Published online Aug 6, 2016. doi: 10.4292/wjgpt.v7.i3.406
Peer-review started: April 18, 2016
First decision: May 17, 2016
Revised: May 27, 2016
Accepted: June 27, 2016
Article in press: June 29, 2016
Published online: August 6, 2016
Processing time: 105 Days and 10.4 Hours
Barrett’s esophagus (BE) is defined as the extension of salmon-colored mucosa into the tubular esophagus ≥ 1 cm proximal to the gastroesophageal junction with biopsy confirmation of intestinal metaplasia. Patients with BE are at increased risk of esophageal adenocarcinoma (EAC), and undergo endoscopic surveillance biopsies to detect dysplasia or early EAC. Dysplasia in BE is classified as no dysplasia, indefinite for dysplasia (IND), low grade dysplasia (LGD) or high grade dysplasia (HGD). Biopsies are diagnosed as IND when the epithelial abnormalities are not sufficient to diagnose dysplasia or the nature of the epithelial abnormalities is uncertain due to inflammation or technical issues. Specific diagnostic criteria for IND are not well established and its clinical significance and management has not been well studied. Previous studies have focused on HGD in BE and led to changes and improvement in the management of BE with HGD and early EAC. Only recently, IND and LGD in BE have become focus of intense study. This review summarizes the definition, neoplastic risk and clinical management of BE IND.
Core tip: Barrett’s esophagus (BE) with indefinite for dysplasia (IND) is diagnosed when the epithelial abnormalities are not sufficient to diagnose dysplasia or the nature of the epithelial abnormalities is uncertain due to inflammation. The risk of prevalent neoplasia in BE with IND varies between 1.9% and 15%. The progression to advanced neoplasia reported varies from 0.43 to 1.2 cases per 100 person-years at risk. Predictors such as the length of BE segment, multi-focality of BE IND, age > 60 years, abnormal p53 expression, active inflammation, and abnormal DNA content as detected by flow cytometry may help in risk-stratifying this patient population.
- Citation: Thota PN, Kistangari G, Esnakula AK, Gonzalo DH, Liu XL. Clinical significance and management of Barrett’s esophagus with epithelial changes indefinite for dysplasia. World J Gastrointest Pharmacol Ther 2016; 7(3): 406-411
- URL: https://www.wjgnet.com/2150-5349/full/v7/i3/406.htm
- DOI: https://dx.doi.org/10.4292/wjgpt.v7.i3.406
Barrett’s esophagus (BE) is a complication of chronic esophageal injury from gastroesophageal reflux disease (GERD) and develops when reflux damaged esophageal squamous cells are replaced by mucous-secreting columnar cells. A definitive diagnosis of BE is established by the extension of salmon-colored mucosa into the tubular esophagus ≥ 1 cm proximal to the gastroesophageal junction (GEJ) with esophageal biopsy showing intestinal metaplasia, defined by the presence of goblet cells[1]. Intestinal metaplasia in BE is a well-established marker of esophageal adenocarcinoma (EAC), and as such patients diagnosed with BE undergo regular endoscopic surveillance and biopsy to detect dysplasia or curable neoplasia. According to the published criteria by Reid et al[2] the biopsies are classified based on five-tiered histologic classification of dysplasia as negative for dysplasia, indefinite for dysplasia (IND), low-grade dysplasia (LGD), highgrade dysplasia (HGD) and intramucosal adenocarcinoma (IMAC).
Dysplasia remains the best available clinical marker for cancer risk. Published guidelines have recommended endoscopic surveillance and treatment strategies based on the grade of dysplasia. The management of LGD and HGD in BE has been reviewed extensively and discussed in many published guidelines. Many studies have focused on the high end of neoplasia in BE, HGD and IMAC, leading to a much improved and less invasive management[3-5]. However, there is a paucity of data to guide the management of BE patients with IND. Besides, due to lack of definitive criteria for diagnosis, and greatest inter-observer variability, and uncertain clinical significance, natural history of progression of BE with IND and management are not clear.
