Published online May 6, 2016. doi: 10.4292/wjgpt.v7.i2.261
Peer-review started: January 19, 2016
First decision: February 22, 2016
Revised: February 26, 2016
Accepted: March 14, 2016
Article in press: March 16, 2016
Published online: May 6, 2016
Processing time: 93 Days and 4.3 Hours
AIM: To investigate the clinical and biochemical factors associated with visceral fat accumulation in the general population.
METHODS: We enrolled 1004 subjects who underwent a medical health checkup between April 2008 and March 2009. The medical health checkup included the following tests: Height, body weight, waist circumference (WC), systolic blood pressure, diastolic blood pressure, urinalysis, blood-cell counts, blood chemistry, electrocardiography, chest radiography, and abdominal computed tomography (CT) for visceral fat accumulation. The patients’ medical history and lifestyle factors were collected privately by nurses using a self-administered questionnaire, and they included questions regarding physical activity, sleep duration, dietary habits, smoking, and alcohol consumption. Visceral fat area (VFA) was defined as the sum of the intraperitoneal fat area at the level of the umbilicus with CT density in the range of -150 to -50 Hounsfield units.
RESULTS: The mean age and body mass index (BMI) of the study subjects were 57.0 years and 24.4 kg/m2. In both male and females, VFA was significantly and positively correlated with WC (r = 0.532, P < 0.01; r = 0.612, P < 0.01). Subjects with high levels of VFA were primarily male with significantly higher age, height, body weight, BMI, systolic blood pressure (BP), diastolic BP, and hemoglobin in all subjects (P < 0.05). A multivariate logistic regression analysis revealed that VFA had a positive relationship with age ≥ 56, BMI ≥ 25 kg/m2, and triglyceride level ≥ 149 in males (P < 0.05), whereas it had a positive relationship with age ≥ 58, BMI ≥ 24.4 kg/m2, high-density lipoprotein cholesterol level < 40 mg/dL, and current drinking in females (P < 0.05).
CONCLUSION: These results suggest that gender differences exist in the clinical and biochemical parameters associated with visceral fat accumulation.
Core tip: Although close association between visceral fat accumulation and metabolic syndrome has been established, little is known about what clinical and biochemical parameters affect visceral fat accumulation. We analyzed the clinical and biochemical parameters of the health checkup subjects and assessed the visceral fat area (VFA). A multivariate logistic regression analysis revealed that VFA had a positive relationship with age, body mass index (BMI), and triglyceride level in males, whereas it had a positive relationship with age, BMI, and current drinking in females. These results suggest that gender differences exist in the clinical and biochemical parameters associated with visceral fat accumulation.
- Citation: Arakaki S, Maeshiro T, Hokama A, Hoshino K, Maruwaka S, Higashiarakawa M, Parrott G, Hirata T, Kinjo K, Fujita J. Factors associated with visceral fat accumulation in the general population in Okinawa, Japan. World J Gastrointest Pharmacol Ther 2016; 7(2): 261-267
- URL: https://www.wjgnet.com/2150-5349/full/v7/i2/261.htm
- DOI: https://dx.doi.org/10.4292/wjgpt.v7.i2.261
Visceral fat accumulation is closely related to atherogenic disorders and metabolic syndrome (MS), including diabetes mellitus, hypertension, and dyslipidemia[1]. It also leads to obesity-related complications. MS further increases the risks for cardiovascular diseases and thus is an important therapeutic target. Although computed tomography (CT) has been applied widely as the gold standard method to evaluate visceral fat accumulation[2], little is known about what clinical and biochemical parameters affect visceral fat accumulation. The aim of this study is to investigate the clinical and biochemical parameters potentially associated with visceral fat accumulation.
We searched the database to find 1151 subjects who underwent abdominal fat CT scans for visceral fat area (VFA), and blood tests for a routine health checkup between 1 April 2008 and 31 March 2009 at Okinawa Health Promotion Foundation, Okinawa, Japan. The health checkups were provided as part of a medical health initiative to promote public health through the early detection of chronic diseases and the evaluation of associated underlying risk factors. Subjects were included if they fulfilled the following criteria: (1) absence of markers for hepatitis B virus infection [hepatitis B surface antigen (HBsAg)] and hepatitis C virus (HCV) infection (anti-HCV antibodies); and (2) absence of excess drinking of alcohol defined as consumption of > 280 g/wk. Among the 1151 subjects, 147 subjects were positive for either HBsAg or anti-HCV, or were defined as excess drinkers. The data for the remaining 1004 people were included in the analysis. All subjects provided written informed consent for the use of their anonymized data for an epidemiological study. The study design was approved by the Ethics Committee of University of the Ryukyus. The study was conducted in accordance with the Declaration of Helsinki.
