Published online Feb 6, 2016. doi: 10.4292/wjgpt.v7.i1.162
Peer-review started: April 16, 2015
First decision: July 1, 2015
Revised: November 13, 2015
Accepted: December 1, 2015
Article in press: December 2, 2015
Published online: February 6, 2016
Processing time: 290 Days and 2.1 Hours
AIM: To systematically review economic evaluations in gastroenterology, relating to Brazil, published between 1980 and 2013.
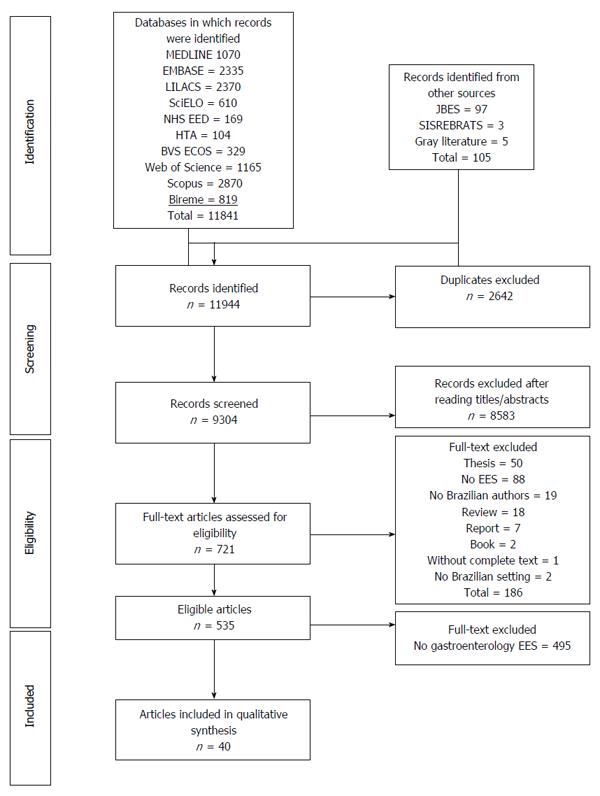
METHODS: We selected full and partial economic evaluations from among those retrieved by searching the following databases: MEDLINE (PubMed); Excerpta Medica; the Latin American and Caribbean Health Sciences Literature database; the Scientific Electronic Library Online; the database of the Centre for Reviews and Dissemination; the National Health Service (NHS) Economic Evaluation Database; the NHS Health Technology Assessment database; the Health Economics database of the Brazilian Virtual Library of Health; Scopus; Web of Science; and the Brazilian Network for the Evaluation of Health Technologies. Two researchers, working independently, selected the studies and extracted the data.
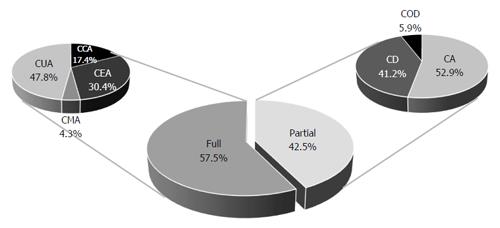
RESULTS: We identified 535 health economic evaluations relating to Brazil and published in the 1980-2013 period. Of those 535 articles, only 40 dealt with gastroenterology. Full and partial economic evaluations respectively accounted for 23 (57.5%) and 17 (42.5%) of the 40 studies included. Among the 23 full economic evaluations, there were 11 cost-utility analyses, seven cost-effectiveness analyses, four cost-consequence analyses, and one cost-minimization analysis. Of the 40 studies, 25 (62.5%) evaluated medications; 7 (17.5%) evaluated procedures; and 3 (7.5%) evaluated equipment. Most (55%) of the studies were related to viral hepatitis, and most (63.4%) were published after 2010. Other topics included gastrointestinal cancer, liver transplantation, digestive diseases and hernias. Over the 33-year period examined, the number of such economic evaluations relating to Brazil, especially of those evaluating medications for the treatment of hepatitis, increased considerably.
CONCLUSION: Further studies are needed in order to ensure that expenditures on health care in Brazil are made as fairly and efficiently as possible.