This paper discusses the current literature and examines available evidence for the histologic criteria for diagnosis, its clinical significance, prevalence and risk of progression to cancer, and also the clinicopathologic and biomarker predictors that are associated with dysplasia progression among patients diagnosed with BE with IND. PubMed search was performed for the term “Barrett’s esophagus indefinite for dysplasia” as of November 1, 2015 and studies were reviewed for prevalence and incidence rates of HGD/EAC in BE IND as well as predictors for progression in IND. One study shared part of the same database and was excluded[6].
The diagnosis “indefinite for dysplasia” is used when the biopsy findings are too marked for being negative, but not absolutely sufficient for the presence of dysplasia. The background regenerative changes may be related to inflammation or ulceration and may overlap with LGD that often makes it difficult to differentiate from true dysplasia. Less commonly technical factors related to biopsy specimen handling such as biopsy crushing artifact, thick tissue sectioning, marked thermal artifact and tangential embedding and sectioning also prevents accurate diagnosis of dysplasia and are categorized as BE with IND; In certain circumstances pathologists unaccustomed to certain types of fixatives, for example, Hollande’s and Bouin fixatives that results in vesicular nucleus and prominent nucleolus, may overinterpret the changes as indicative of BE with IND[7]. Rarely, the diagnosis of IND may be due to the dysplasia like changes present only in the bases of the crypts, also called “basal crypt dysplasia-like atypia”, where the surface epithelium may not be involved[8].
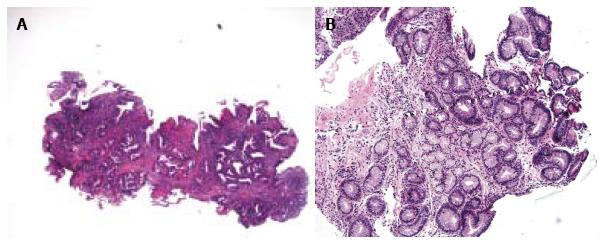
BE IND is diagnostically challenging and it is clear that its diagnostic reproducibility is poor[7,9,10]. Histologic criteria used to diagnose BE IND varied in different studies (Table 1) and even more so by pathologists in routine practice. For instance, the criteria for IND described by Reid et al[2] included moderate architectural distortion, nuclear abnormalities less marked than those seen in dysplasia, frequent dystrophic goblet cells, more extensive nuclear stratification, diminished or absent mucus production, increased cytoplasmic basophilia, and increased mitoses (Figure 1A). The diagnosis of IND should be limited to cases in which the changes are worrisome but not sufficient for the diagnosis of dysplasia (Figure 1B). Using similar criteria, other groups performed intraobserver and interobserver reproducibility studies and found that BE IND has significant interobserver variability[7,11]. In daily pathology practice, the BE IND category appears to expand, one such example being basal crypt dysplasia-like atypia. The concept of basal crypt dysplasia-like atypia remains controversial and is interpreted by some groups as IND while others believe that it truly represents dysplasia without surface involvement.
| Ref. | Criteria |
| Reid et al[2], 1988; Montgomery et al[7], 2001 | The architecture may be moderately distorted. Nuclear abnormalities are less marked than those seen in dysplasia. Other features that may lead to a diagnosis of IND include more numerous dystrophic goblet cells, more extensive nuclear stratification, diminished or absent mucus production, increased cytoplasmic basophilia, and increased mitoses |
| Sonwalkar et al[9], 2010 | Preserved gland architecture, mild crypt distortion, minimal nuclear stratification and slight nuclear atypia or enlargement |
| Kestens et al[15], 2015 | When a diagnosis of genuine dysplasia cannot be made. This is often due to the co-occurrence of inflammatory changes or when evaluation of surface maturation is not possible |
| Sinh et al[16], 2015 | Cytologic changes similar to those seen in LGD but with surface maturation or presence of inflammation |
| Duits et al[13], 2015 | Downgraded from BE LGD to BE IND by an expert pathology panel |
| Horvath et al[12], 2015 | The presence of architectural and cytologic atypia in small and mal-oriented biopsy specimen or those with inflammation or ulceration exceeding those expected for reactive changes. In some cases, it is due to basal dysplasia with surface maturation |
Regardless of the definition, illustration, and intraobserver /interobserver variability, BE IND category is not uncommonly used in daily pathology practice. Several studies recently investigated the clinical significance of BE IND and the results are reviewed and summarized in Tables 2 and 3.