The medical health checkup included the following tests: Height, body weight, waist circumference (WC), systolic blood pressure (SBP), diastolic BP (DBP), urinalysis, blood-cell counts, blood chemistry, electrocardiography, chest radiography, and abdominal CT for VFA. The patients’ medical history and lifestyle factors were collected privately by nurses using a self-administered questionnaire, and they included questions regarding physical activity, sleep duration, dietary habits, smoking, and alcohol consumption. For our study purposes, individuals who consumed at least one alcoholic beverage per week were defined as a “current drinker”. Patients who reported alcohol consumption of > 280 g/wk were identified as “excess drinkers”[3]. Alcohol consumption was evaluated by asking the participants about the amount and type of alcoholic beverages they consumed per week, an estimated total alcohol intake was calculated in grams. Blood samples were taken after > 10 h of overnight fasting. Laboratory tests were performed with standard laboratory methods and included measurements of hemoglobin (HGB), platelet count (PLT), aspartate aminotransferase, alanine aminotransferase, γ-glutamyl transferase, alkaline phosphatase, cholinesterase, total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein-cholesterol (LDL-C), triglycerides (TG), fasting plasma glucose, glycosylated hemoglobin A1c, serum uric acid, HBsAg, and anti-HCV.
Abdominal visceral fat distribution was assessed with a single-slice CT image taken at the level of the umbilicus by a helical CT scanner (Siemens, Germany). The area of visceral fat was defined as the sum of the intraperitoneal fat area with CT density in the range of -150 to -50 Hounsfield units.
Descriptive statistics (means and standard deviations) were calculated for all continuous variables. Differences between the two groups were compared using χ2 test. The comparisons of continuous variables between the 2 groups were performed using the Student t test. The linear association of WC and VFA was evaluated by the Spearman’s rank correlation. The statistical analyses were performed using SPSS 19.0 (SPSS Inc, Chicago, IL, United States). Statistical significance was achieved at P < 0.05.
The clinical and biochemical characteristics of 1004 subjects are summarized in Table 1. The subjects were predominantly middle-aged (55.6 ± 11.6 years; range 25-88 years), and 53.8% were male. The mean BMI of all subjects was 24.9 ± 3.4 kg/m2, and 44.8% of the subjects met the criteria for obesity (BMI ≥ 25 kg/m2).
| Gender (M/F) | 540/464 |
| Age (yr) | 55.6 ± 11.6 |
| Height (cm) | 159.7 ± 9.1 |
| Body weight (kg) | 63.8 ± 11.7 |
| BMI (kg/m2) | 24.9 ± 3.4 |
| WC (cm) | 88.0 ± 8.4 |
| SBP (mmHg) | 123.1 ± 14.5 |
| DBP (mmHg) | 76.9 ± 9.6 |
| VFA (cm2) | 99.3 ± 50.5 |
| HGB (g/dL) | 14.3 ± 1.4 |
| PLT (× 104/μL) | 22.2 ± 4.9 |
| AST (IU/L) | 23.1 ± 8.3 |
| ALT (IU/L) | 25.4 ± 16.0 |
| GGT (IU/L) | 34.6 ± 30.7 |
| ALP (IU/L) | 226.1 ± 64.2 |
| ChE (IU/L) | 355.1 ± 65.9 |
| TC (mg/dL) | 206.5 ± 31.7 |
| LDL-C (mg/dL) | 126.1 ± 29.0 |
| HDL-C (mg/dL) | 56.0 ± 13.2 |
| TG (mg/dL) | 121.0 ± 74.3 |
| FPG (g/dL) | 99.7 ± 16.1 |
| HbA1c (%) | 5.3 ± 0.5 |
| UA (mg/dL) | 5.73 ±1.4 |
| Alcohol consumption (non/current drinkers) | 561/443 |
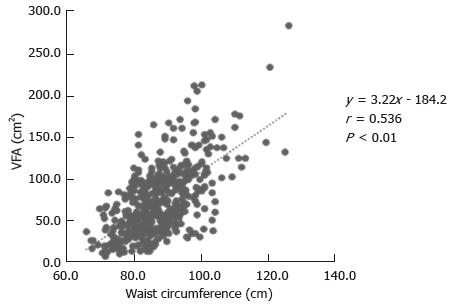
Figure 1 shows the relationship between WC and VFA of all subjects, indicating a significant positive correlation (Figure 1; r = 0.536, P < 0.01). In both males and females, VFA was significantly positively correlated with WC (r = 0.532, P < 0.01; r = 0.612, P < 0.01), respectively. VFA in females was more strongly correlated with WC than in males.
We divided the sample using a cut-off point of VFA (100 cm2)[4]. Subjects with high levels of VFA had primarily male with a significantly higher height, body weight, BMI, SBP, DBP, and HGB in all subjects (P < 0.05, Table 2). In male subjects, TC were also significantly higher in high VFA group (P < 0.05, Table 3), whereas PLT were significantly higher in females with high VFA group (P < 0.05, Table 4). Of note, the rate of current drinking was significant lower in females with high VFA group.
| VFA ≥ 100 (n = 459) | VFA < 100 (n = 545) | P value | |
| Gender (M/F) | 384/111 | 192/353 | < 0.001 |
| Age (yr) | 56.9 ± 11.5 | 54.5 ± 11.5 | 0.319 |
| Height (cm) | 162.61 ± 8.6 | 157.3 ± 8.8 | < 0.001 |
| Body weight (kg) | 70.1 ± 11.1 | 58.5 ± 9.4 | < 0.001 |
| BMI (kg/m2) | 26.5 ± 3.4 | 23.6 ± 2.6 | < 0.001 |
| WC (cm) | 91.9 ± 7.9 | 84.8 ± 7.3 | < 0.001 |
| SBP(mmHg) | 126.5 ± 13.6 | 120.3 ± 14.6 | < 0.001 |
| DBP(mmHg) | 79.1 ± 9.2 | 75.0 ± 9.5 | < 0.001 |
| HGB (g/dL) | 14.8 ± 1.2 | 13.9 ± 1.3 | < 0.001 |
| PLT (× 104/μL) | 22.0 ± 4.9 | 23.3 ± 8.5 | 0.24 |
| AST (IU/L) | 22.8 ± 7.9 | 25.4 ± 10.2 | 0.32 |
| ALT (IU/L) | 24.6 ± 15.2 | 25.9 ± 16.7 | 0.19 |
| GGT (IU/L) | 32.5 ± 27.3 | 36.3 ± 33.3 | 0.048 |
| ALP (IU/L) | 226.4 ± 62.6 | 225.9 ± 65.7 | 0.88 |
| ChE (IU/L) | 357.8 ± 67.2 | 352.8 ± 64.7 | 0.23 |
| TC (mg/dL) | 208.5 ± 31.7 | 204.9 ± 31.7 | 0.07 |
| LDL-C (mg/dL) | 126.8 ± 29.6 | 125.4 ± 28.6 | 0.45 |
| HDL-C (mg/dL) | 56.5 ± 13.9 | 55.5 ± 12.6 | 0.23 |
| TG (mg/dL) | 119.5 ± 58.2 | 122.3 ± 79.1 | 0.56 |
| FPG (g/dL) | 98.7 ± 14.5 | 100.5 ± 17.3 | 0.07 |
| HbA1c (%) | 5.2 ± 0.47 | 5.3 ± 0.59 | 0.11 |
| UA (mg/dL) | 5.7 ± 1.4 | 5.8 ± 1.3 | 0.09 |
| Alcohol consumption (non/current drinkers) | 254/205 | 307/238 | 0.401 |
| VFA ≥ 100 (n = 348) | VFA < 100 (n = 192) | P value | |
| Age (yr) | 55.3 ± 11.7 | 50.6 ± 12.9 | < 0.001 |
| Height (cm) | 166.1 ± 6.0 | 166.2 ± 6.4 | 0.93 |
| Body weight (kg) | 72.1 ± 9.9 | 66.7 ± 8.2 | < 0.001 |
| BMI (kg/m2) | 26.1 ± 3.0 | 24.1 ± 2.4 | 0.001 |
| WC (cm) | 90.7 ± 7.0 | 84.9 ± 6.7 | < 0.001 |
| SBP (mmHg) | 126.6 ± 14.1 | 121.8 ± 14.2 | < 0.001 |
| DBP (mmHg) | 79.8 ± 9.5 | 77.0 ± 8.8 | 0.001 |
| HGB (g/dL) | 15.2 ± 1.0 | 15.1 ± 1.1 | 0.29 |
| PLT (× 104/μL) | 21.4 ± 4.4 | 21.5 ± 4.2 | 0.76 |
| AST (IU/L) | 22.9 ± 8.0 | 23.4 ± 8.7 | 0.51 |
| ALT (IU/L) | 24.4 ± 15.9 | 25.8 ± 16.6 | 0.34 |
| GGT (IU/L) | 32.3 ± 29.3 | 25.8 ± 16.6 | 0.29 |
| ALP (IU/L) | 227.0 ± 64.1 | 228.2 ± 72.2 | 0.84 |
| ChE (IU/L) | 359.0 ± 68.5 | 353.9 ± 68.2 | 0.41 |
| TC (mg/dL) | 209.6 ± 32.8 | 203.4 ± 32.7 | 0.03 |
| LDL-C (mg/dL) | 127.1 ± 31.0 | 124.5 ± 30.1 | 0.35 |
| HDL-C (mg/dL) | 57.4 ± 13.7 | 55.3 ± 13.4 | 0.09 |
| TG (mg/dL) | 118.4 ± 68.3 | 118.8 ± 79.1 | 0.95 |
| FPG (g/dL) | 98.6 ± 15.3 | 101.4 ± 19.6 | 0.07 |
| HbA1c (%) | 5.2 ± 0.5 | 5.3 ± 0.7 | 0.06 |
| UA (mg/dL) | 5.6 ± 1.4 | 5.7 ± 1.2 | 0.28 |
| Alcohol consumption (non/current drinkers) | 209/139 | 119/73 | 0.37 |
| VFA ≥ 100 (n = 111) | VFA < 100 (n = 353) | P value | |
| Age (yr) | 62.1 ± 9.4 | 56.7 ± 10.0 | < 0.001 |
| Height (cm) | 151.4 ± 5.6 | 152.5 ± 5.5 | 0.07 |
| Body weight (kg) | 63.9 ± 12.2 | 54.1 ± 6.7 | < 0.001 |
| BMI (kg/m2) | 27.8 ± 4.4 | 23.3 ± 2.7 | < 0.001 |
| WC (cm) | 95.6 ± 9.5 | 84.7 ± 7.6 | < 0.001 |
| SBP (mmHg) | 126.0 ± 11.9 | 119.4 ± 14.8 | < 0.001 |
| DBP (mmHg) | 76.9 ± 8.0 | 74.0 ± 9.7 | 0.004 |
| HGB (g/dL) | 13.6 ± 1.1 | 13.3 ± 0.9 | 0.02 |
| PLT (× 104/μL) | 24.1 ± 5.9 | 22.9 ± 5.1 | 0.03 |
| AST (IU/L) | 22.5 ± 7.6 | 23.3 ± 8.4 | 0.38 |
| ALT (IU/L) | 25.3 ± 12.8 | 26.0 ± 16.8 | 0.67 |
| GGT (IU/L) | 33.0 ± 20.2 | 36.9 ± 30.3 | 0.21 |
| ALP (IU/L) | 224.8 ± 57.8 | 224.6 ± 61.9 | 0.97 |
| ChE (IU/L) | 354.0 ± 62.8 | 352.2 ± 62.8 | 0.79 |
| TC (mg/dL) | 204.8 ± 27.9 | 205.7 ± 31.1 | 0.77 |
| LDL-C (mg/dL) | 125.8 ± 24.7 | 125.9 ± 27.7 | 0.98 |
| HDL-C (mg/dL) | 53.8 ± 14.2 | 55.6 ± 12.2 | 0.2 |
| TG (mg/dL) | 123.1 ± 68.0 | 124.1 ± 79.1 | 0.9 |
| FPG (g/dL) | 98.9 ± 11.7 | 100.1 ± 15.9 | 0.5 |
| HbA1c (%) | 5.2 ± 0.4 | 5.3 ± 0.5 | 0.11 |
| UA (mg/dL) | 5.7 ± 1.4 | 5.8 ± 1.3 | 0.66 |
| Alcohol consumption (non/current drinkers) | 45/66 | 188/165 | 0.01 |
We further evaluated the risk factors for VFA using the multivariate logistic regression analysis. VFA showed positive relationships with age ≥ 56, male gender, BMI ≥ 24.4 kg/m2, BP ≥ 149 and/or 90 mmHg, TG ≥ 149 mg/dL, and current drinking in all subjects (P < 0.05, Table 5). In male subjects, VFA was positively associated with age ≥ 56, BMI ≥ 25.0 kg/m2, and TG ≥ 149 mg/dL (P < 0.05, Table 6), whereas in female subjects positive associations were observed with age ≥ 58, BMI ≥ 24.4 kg/m2, HDL-C < 40 mg/dL, and current drinking (P < 0.05, Table 7).
| Variable | β | SE | Wald | P value | OR | 95%CI |
| Age (≥ 56 yr) | 0.692 | 0.165 | 17.714 | < 0.001 | 1.999 | 1.448-2.759 |
| Male gender | 1.746 | 0.202 | 74.406 | < 0.001 | 5.73 | 3.854-8.52 |
| BMI (≥ 24.4 kg/m2) | 1.621 | 0.158 | 105.265 | < 0.001 | 5.06 | 3.712-6.897 |
| BP (≥ 149/90 mmHg) | 0.415 | 0.196 | 4.503 | 0.034 | 1.515 | 1.032-2.223 |
| HGB (≥ 14.3 g/dL) | 0.251 | 0.193 | 1.696 | 0.193 | 1.286 | 0.881-1.876 |
| PLT (≥ 22.2 × 104/μL) | -0.014 | 0.187 | 0.006 | 0.938 | 0.986 | 0.683-1.422 |
| TC (≥ 219 mg/dL) | -0.206 | 0.234 | 0.775 | 0.379 | 0.814 | 0.514-1.288 |
| TG (≥ 149 mg/dL) | 0.473 | 0.203 | 5.416 | 0.02 | 1.604 | 1.077-2.389 |
| HDL-C (≥ 40 mg/dL) | -0.197 | 0.279 | 0.499 | 0.48 | 0.821 | 0.475-1.42 |
| LDL-C (≥ 139 mg/dL) | 0.184 | 0.242 | 0.574 | 0.449 | 1.201 | 0.747-1.932 |
| AST (≥ 30 IU/L) | -0.191 | 0.276 | 0.477 | 0.49 | 0.826 | 0.481-1.42 |
| ALT (≥ 30 IU/L) | 0.075 | 0.238 | 0.099 | 0.753 | 1.078 | 0.676-1.72 |
| GGT (≥ 51 IU/L) | -0.324 | 0.233 | 1.927 | 0.165 | 0.724 | 0.458-1.143 |
| ALP (≥ 325 IU/L) | 0.156 | 0.305 | 0.263 | 0.608 | 1.169 | 0.643-2.126 |
| ChE (≥ 350 IU/L) | -0.065 | 0.165 | 0.155 | 0.694 | 0.937 | 0.678-1.294 |
| UA (≥ 5.8 mg/dL) | -0.117 | 0.177 | 0.439 | 0.508 | 0.89 | 0.629-1.257 |
| FPG (≥ 110 g/dL) | -0.473 | 0.257 | 3.369 | 0.066 | 0.623 | 0.376-1.033 |
| HbA1c (≥ 6.2%) | -0.205 | 0.45 | 0.208 | 0.649 | 0.814 | 0.337-1.969 |
| Current drinking | 0.371 | 0.171 | 4.713 | 0.03 | 1.449 | 1.037-2.026 |
| Variable | β | SE | Wald | P value | OR | 95%CI |
| Age (≥ 56 yr) | 0.688 | 0.21 | 10.734 | 0.001 | 1.99 | 1.319-3.004 |
| BMI (≥ 25 kg/m2) | 1.459 | 0.204 | 51.055 | < 0.001 | 4.301 | 2.883-6.417 |
| BP (≥ 149/90 mmHg | 0.418 | 0.247 | 2.859 | 0.091 | 1.518 | 0.936-2.464 |
| Hb (≥ 15.1 g/dL) | 0.293 | 0.203 | 2.098 | 0.148 | 1.341 | 0.902-1.995 |
| Plt (≥ 20.9 × 104/μL) | -0.324 | 0.236 | 1.874 | 0.171 | 0.724 | 0.455-1.15 |
| TC (≥ 219 mg/dL) | -0.104 | 0.316 | 0.108 | 0.743 | 0.901 | 0.485-1.674 |
| TG (≥ 149 mg/dL) | 0.602 | 0.273 | 4.862 | 0.027 | 1.826 | 1.069-3.117 |
| HDL-C (≥ 40 mg/dL) | 0.218 | 0.341 | 0.408 | 0.523 | 1.243 | 0.637-2.427 |
| LDL-C (≥ 139 mg/dL) | 0.037 | 0.322 | 0.013 | 0.908 | 1.038 | 0.552-1.952 |
| AST (≥ 30 IU/L) | -0.161 | 0.343 | 0.22 | 0.639 | 0.851 | 0.434-1.669 |
| ALT (≥ 30 IU/L) | 0.022 | 0.302 | 0.005 | 0.942 | 1.022 | 0.566-1.847 |
| GGT (≥ 51 IU/L) | -0.003 | 0.309 | 0 | 0.992 | 0.997 | 0.544-1.828 |
| ALP (≥ 325 IU/L) | 0.124 | 0.37 | 0.113 | 0.737 | 1.132 | 0.548-2.34 |
| ChE (≥ 350 IU/L) | -0.049 | 0.217 | 0.052 | 0.819 | 0.952 | 0.622-1.455 |
| UA (≥ 5.8 mg/dL) | -0.085 | 0.232 | 0.133 | 0.716 | 0.919 | 0.583-1.448 |
| FPG (≥ 110 g/dL) | -0.485 | 0.327 | 2.201 | 0.138 | 0.615 | 0.324-1.169 |
| HbA1c (≥ 6.2%) | -0.162 | 0.532 | 0.093 | 0.761 | 0.85 | 0.3-2.414 |
| Current drinking | 0.345 | 0.222 | 2.421 | 0.12 | 1.412 | 0.914-2.18 |
| Variable | β | SE | Wald | P value | OR | 95%CI |
| Age (≥ 58 yr) | 0.793 | 0.259 | 9.349 | 0.002 | 2.211 | 1.33-3.676 |
| BMI (≥ 24.4 kg/m2) | 2.074 | 0.298 | 48.386 | < 0.001 | 7.957 | 4.436-14.275 |
| BP (≥ 149/90 mmHg) | 0.576 | 0.346 | 2.768 | 0.096 | 1.78 | 0.902-3.509 |
| Hb (≥ 14.3 g/dL) | 0.39 | 0.26 | 2.25 | 0.134 | 1.477 | 0.887-2.457 |
| Plt (≥ 22.2 × 104/μL) | 0.186 | 0.295 | 0.399 | 0.528 | 1.205 | 0.676-2.146 |
| TC (≥ 219 mg/dL) | -0.297 | 0.388 | 0.586 | 0.444 | 0.743 | 0.347-1.59 |
| TG (≥ 149 mg/dL) | 0.148 | 0.334 | 0.196 | 0.658 | 1.16 | 0.602-2.233 |
| HDL-C (≥ 40 mg/dL) | -0.83 | 0.465 | 4.463 | 0.035 | 0.374 | 0.15-0.931 |
| LDL-C (≥ 139 mg/dL) | 0.355 | 0.4 | 0.791 | 0.374 | 1.427 | 0.652-3.122 |
| AST (≥ 30 IU/L) | -0.137 | 0.489 | 0.078 | 0.78 | 0.872 | 0.334-2.275 |
| ALT (≥ 30 IU/L) | 0.247 | 0.394 | 0.392 | 0.531 | 1.28 | 0.591-2.773 |
| GGT (≥ 51 IU/L) | -0.928 | 0.393 | 5.567 | 0.018 | 0.395 | 0.183-0.855 |
| ALP (≥ 325 IU/L) | -0.093 | 0.571 | 0.027 | 0.87 | 0.911 | 0.298-2.787 |
| ChE (≥ 350 IU/L) | -0.106 | 0.267 | 0.158 | 0.691 | 0.899 | 0.533-1.517 |
| UA (≥ 5.8 mg/d) | -0.011 | 0.285 | 0.001 | 0.97 | 0.989 | 0.566-1.729 |
| FPG (≥ 110 g/dL) | -0.567 | 0.412 | 1.895 | 0.169 | 0.567 | 0.253-1.272 |
| HbA1c (≥ 6.2%) | 0.225 | 0.824 | 0.075 | 0.785 | 1.252 | 0.249-6.299 |
| Current drinking | 0.574 | 0.285 | 4.062 | 0.044 | 1.776 | 1.016-3.104 |
This study was conducted in Okinawa is a subtropical island with 1.4 million population located in the southwest of Japan. BMI of 24.9 ± 3.4 kg/m2 in this study was higher than that (23.0 ± 3.3 kg/m2) of a multicenter large health checkup study conducted in the main land Japan[5]. United States ruled Okinawa for 27 years after the World War II, thus a westernized food and life styles have been popular in Okinawa[6]. This westernization may attribute to the obesity (higher BMI) in Okinawa.
The Japanese Visceral Fat Syndrome Study Committee of the Ministry of Health and Welfare of Japan was organized to establish the diagnostic criteria of obesity disease and the importance of visceral fat accumulation among the multiple obesity-related cardiovascular risk factors[4]. They also demonstrated that among the various anthropometric parameters measured in their study, WC showed the closest relationship with VFA in both men and women. WC is used as an index of visceral fat accumulation in the diagnosis of MS because of its ease of measurement. According to the modified National Cholesterol Education Program’s Adult Treatment Panel III (NCEP-ATP) guidelines[7], the prevalence of MS in Japan and China was 20.6% and 27.6%, respectively[8,9]. In this study, the CT-evaluated visceral fat accumulation also correlated with WC; our findings confirm that WC is a reliable surrogate measurement for visceral fat accumulation, and the findings are in concordance with results from previous studies conducted within the Japanese population[10-12].
We focused on gender differences and the relationship between visceral fat accumulation and other clinical parameters. It is well known that visceral fat accumulation exhibits age, sex, and race differences in both prevalence and severity[13]. Age and gender differences are further affected by country-specific differences in the prevalence of obesity and lifestyle-related diseases[14]. In the present multivariate logistic regression analysis, current drinking was significantly associated with VFA in females but not in male subjects. These differences may be a result of alcoholic drink choice, such as beer vs liquor. The effect of alcohol on fat metabolism remains controversial. In a cross-sectional study of healthy South Korean men, Kim et al[15] reported that alcohol consumption showed a significant association with increased VFA, which was independent of other factors. On the other hand, Fan et al[16] have reported that current alcohol consumption was associated with a lower prevalence of MS, irrespective of alcohol intake, and alcohol consumption had a favorable influence on HDL-C and WC in a Shanghai study. Excessive alcohol consumption is known to cause alcoholic liver diseases; however, Moriya et al[3] from Japan reported that light to moderate alcohol consumption by men was likely to protect individuals against fatty liver over time. Most recently, Takahashi et al[17] from Japan showed clearly in a cohort study that alcohol had a biphasic effect on fatty liver. Although the available evidence is conflicting, moderation of alcohol consumption is still a consistent recommendation for a healthy lifestyle[18].
The strengths of our study are the large sample size and the direct assessment of VFA using a CT scan which allowed for the precise determination of the WC component[12]. In addition, the study subjects were representative of the general population undergoing a health checkup. There are some limitations of this study. First, because of the cross-sectional design of this study, we could not identify the causal relationship between VFA and the various parameters in depth. Second, the self-administered questionnaire on alcohol consumption may have resulted in under-reported alcohol intake for some subjects. Third, this study lacks data regarding patient medication use, nutritional intake, and physical fitness, all variables that can influence visceral fat accumulation. There was a possibility of selection bias because subjects were volunteers who opted to complete a health checkup. Thus, it is possible the study subjects also had an increased awareness of healthy behaviors.
In conclusion, despite these limitations, the present study showed gender differences in the clinical and biochemical parameters associated with visceral fat accumulation in the general population in Okinawa, Japan. Visceral obesity is probably the most important target for future interventions in MS. Future studies are needed to clarify preventive methods among different gender and age groups.
We are grateful to staff members in OHPF for their great contribution.
Visceral fat accumulation is closely related to atherogenic disorders and metabolic syndrome (MS), including diabetes mellitus, hypertension, and dyslipidemia. It also leads to obesity-related complications. MS further increases the risks for cardiovascular diseases and thus is an important therapeutic target.
Although computed tomography has been applied widely as the gold standard method to evaluate visceral fat accumulation, little is known about what clinical and biochemical parameters affect visceral fat accumulation. The aim of this study is to investigate the clinical and biochemical parameters potentially associated with visceral fat accumulation.
The present study showed gender differences in the clinical and biochemical parameters which associated with visceral fat accumulation in the general population in Okinawa, Japan.
Visceral obesity is probably the most important target for future interventions in MS.
Visceral fat accumulation is defined as the sum of the intraperitoneal fat area with computed tomography (CT) density in the range of -150 to -50 Hounsfield units.
The authors retrospectively analyzed data of 1004 check up patients. They found that visceral fat area measured by CT is correlated with waist circumference and metabolic parameters in both sex.
P- Reviewer: Balaban YH, Hallgren T S- Editor: Gong ZM L- Editor: A E- Editor: Wu HL
| 1. | Matsuzawa Y, Funahashi T, Nakamura T. The concept of metabolic syndrome: contribution of visceral fat accumulation and its molecular mechanism. J Atheroscler Thromb. 2011;18:629-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 307] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 2. | Ryo M, Kishida K, Nakamura T, Yoshizumi T, Funahashi T, Shimomura I. Clinical significance of visceral adiposity assessed by computed tomography: A Japanese perspective. World J Radiol. 2014;6:409-416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 64] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 3. | Moriya A, Iwasaki Y, Ohguchi S, Kayashima E, Mitsumune T, Taniguchi H, Ando M, Yamamoto K. Roles of alcohol consumption in fatty liver: a longitudinal study. J Hepatol. 2015;62:921-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 87] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 4. | Examination Committee of Criteria for ‘Obesity Disease’ in Japan; Japan Society for the Study of Obesity. New criteria for ‘obesity disease’ in Japan. Circ J. 2002;66:987-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1256] [Cited by in RCA: 1420] [Article Influence: 61.7] [Reference Citation Analysis (1)] |
| 5. | Eguchi Y, Hyogo H, Ono M, Mizuta T, Ono N, Fujimoto K, Chayama K, Saibara T. Prevalence and associated metabolic factors of nonalcoholic fatty liver disease in the general population from 2009 to 2010 in Japan: a multicenter large retrospective study. J Gastroenterol. 2012;47:586-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 400] [Article Influence: 30.8] [Reference Citation Analysis (2)] |
| 6. | Sugama C, Isa K, Okumura K, Iseki K, Kinjo K, Ohya Y. Trends in the incidence of stroke and cardiovascular risk factors on the isolated island of Okinawa: the Miyakojima study. J Stroke Cerebrovasc Dis. 2013;22:e118-e123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735-2752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7515] [Cited by in RCA: 8277] [Article Influence: 413.9] [Reference Citation Analysis (0)] |
| 8. | Takami H, Nakamoto M, Uemura H, Katsuura S, Yamaguchi M, Hiyoshi M, Sawachika F, Juta T, Arisawa K. Inverse correlation between coffee consumption and prevalence of metabolic syndrome: baseline survey of the Japan Multi-Institutional Collaborative Cohort (J-MICC) Study in Tokushima, Japan. J Epidemiol. 2013;23:12-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 9. | Xi B, He D, Hu Y, Zhou D. Prevalence of metabolic syndrome and its influencing factors among the Chinese adults: the China Health and Nutrition Survey in 2009. Prev Med. 2013;57:867-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 202] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 10. | Ishibashi E, Eguchi Y, Eguchi T, Matsunobu A, Oza N, Nakashita S, Kitajima Y, Kuroki S, Ozaki I, Kawaguchi Y. Waist circumference correlates with hepatic fat accumulation in male Japanese patients with non-alcoholic fatty liver disease, but not in females. J Gastroenterol Hepatol. 2008;23:908-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Oka R, Kobayashi J, Yagi K, Tanii H, Miyamoto S, Asano A, Hagishita T, Mori M, Moriuchi T, Kobayashi M. Reassessment of the cutoff values of waist circumference and visceral fat area for identifying Japanese subjects at risk for the metabolic syndrome. Diabetes Res Clin Pract. 2008;79:474-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 119] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 12. | Matsushita Y, Nakagawa T, Shinohara M, Yamamoto S, Takahashi Y, Mizoue T, Yokoyama T, Noda M. How can waist circumference predict the body composition? Diabetol Metab Syndr. 2014;6:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Camhi SM, Bray GA, Bouchard C, Greenway FL, Johnson WD, Newton RL, Ravussin E, Ryan DH, Smith SR, Katzmarzyk PT. The relationship of waist circumference and BMI to visceral, subcutaneous, and total body fat: sex and race differences. Obesity (Silver Spring). 2011;19:402-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 411] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 14. | Yatsuji S, Hashimoto E, Tobari M, Tokushige K, Shiratori K. Influence of age and gender in Japanese patients with non-alcoholic steatohepatitis. Hepatol Res. 2007;37:1034-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Kim KH, Oh SW, Kwon H, Park JH, Choi H, Cho B. Alcohol consumption and its relation to visceral and subcutaneous adipose tissues in healthy male Koreans. Ann Nutr Metab. 2012;60:52-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Fan JG, Farrell GC. VAT fat is bad for the liver, SAT fat is not! J Gastroenterol Hepatol. 2008;23:829-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Takahashi H, Ono M, Hyogo H, Tsuji C, Kitajima Y, Ono N, Eguchi T, Fujimoto K, Chayama K, Saibara T. Biphasic effect of alcohol intake on the development of fatty liver disease. J Gastroenterol. 2015;50:1114-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Traversy G, Chaput JP. Alcohol Consumption and Obesity: An Update. Curr Obes Rep. 2015;4:122-130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 314] [Cited by in RCA: 390] [Article Influence: 39.0] [Reference Citation Analysis (0)] |