Core tip: The volume and scope of economic evaluations relating to Brazil remain unknown. To improve understanding of what studies are available as inputs for resource-allocation decisions, as well as of how that body of knowledge can be expanded, we conducted a systematic review of such economic evaluations. Although there have been many economic evaluations related to gastroenterology in Brazil, most have analyzed medications for the treatment of viral hepatitis. In most cases, decisions to incorporate new technologies into the public health care system were made before such studies were conducted and were therefore not based on local cost-effectiveness analyses.
- Citation: de Paiva Haddad LB, Decimoni TC, Turri JA, Leandro R, de Soárez PC. Economic evaluations in gastroenterology in Brazil: A systematic review. World J Gastrointest Pharmacol Ther 2016; 7(1): 162-170
- URL: https://www.wjgnet.com/2150-5349/full/v7/i1/162.htm
- DOI: https://dx.doi.org/10.4292/wjgpt.v7.i1.162
In addition to safety and efficacy, economic considerations have increasingly been taken into account in decisions regarding the use of health care technologies. Health economic evaluations typically draw comparisons between and among alternative treatments, devices and health programs, in terms of their costs and consequences. Such studies analyze data regarding clinical effectiveness, in relation to cost. Although most health economic evaluations examine only the direct costs of a technology-those related to medications, services and hospitalization-some take a broader approach, also evaluating the indirect costs-those related to lost productivity (on the part of the patients and caregivers), as well as to other aspects[1].
In a number of countries, the decision-making process regarding the reimbursement for and incorporation of new technologies have been informed by health economic evaluations. In Brazil, the performance of such evaluations has been an obligatory part of the process of incorporating new technologies into the Brazilian Sistema Único de Saúde (SUS, Unified Health Care System), as mandated by Federal Law no. 12401, since 2011. However, health economic evaluations have been conducted in Brazil since the 1980s. One recent study demonstrated that, as of 2009, Brazil had produced more health economic evaluations than had any other country in South America[2].
In the field of gastroenterology, economic evaluations are performed in order to evaluate or compare new alternative medications, surgical techniques, diagnostic tests or procedures. Now-classic studies include that in which omeprazole was shown to be more cost-effective than ranitidine in the treatment of gastroesophageal reflux[3], as well as those demonstrating that it was more cost-effective to eradicate Helicobacter pylori (H. pylori) than to provide symptomatic treatment to H. pylori-positive patients with suspected peptic ulcer disease[4,5].
Knowledge of economic evaluations related to gastroenterology can help health care professionals make better choices from among the available technologies and can help researchers identify segments in which there is a need for further studies. It is incumbent upon researchers to identify technologies that have already been assessed and to understand the relationship between the emergence of new technologies (clinical or surgical) and the need for specific studies. Such knowledge also provides health care managers with additional input that can be used in the decision-making processes related to the incorporation of new technologies into a health care system.
The volume and scope of economic evaluations relating to gastroenterology in Brazil remain unknown. To gain a better understanding of what kind of research is available as input for resource-allocation decisions, as well as of how that body of knowledge can be expanded, we conducted a systematic review of such economic evaluations.
We conducted this review, which is specific to gastroenterology and to Brazil, in accordance with the guidelines for systematic review of economic evaluations published by the UK National Health Service (NHS) Centre for Reviews and Dissemination[6]. The methodology of the systematic review and the search strategy have been detailed elsewhere[7]. In brief, we searched multiple databases, including MEDLINE (PubMed); Excerpta Medica; the Latin American and Caribbean Health Sciences Literature database; the Scientific Electronic Library Online; the database of the Centre for Reviews and Dissemination; the NHS Economic Evaluation Database; the NHS Health Technology Assessment database; and Health Economics database of the Brazilian Virtual Library of Health. The last three sources are repositories of economic evaluations. We searched the citation indexes: Scopus; Web of Science; and the Brazilian Network for the Evaluation of Health Technologies, a bibliographic database of Brazilian health technology assessment studies. We also performed hand searches of the Brazilian Journal of Health Economics, which is not indexed for any of the previously mentioned databases. All searches were limited to the 1980-2013 period.
Articles were included if they were partial or full economic evaluations, according to the classification devised by Drummond et al[8], if they dealt with gastroenterology, were conducted in or related to Brazil, and if at least one of the authors was affiliated with an institution in Brazil. We defined partial economic evaluations as those that examined only costs (cost description studies), described the costs of a particular disease to society (cost-of-illness studies), described the costs and consequences of a single service or program (cost-outcome description studies), or compared two or more interventions only in terms of their costs (cost analyses). Studies were considered full economic evaluations if they compared the costs and consequences of two or more health care interventions or alternatives, designs including cost-consequences analysis, cost-minimization analysis, cost-effectiveness analysis, cost-utility analysis and cost-benefit analysis.
The titles and abstracts of identified citations were screened for relevance by two independent reviewers. The full texts of “relevant” and “potentially relevant” articles were retrieved and evaluated independently by both reviewers. From each of the selected studies, two reviewers independently extracted data on the year and journal of publication; type of economic evaluation; the category of technology assessed (medications, vaccines, equipment, clinical practices, surgical techniques, diagnostic procedures, public health programs or health promotion programs); the purpose of the technology assessed (treatment, prevention, screening or diagnosis); the category of health problem studied (according to the tenth revision of the International Classification of Diseases); the type of affiliation of the first author (academia, government, research institute, health organization, consultancy, pharmaceutical industry, equipment industry or international body); the geographical location of the first author; and conflicts of interest, as defined by Valachis et al[9]. Disagreements regarding the extracted data were resolved by consensus or through consultation with a third reviewer. We summarized the characteristics of the selected articles and evaluated them using narrative synthesis. The statistical review of the study was performed by a biomedical statistician.
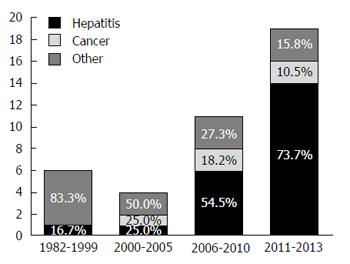
We identified 535 health economic evaluations relating to Brazil and published in the 1980-2013 period (Figure 1). Of those 535 articles, only 40 dealt with gastroenterology, the first of those having been published in 1982. Partial and full economic evaluations respectively accounted for 17 (42.5%) and 23 (57.5%) of the articles selected. Of the 23 full evaluations, 11 were cost-utility analyses, seven were cost-effectiveness analyses, four were cost-consequences analyses and one was a cost-minimization analysis. Of the 17 partial evaluations, nine were cost analyses, seven were cost-description studies, and one was a cost-outcome description study (Figure 2). Over the period under study, there was an increase in the frequency of such economic evaluations (Figure 3), five articles being published in the 1980s, only one being published in the 1990s, 14 being published in the 2000s and 19 being published after 2010.
The majority (90%) of the selected studies evaluated technologies that are employed in the treatment of diseases of the gastrointestinal tract. There were two studies evaluating diagnostic technologies and one evaluating a technology for the prevention of viral hepatitis. Twenty-five studies evaluated medications; seven evaluated clinical or surgical procedures; three evaluated equipment; and one evaluated vaccines. There were four studies that evaluated more than one technology simultaneously.
The first authors of the selected articles had the following types of affiliations: Academia, in 33 studies; health care facilities, in four; consultancy, in two; and industry, in one. There were only four articles in which the authors declared conflicts of interest, all related to funding provided by industry or consultancy sources. However, when we evaluated the articles using the criteria proposed by Valachis et al[9], we identified conflicts of interest in four other studies. In three of those four articles, the authors had declared no conflicts, whereas the remaining article contained no conflict of interest statement. According to the published statements of financial support, six of the 40 studies received financial support from industry sources, seven received financial support from funding agencies, and two received no funding from external sources. The remaining 25 articles contained no information regarding financial support.
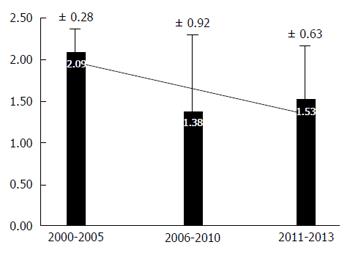
Of the 40 institutions at which the studies were conducted, 30 were located in Southeastern Brazil, nine were located in Southern Brazil and one was located in Northeastern Brazil. The majority (67.5%) of the selected studies were published in national journals. For the period evaluated, as a whole, the mean impact factor of the journals in which the studies were published was 1.57 ± 0.66. For the 2000-2005 period, the mean impact factor was 2:09, compared with 1.38 for the 2006-2010 period and 1.53 for the 2011-2013 period (Figure 4).
