:501-523; quiz 524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 899] [Cited by in RCA: 942] [Article Influence: 62.8] [Reference Citation Analysis (0)] |
| 12. | Stenke E, Hussey S. Ulcerative colitis: management in adults, children and young people (NICE Clinical Guideline CG166). Arch Dis Child Educ Pract Ed. 2014;99:194-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |