INTRODUCTION
Eosinophilic esophagitis (EoE) also known as “asthma of the esophagus” is a chronic immune/antigen mediated disorder of the esophagus affecting both children and adults. It is a clinicopathologic disease characterized clinically by dysphagia and pathologically by esophageal eosinophilia. Diagnosis is made by 3 criteria: (1) symptoms of esophageal dysfunction; (2) presence of ≥ 15 eosinophils/high power field in at least 1 esophageal biopsy with few exceptions; and (3) eosinophilia limited to the esophagus, with exclusion of other possible causes of esophageal eosinophilia, including proton pump inhibitors (PPI) responsive esophageal eosinophilia[1].
EPIDEMIOLOGY
The disease is more common in Caucasian population with a male to female ratio of 3:1[2]. Eosinophilic esophagitis has also been seen in African Americans, Asians and Hispanic population. The disease is increasingly being recognized over the last few decades. The prevalence of EoE is currently as high as 50 patients per 100000 population in the United States and Europe[2]. The disease can affect both children and adults. In adults, it mostly affects middle aged men between the age of 30 and 50. Most of the patients with eosinophilic esophagitis have personal history of allergic disorders like bronchial asthma, allergic rhinitis, allergic conjunctivitis or food allergy.
PATHOGENESIS
Exposure of the esophagus to food and aeroallergens in genetically predisposed individuals may initiate the process of eosinophilic esophagitis although the exact mechanism is currently unknown[3]. Foods most commonly implicated in EoE are: Milk, egg, wheat, soy, peanuts, beans, rye and beef. Genome-wide association analysis (GWAS) suggested that CAPN14 at 2p23 locus is upregulated after epithelial exposure to interleukin (IL)-13[4]. Recently, epithelial-derived cytokine thymic stromal lymphopoietin (TSLP) gene at 5q22 locus has been identified as a candidate gene in a multicenter GWAS. There is an increased expression of TSLP in patients with EoE. TSLP activates dendritic cells (antigen presenting cells). Food allergen is initially recognized by antigen presenting cells which differentiate CD4 cells into TH1 cells and TH2 cells. TH1 cells secrete interferon-γ and transforming growth factor-β. TH2 cells secrete IL-4, IL-5 and IL-13. There is also single nucleotide polymorphism (SNP) in this TSLP receptor gene in male patients with EoE. This gene is found on the pseudoautosomal region on Xp22.3 and Yp11.3. This finding may explain increased prevalence of EoE in male patients. There is also a suggestion of second hit for the development of EoE. Toll-like receptor-3 (TLR-3) can recognize double-stranded RNA (found in some viruses) and can induce TSLP[5]. IL-5 is responsible for eosinophilic infiltration, growth and survival. Eosinophils secrete various inflammatory cytokines and chemokines including macrophage migration inhibitory factor, tumor necrosis factor, granulocyte-monocyte colony stimulating factors (GMCSF) and toxic granules[6]. Transforming growth factor β1 is a profibrotic molecule and helps in remodeling of the esophagus in EoE. This may explain esophageal luminal narrowing, stricture formation and dysmotility. Eotaxin-3 is a strong chemotactic agent for esophageal eosinophilia. A single-nucleotide polymorphism in the human eotaxin-3 gene was associated with disease susceptibility. IL-4 and IL-13 secreted by TH2 can stimulate eotaxin-3. In telomerase-immortalized esophageal squamous cells of EoE patients, IL-4 stimulated eotaxin-3 secretion was blocked by PPI - omeprazole and lansoprazole[7]. The may explain PPI responsiveness of esophageal eosinophilia. Twin and family studies suggest that there is not only increased prevalence of EoE in male sex but also in monozygotic twins and other family member[8].