| Ref. | Number of cases | Prevalent LGD, n (%) | Prevalent HGD, n (%) | Prevalent adenocarcinoma n (%) | Prevalent advanced neoplasia |
| Montgomery et al[11], 2001 | 7 | 0 (0) | 0 (0) | 1 (15) | At least 1 (15) |
| Sonwalkar et al[9], 2010 | 41 | At least 1 (2.4) | 0 (0) | At least 1 (2.4) | At least 1 (2.4) |
| Choi et al[14], 2015 | 96 | At least 14 (14.5) | Not known | Not known | At least 10 (10) |
| Horvath et al[12], 2015 | 107 | 7 (8.2) | 2 (2.35) | 2 (2.35) | 4 (4.7) |
| Kestens et al[15], 2015 | 842 | 101 (12.1) | Not known | Not known | 16 (1.9) |
| Sinh et al[16], 2015 | 83 | Not known | 0 (0) | 0 (0) | 0 (0) |
| Ref. | No. of cases | Follow up in months (range) | Incident LGD n (%) | Incident HGD n (%) | Incident adeno carcinoma n (%) | Incident advanced neoplasia (per 100 person-years | Risk factors for progression to advanced neoplasia |
| Duits et al[13], 2015 | 40 | Median 31 (16-59) | 0 | 1 (2.5) | 0 (0) | 0.9 | Not done |
| Horvath et al[12], 2015 | 82 | Mean 59 (13-182) | 14 (8.3) | 3 (2.3) | 2 (2.3) | 1.2 | p53 expression in >5% nuclei |
| Kestens et al[15], 2015 | 631 | Not known | No data | 10 (1.6) | 6 (1.0) | 0.43 | Older age |
| Sinh et al[16], 2015 | 83 | Mean 68.4 (SD: 37.2) | No data | 3 (3.6) | 1 (1.2) | 0.86 | Not done for BE IND group |
| Sonwalkar et al[9], 2010 | 37 | Median 38.7 (6-122) | 3 (8.1) | 0 (0) | 3 (8.1) | Not done | Expression of AMACR in more than 1% of cells |
Only few studies investigated the risk of neoplasia in BE IND. Prevalent neoplasia risk, defined as LGD, HGD or EAC detected within 1 year of the diagnosis of BE IND, was reported in 3 studies and ranged from 12.9% to 25%. Prevalence of advanced neoplasia, i.e., detection of HGD or EAC within 1 year of the diagnosis of BE IND, varied between 1.9% and 15%[9,11,12,14,15]. When a 6-mo interval was used as a cut-off, the prevalence of LGD and advanced neoplasia in BE IND was at least 2.8%[9]. In one case, the mucosal ulceration was associated with EAC[11].
The incidence of neoplasia in BE IND is summarized in Table 3. The incidence of all neoplasia in BE-IND is reported to be 4.5 cases per 100 person-years at risk. The progression to advanced neoplasia was 0.43 to 1.2 cases per 100 person-years at risk. The progression to EAC varied between 0.18 to 1.10 cases per 100 person-years at risk. In a study of 82 patients with BE IND, the mean length of BE segment was 6 cm in progressors vs 3 cm in non progressors (P = 0.01). The length of BE segment (HR = 1.2, 1.03-1.3) and multi-focality of BE IND (HR = 2.9, 1.09-7.6) were significantly associated with a higher risk of progression[12]. One study examined the progression to advanced neoplasia in a cohort of BE IND (n = 36) which was downgraded from an original diagnosis of BE LGD and reported an advanced neoplasia incidence of 0.9 cases per 100 person-years at risk, similar to a rate of 0.6 cases per 100 person-years at risk in patients with BE negative for dysplasia (n = 153)[13]. In contrast, BE LGD (n = 75) agreed upon by a panel of expert pathologists had an advanced neoplasia incidence of 9.1 cases per 100 person-years at risk[13]. Using 6-mo follow-up as a cutoff, Sonwalkar et al[9] (2010) reported that 8.1% of BE IND patients progressed to LGD and 8.1% BE IND progressed to EAC during a median follow up of 38.7 mo (range: 6-122). Interestingly, none of the 6 patients with BE IND progression had a consensus diagnosis of IND by all three reviewing pathologists.