PATHOLOGY
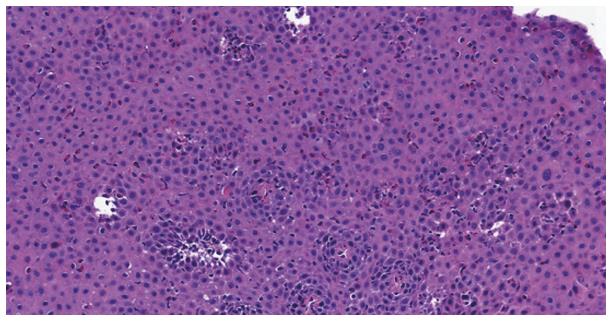
The major features (Figure 1) include infiltration of numerous eosinophils (usually > 15 per high power field) into the squamous epithelium, layering of eosinophils on the surface layer and eosinophilic microabscess formation (clusters of ≥ 4 eosinophils). Often necrotic squamous cells are also seen on the surface layer[9]. Minor features include chronic inflammatory infiltrate into the lamina propria with fibrosis of the lamina propria[10], hyperplasia of muscular layers and basal epithelial cells with lengthening of lamina propria papillae, and intercellular edema. One study showed plenty of IgG4-containing plasma cells in the lamina propria[11]. The pathological changes are patchy in distribution, and generally affect the whole length of the esophagus. None of the histologic findings is specific for eosinophilic esophagitis. Esophageal eosinophilia can be found in a variety of disorders including gastroesophageal reflux disease (GERD), proton pump responsive esophageal eosinophilia (PPI-REE), eosinophilic gastroenteritis, hypereosinophilic syndrome, Crohn’s disease, connective tissue diseases, drug hypersensitivity, parasitic and fungal infections and achalasia. In clinical practice, the real challenge comes to differentiate EoE from GERD and PPI-REE[12]. Eosinophilic degranulation is seen more profoundly in EoE than in GERD biopsy specimen[13]. In EoE, the eosinophilic inflammation extends beyond mucosa into the submucosa and muscularis propria.
Figure 1 HE staining from the same patient showing many eosinophils, dilated intercellular spaces and basal layer hyperplasia.
CLINICAL FEATURE
The major symptoms of eosinophilic esophagitis are solid food dysphagia and esophageal food impaction requiring endoscopic removal of food bolus as an emergency case[14]. In one study, EoE was found in 9% of all cases of esophageal food impaction[15]. Commonly, the diagnosis is suspected after a first episode of esophageal food impaction and biopsy showing esophageal eosinophilia. Less commonly, patients present with heartburn and chest pain mimicking gastroesophageal reflux disease. One study found that gender was an important factor in the initial clinical presentation of eosinophilic esophagitis. Men presented with dysphagia and esophageal food impaction more commonly than women. Women presented with heartburn and chest pain more commonly than men[16]. Diffuse narrowing of the esophageal lumen has been seen in clinical practice as a result of chronic inflammation and fibrosis. Esophageal mucosa is friable and esophageal perforation has been reported during endoscopic esophageal foreign body removal and during esophageal stricture dilation[17]. As aeroallergens play an important role in the pathogenesis, eosinophilic esophagitis was diagnosed more frequently when the environmental pollen counts (grass, trees and weeds) are high; the highest percentage of EoE occurred in the Spring and the lowest percentage in the Winter[18]. Another study showed symptomatic esophageal eosinophilia was diagnosed more frequently in the December/January and May/June periods[19].
INVESTIGATIONS
Lab tests
There is no single Lab test which can support the diagnosis of EoE. Mild peripheral eosinophilia may or may not be present. Peripheral eosinophilia, elevated serum eosinophil-derived neurotoxin and eotaxin-3 (CCL26) may have the potential to act as a biomarker for monitoring EoE[20].
Endsocopy
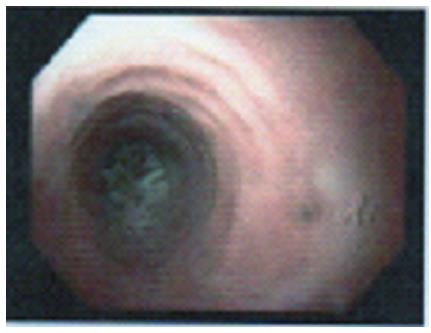
The esophageal mucosa may look normal in 7% to 10% of cases of EoE[21]. A variety of non-specific features of inflammation can be seen in EoE during endoscopy. The five major endoscopic features of EoE as per EoE endoscopic reference score (EREFS) are edema, rings (Figure 2), exudates, furrows and strictures[22]. Edema is identified by loss of vascular markings and mucosal pallor. Transient concentric rings or trachealization may indicate esophageal longitudinal muscle contraction[23] and fixed rings may indicate fibrous stricture formation due to tissue remodeling. Exudates or white spots or white plaques may mimic candida esophagitis, histologically they are eosinophilic microabscesses. Furrows are vertical lines running parallel to the axis of the esophagus probably due to epithelial edema. Chronic eosinophilic esophagitis may lead to long segment or short segment stricture. Narrow-caliber esophagus due to luminal narrowing of most of the esophagus is infrequently seen in EoE. Crepe paper esophagus occurs due to esophageal mucosal fragility and is recognized by a mucosal tear that occurs during passage of a diagnostic endoscope but neither during endoscope withdrawal nor after esophageal dilation. Although more than one of the above endoscopic findings can be seen in the same patient, none of them is specific for EoE. Recently, esophageal “pull” sign (substantial resistance and mucosal tenting during pulling of the biopsy forcep) was found to be highly specific and responsive to successful therapy in EoE patients[24].
Figure 2 Esophageal rings with food bolus impaction in esophagus.
Current recommendation is to take at least 2 to 4 biopsies both proximal and distal halves of esophagus (5 cm above GE junction) and also to take targeted biopsies from abnormal mucosa, i.e., exudates, rings, edema, furrows and strictures. Gastric and duodenal biopsies should also be taken to evaluate eosinophilic gastroenteritis.
BARIUM SWALLOW
Imaging studies are generally not done to diagnose EoE. Barium swallow may show normal esophagus. Sometimes featureless narrow-caliber esophagus, ringed esophagus, and isolated esophageal stricture are seen in EoE. But none is pathognomonic of EoE.
ESOPHAGEAL MANOMETRY
Generally normal peristalsis is seen in EoE. Prolonged esophageal manometry and pH-metry showed ineffective esophageal peristalsis in children with EoE[25]. Twenty-four hours pH study would be normal in EoE unless there is coexistent GERD.
ECHOENDOSCOPY
Echoendoscopy may show hypoechogenesity and thickening of all the layers of the esophageal wall due to inflammation and edema[26].
MANAGEMENT
Firm diagnosis of EoE is essential before offering any treatment. The three most common differential diagnoses of esophageal eosinophilia include EoE, PPI-REE and GERD. EoE and PPI-REE can be indistinguishable clinically, endoscopically and pathologically. So repeat endoscopy with biopsy after an eight weeks trial of twice daily PPI is essential to differentiate EoE from PPI-REE. Esophageal eosinophilia will persist in EoE. In suspected cases, 24 h pH study should be done to exclude GERD. In EoE, tissue eosinophilia is restricted to the esophagus in contrast to eosinophilic gastroenteritis.
Currently, drugs, diet and dilation are the three main modalities of treatment of EoE.
DRUGS
Topical corticosteroids
Have become the first line medications for the treatment of EoE[27]. Fluticasone metered dose inhaler 880 microgram puffed directly into the mouth without breathing and then dry swallowed twice a day for 6 wk has been found to be effective in reducing symptoms and esophageal eosinophilia[28]. Patients are advised not to take any food or drink or rinse their mouth for half an hour to prevent the medication from washing off the esophageal mucosa. The maximal anti-inflammatory effect is found in proximal esophagus. Oral viscous budesonide (OVB) 1 mg twice a day also decreases dysphagia and esophageal eosinophilia. OVB is easy to swallow, more mucoadherent and is made by mixing aqueous solution of budesonide (1 mg/2 mL) with the sugar substitute sucralose (5 g), chocolate syrup or honey[29]. Both forms of topical corticosteroids are more effective in histologic improvement than symptomatic improvement. Only 1% of the topical steroid is absorbed, so systemic side effects are extremely rare although oral and esophageal candidiasis can occur in up to one third of the time and herpes simplex esophagitis have been reported rarely.
Topical steroid is generally given for 8 wk. If that fails, prolonged or higher doses of topical steroids or systemic steroids or dietary treatment or esophageal dilation should be tried to get symptomatic improvement.
SYSTEMIC STEROIDS
Oral methylprednisolone induced marked clinical and histological improvement in pediatric EoE patients[30]. Because of systemic side effects, this therapy is reserved when other therapeutic interventions fail.
Steroids work by reducing the synthesis of eotaxin-3, IL-5 and GMCSF, and inducing the apoptosis of eosinophils. But recurrence of the EoE occurs after withdrawal of the steroids.
IMMUNOMODULATORS
Azathiopurine and 6-mercaptopurine induced and maintained clinical and histological remission in steroid dependent EoE patients in a case series[31]. They are not currently recommended for routine clinical use in EoE.
MAST CELL STABILIZERS
In a small case series, Cromolyn sodium failed to show any clinical or histologic improvement in EoE patients[32].
LEUKOTRIENE INHIBITORS
Montelukast is an eosinophil stabilizing agent. It improved clinical symptoms in EoE but there was no histological improvement[33].
IL-5 ANTIBODY
Anti-IL-5 antibody has been studied in both pediatric and adult patients with EoE. Mepolizumab significantly reduced esophageal eosinophilia but there was minimum symptomatic improvement[34]. Reslizumab also improved esophageal eosinophilia in EoE but there was no difference in clinical improvement in comparison to placebo[35].
IL-13 ANTIBODY
Anti-IL-13 monoclonal antibody QAX576 was studied in a small number of patients with EoE. There was a significant and sustained decrease of intraepithelial esophageal eosinophil count and a tendency towards clinical improvement[36].
DIET
Dietary therapy is very effective in the management of EoE. It can be used as an initial therapy or when other modalities of treatments fail. Dietary therapy depends on the resources available and can be expensive. As the dietary food allergen is removed, dietary therapy is very effective in inducing and maintaining clinicopathological remission. The three ways of dietary modification include: (1) elemental diet: Amino acid based formula to remove food allergens. This therapy when given for a minimum of 6 wk did both symptomatic and histologic improvement (95%-98%) in EoE patients[37]. But the amino acid formula is expensive and unpalatable and affects patients’ quality of life; and (2) six-food group elimination diet (SFGED): The most common food allergens in EoE include milk, egg, wheat, soy, peanuts/tree nuts and sea food (fish/shellfish). Significant clinical and histological (74%) improvement occurred in EoE patients (children) when they were on this SFGED[38]. Another study showed four-food group elimination diet (FFGED) which includes milk, egg, wheat and legumes, when given for 6 wk, clinicopathological remission occurred in 54% of adult EoE patient[39]. Targeted or tailored elimination diet: This therapy is guided by detection of food allergens by skin prick/patch tests and blood tests. These tests can be not only time consuming but also can give false positive and false negative results. This therapy is offered as per the preference of the patient. Sixty-eight percent of EoE patients had symptomatic improvement on targeted therapy[40].
A dietitian interested in food allergies and EoE should be consulted. An Allergist should also be involved to find out the allergens triggering EoE. Food challenge by introducing one food or food group every 4 to 6 wk should be offered. If the patient is allergic to food, there will be recurrence of symptoms and esophageal eosinophilia[41].
ENDOSCOPIC TREATMENT
Esophageal dilation has definitive role in the management of EoE. It is indicated if the patients do not respond to pharmacological or dietary therapy. It is also very effective in symptomatic esophageal stricture (esophageal diameter < 10 mm), long segment narrowing and narrow caliber esophagus. This modality of treatment improves dysphagia and quality of life but does not reduce esophageal eosinophilia[42]. Either hydrostatic balloon dilation or wire guided bougie dilation can be done. Esophageal diameter should be 15 to 18 mm to relieve dysphagia. Patients may need multiple sessions to achieve this. There is an increased risk of mucosal tear causing post-dilation chest pain for several days[43]. Although initially thought that EoE patients carry higher risk of perforation after esophageal dilation, systematic review did not show any higher risk of perforation (0.1%) in these group of patients[44].
PROGNOSIS
As mentioned earlier, EoE is a chronic inflammatory disease of the esophagus. The inflammation leads to remodeling, fibrosis and stricture. Fortunately, no case of esophageal malignancy has been reported in EoE. Patients are generally diagnosed after several years of their symptoms. Although symptomatic improvement occurs after treatment, recurrence is common after discontinuation of treatment. So maintenance therapy is needed to prevent recurrences. At the present time there is no head to head study to suggest the best maintenance treatment. Continuation of swallowed corticosteroid and/or dietary therapy should be done in all EoE patients particularly in those with history of food impaction, dysphagia, esophageal stricture, and in those with rapid symptomatic and histologic relapse following initial treatment[45].
SUMMARY
EoE has become a common clinical entity in patients with dysphagia and esophageal food impaction. Although the disease is more common in young male patients with allergic disorders, any person can get affected. High degree of suspicion is essential to diagnose this disease. So multiple proximal and distal esophageal biopsies should be taken in EoE suggestive mucosa (EREFS) and even in normal looking mucosa. Other causes of esophageal eosinophilia particularly GERD, PPIREE, eosinophilic gastroenteritis and hypereosinophilic syndrome should be excluded. Patients should be referred to the dietitians interested in food allergies and EoE patients. Patients with endoscopic findings of edema, exudates and furrows should be given topical corticosteroids for 6 to 8 wk. If there is no clinicoopathological improvement, esophageal dilation should be offered. Esophageal dilation followed by topical corticosteroid therapy should be offered to patients with esophageal rings, strictures and narrow caliber lumen. Lowest effective dose of topical corticosteroid should be continued to all EoE patients as maintenance therapy to reduce progression of the disease and relapse.
Macrophage migration inhibitory factor (MIF) is overexpressed in the esophageal mucosa of EoE patients. Recently, in the mice model of EoE, early administration a drug that blocked the action of MIF prevented eosinophilic infiltration in the esophagus. This study can lead to a novel therapy in future if MIF effect can be blocked in EoE patients.
P- Reviewer: Fabozzi M, Peng SY, Zhu X S- Editor: Qi Y L- Editor: A E- Editor: Wu HL