Some studies did not distinguish between incident and prevalent dysplasia in BE IND. In a study by Montgomery et al[11] the neoplasia detection rate among patients with BE IND during a median follow-up of 36 mo was 18% where 4 of 22 patients developed carcinoma. In another study, Choi et al[14] reported 1-, 2-, and 3-year detection rates of HGD or EAC among patients with BE IND as 10%, 13% and 20%, respectively.
Few studies evaluated the role of biomarkers to aid in predicting the progression of dysplasia and/or cancer. In a study of 96 BE IND patients, Choi et al[14] identified active inflammation (by histology) and DNA flow cytometric abnormalities (either aneuploidy and/or increased 4N fractions greater than 6% of the nuclei) as significant risk factors associated with subsequent detection of dysplasia or neoplasia (hazard ratio for the combiner marker was 18.8, P < 0.0001). Sonwalkar et al[9] reported that the expression of alpha-methylacyl-CoA racemase (AMACR) in more than 1% of cells correlated with progression in BE IND. However, this role of AMACR expression in risk stratifying BE IND was not seen in a subsequent study by Horvath et al[17] and they instead showed that high expression of p53 (defined as intense staining in > 5% nuclei), later associated with prevalent advanced neoplasia and progression to advanced neoplasia in BE IND.
The diagnosis of BE IND is challenging due to varying definitions and inter and intraobserver variability. Therefore, all biopsies should be reviewed by a second pathologist preferably a gastrointestinal pathologist. The patients are treated with aggressive acid suppression. Then, a surveillance endoscopy is performed within 6-12 mo. The biopsy protocol consists of four quadrant biopsies every 1 cm interval. If nondysplastic BE is found, then surveillance interval can be lengthened beyond one year. If LGD or HGD are found, then endoscopic eradication therapy should be considered after confirmation of the diagnosis. The guidelines for management of BE IND are presented by major societies[1,18-20] and are summarized in Table 4.
| Guidelines | Diagnosis | Treatment and surveillance |
| ACG guidelines[1] | Acid suppressive medications for 3-6 mo A repeat endoscopy after optimization of should be performed If BE IND, surveillance in 12 mo | |
| BSG guidelines[18] | Review by a second GI pathologist, and the reasons for use of the ‘indefinite for dysplasia’ category should be given in the histology report in order to aid patient management | Optimisation of antireflux medication Repeat endoscopy in 6 mo If no dysplasia is found, then the surveillance per non-dysplastic Barrett’s oesophagus |
| ASGE[19] | Clarify presence and grade of dysplasia with expert GI pathologist | Increase antisecretory therapy to eliminate esophageal inflammation. Repeat EGD and biopsy to clarify dysplasia status |
| Australian Guidelines[20] | Confirm by a second pathologist, ideally an expert gastrointestinal pathologist. | Repeat endoscopy in 6 mo with Seattle protocol biopsies for suspected dysplasia (biopsy of any mucosal irregularity and quadrantic biopsies every 1 cm) on maximal acid suppression If repeat shows no dysplasia, then follow as per non-dysplastic protocol If repeat shows low-grade or high-grade dysplasia or adenocarcinoma, then follow protocols for these respective conditions If repeat again shows confirmed indefinite for dysplasia, then repeat endoscopy in 6 mo with Seattle protocol biopsies for suspected dysplasia |
In summary, the diagnosis of BE IND is difficult. Recent studies reveal that BE IND carries a significant risk of prevalent advanced neoplasia (at least 2.8%, 31 out of 1135 patients, ranging from 0% to 15%) (Table 2). In addition, the diagnosis of BE IND is associated with risk of progression to advanced neoplasia (0.43 to 1.2 cases person-years at risk) (Table 3). These figures are similar to the risk of LGD without histology review[16], but much lower than the progression risk in consensus diagnosis of LGD[13]. It is worth bearing in mind that 73% of cases with a diagnosis of BE LGD originally rendered by practicing pathologists were down-graded to BE IND or BE negative for dysplasia by an expert pathology panel[13]. Therefore, cases with initial impression of BE IND or LGD should be reviewed by additional GI pathologists to confirm the diagnosis. Patients with a confirmed diagnosis of BE IND should be placed on intensive acid suppressive therapy and have a surveillance endoscopy with four quadrant biopsies every 1 cm interval in BE segment within one year. BE IND patients with follow-up biopsies which are negative for dysplasia have low risk of neoplasia progression and may be reverted to routine surveillance. The length of BE, multi-focality of BE IND, older age (> 60 years old), abnormal p53 expression, active inflammation, and abnormal DNA content as detected by flow cytometry are useful to risk-stratify this patient population. The role of these predictors in clinical management of patients with BE IND requires further scrutiny.
Manuscript Source: Invited manuscript
Specialty Type: Gastroenterology and Hepatology
Country of Origin: United States
Peer-Review Report Classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Elpek GO, Guo YM, Ono T S- Editor: Qi Y L- Editor: A E- Editor: Lu YJ
| 1. | Shaheen NJ, Falk GW, Iyer PG, Gerson LB. ACG Clinical Guideline: Diagnosis and Management of Barrett’s Esophagus. Am J Gastroenterol. 2016;111:30-50; quiz 51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 990] [Cited by in RCA: 1060] [Article Influence: 117.8] [Reference Citation Analysis (0)] |
| 2. | Reid BJ, Haggitt RC, Rubin CE, Roth G, Surawicz CM, Van Belle G, Lewin K, Weinstein WM, Antonioli DA, Goldman H. Observer variation in the diagnosis of dysplasia in Barrett’s esophagus. Hum Pathol. 1988;19:166-178. [PubMed] |
| 3. | Nijhawan PK, Wang KK. Endoscopic mucosal resection for lesions with endoscopic features suggestive of malignancy and high-grade dysplasia within Barrett’s esophagus. Gastrointest Endosc. 2000;52:328-332. [PubMed] |
| 4. | Li Z, Rice TW, Liu X, Goldblum JR, Williams SJ, Rybicki LA, Murthy SC, Mason DP, Raymond DP, Blackstone EH. Intramucosal esophageal adenocarcinoma: primum non nocere. J Thorac Cardiovasc Surg. 2013;145:1519-1524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Li ZG, Zhu H, Shi H, Xie H, Goldblum JR, Thota PN, Liu X. Lymphovascular invasion and nodal metastasis in intramucosal adenocarcinoma of the esophagus and esophagogastric junction. J Dig Dis. 2015;16:197-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Thota PN, Lee HJ, Goldblum JR, Liu X, Sanaka MR, Gohel T, Kanadiya M, Lopez R. Risk stratification of patients with barrett’s esophagus and low-grade dysplasia or indefinite for dysplasia. Clin Gastroenterol Hepatol. 2015;13:459-465.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 7. | Montgomery E, Bronner MP, Goldblum JR, Greenson JK, Haber MM, Hart J, Lamps LW, Lauwers GY, Lazenby AJ, Lewin DN. Reproducibility of the diagnosis of dysplasia in Barrett esophagus: a reaffirmation. Hum Pathol. 2001;32:368-378. [PubMed] |
| 8. | Lomo LC, Blount PL, Sanchez CA, Li X, Galipeau PC, Cowan DS, Ayub K, Rabinovitch PS, Reid BJ, Odze RD. Crypt dysplasia with surface maturation: a clinical, pathologic, and molecular study of a Barrett’s esophagus cohort. Am J Surg Pathol. 2006;30:423-435. |
| 9. | Sonwalkar SA, Rotimi O, Scott N, Verghese E, Dixon M, Axon AT, Everett SM. A study of indefinite for dysplasia in Barrett’s oesophagus: reproducibility of diagnosis, clinical outcomes and predicting progression with AMACR (alpha-methylacyl-CoA-racemase). Histopathology. 2010;56:900-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Coco DP, Goldblum JR, Hornick JL, Lauwers GY, Montgomery E, Srivastava A, Wang H, Odze RD. Interobserver variability in the diagnosis of crypt dysplasia in Barrett esophagus. Am J Surg Pathol. 2011;35:45-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 77] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 11. | Montgomery E, Goldblum JR, Greenson JK, Haber MM, Lamps LW, Lauwers GY, Lazenby AJ, Lewin DN, Robert ME, Washington K. Dysplasia as a predictive marker for invasive carcinoma in Barrett esophagus: a follow-up study based on 138 cases from a diagnostic variability study. Hum Pathol. 2001;32:379-388. [PubMed] |
| 12. | Horvath B, Singh P, Xie H, Thota PN, Allende DS, Pai RK, Patil DT, Plesec TP, Goldblum JR, Liu X. Risk for esophageal neoplasia in Barrett’s esophagus patients with mucosal changes indefinite for dysplasia. J Gastroenterol Hepatol. 2015;30:262-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Duits LC, Phoa KN, Curvers WL, Ten Kate FJ, Meijer GA, Seldenrijk CA, Offerhaus GJ, Visser M, Meijer SL, Krishnadath KK. Barrett’s oesophagus patients with low-grade dysplasia can be accurately risk-stratified after histological review by an expert pathology panel. Gut. 2015;64:700-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 180] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 14. | Choi WT, Emond MJ, Rabinovitch PS, Ahn J, Upton MP, Westerhoff M. “Indefinite for Dysplasia” in Barrett’s Esophagus: Inflammation and DNA Content Abnormality are Significant Predictors of Early Detection of Neoplasia. Clin Transl Gastroenterol. 2015;6:e81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Sinh P, Anaparthy R, Young PE, Gaddam S, Thota P, Balasubramanian G, Singh M, Higbee AD, Wani S, Gupta N. Clinical outcomes in patients with a diagnosis of “indefinite for dysplasia” in Barrett’s esophagus: a multicenter cohort study. Endoscopy. 2015;47:669-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Kestens C, Leenders M, Offerhaus GJ, van Baal JW, Siersema PD. Risk of neoplastic progression in Barrett’s esophagus diagnosed as indefinite for dysplasia: a nationwide cohort study. Endoscopy. 2015;47:409-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Horvath B, Singh P, Xie H, Thota PN, Sun X, Liu X. Expression of p53 predicts risk of prevalent and incident advanced neoplasia in patients with Barrett’s esophagus and epithelial changes indefinite for dysplasia. Gastroenterol Rep (Oxf). 2015; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Fitzgerald RC, di Pietro M, Ragunath K, Ang Y, Kang JY, Watson P, Trudgill N, Patel P, Kaye PV, Sanders S. British Society of Gastroenterology guidelines on the diagnosis and management of Barrett’s oesophagus. Gut. 2014;63:7-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1016] [Cited by in RCA: 878] [Article Influence: 79.8] [Reference Citation Analysis (0)] |
| 19. | Evans JA, Early DS, Fukami N, Ben-Menachem T, Chandrasekhara V, Chathadi KV, Decker GA, Fanelli RD, Fisher DA, Foley KQ. The role of endoscopy in Barrett’s esophagus and other premalignant conditions of the esophagus. Gastrointest Endosc. 2012;76:1087-1094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 241] [Article Influence: 18.5] [Reference Citation Analysis (1)] |
| 20. | Whiteman DC, Appleyard M, Bahin FF, Bobryshev YV, Bourke MJ, Brown I, Chung A, Clouston A, Dickins E, Emery J. Australian clinical practice guidelines for the diagnosis and management of Barrett’s esophagus and early esophageal adenocarcinoma. J Gastroenterol Hepatol. 2015;30:804-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 97] [Article Influence: 9.7] [Reference Citation Analysis (0)] |