We analyzed the type of disease addressed in the economic evaluations under study. On that basis, we divided the studies into three main groups, by topic (Figure 3): Gastrointestinal cancer; viral hepatitis; and other.
We identified five economic evaluations related to gastrointestinal cancer: Four related to colorectal cancer (three dealing with the metastatic form); and one related to esophageal cancer. All five studies evaluated treatments, and three were full economic evaluations. Funding from the pharmaceutical industry was reported in only one of the five studies.
The first of the five studies was published in 2001 and evaluated palliative treatment for advanced esophageal cancer, comparing the costs of using a self-expanding metallic stent with those of esophageal bypass[10]. The authors concluded that endoscopic treatment of dysphagia was as effective as was surgical bypass and could be performed at a lower cost.
A cost-description study, published in 2010, evaluated the costs of hospitalizations related to colorectal cancer in Brazil, drawing upon data obtained from the Information Technology Department of the Brazilian Unified Health Care System[11] The study showed that such costs increased from US$16.5 million in 1996 to US$33.5 million in 2008.
The first of the three economic evaluations of metastatic colorectal cancer in Brazil, published in 2008, was a partial evaluation, a cost analysis comparing the various first-line chemotherapy treatments. The authors of that study concluded that chemotherapy regimens containing capecitabine, especially capecitabine plus oxaliplatin, are less expensive than are those containing 5-fluorouracil and leucovorin[12]. The next of those studies, published in 2012, also compared first-line chemotherapy treatments for metastatic colorectal cancer[13]. That study was a cost-effectiveness analysis comparing 5-fluorouracil plus leucovorin, followed by irinotecan, vs oxaliplatin, 5-fluorouracil and leucovorin, followed by irinotecan, 5-fluorouracil and leucovorin. The two strategies provided estimated gains of 0.17 and 0.91 life-years (LYs), respectively, in comparison with support, the incremental cost-effectiveness ratios (ICERs), in Brazilian reals (R$), being R$50504 and R$73626, respectively, per LY gained. When comparing the new and previous strategies, the authors found the gain to be 0.74 LYs, with an ICER of R$78188 per LY gained[13].
The last of the studies dealing with the treatment of metastatic colorectal cancer was a cost-utility analysis comparing two chemotherapy regimens, modified 5-fluorouracil, leucovorin and oxaliplatin (mFLOX) and modified 5-fluorouracil, folinic acid and oxaliplatin (mFOLFOX6). Over a 20-wk period of treatment, the cost of the mFLOX regimen was R$9000, compared with R$22000 for the mFOLFOX6 regimen. The effective gain for the mFOLFOX6 regimen was 0.117 QALYs, with an ICER of R$110344 per QALY gained[14].
Of the 40 studies evaluated, 19 dealt with technologies related to the treatment of viral hepatitis. Those studies were published more recently phase, the first in 2007 and the majority (14 of the 19) after 2010. Of those 19 studies, 14 evaluated medications for the treatment of hepatitis, one evaluated a vaccine, one evaluated a piece of diagnostic equipment and three evaluated multiple technologies (combinations of procedures, medications and diagnostic equipment). Most of the studies (15 of the 19) were full economic evaluations. Eleven of the articles dealt with hepatitis C, and eight dealt with hepatitis B.
The study published in 2007 was a cost-of-illness study showing that, if hepatitis B was left untreated, the related health care expenditures rose in parallel with advances in the stage of the disease[15]. Of the studies evaluated, the first to evaluate the treatment of hepatitis B was a cost-utility analysis, published in 2008, comparing lamivudine and entecavir[16]. The authors concluded that entecavir reduced the incremental cost per QALY gained, making it the more cost-effective option for the treatment of hepatitis B. A cost-effectiveness analysis published in 2011 compared interferon and lamivudine[17], the result being that interferon proved to be the more cost-effective therapeutic option for the treatment of hepatitis B. Another economic evaluation of hepatitis B treatments compared the cost-effectiveness of telbivudine with that of lamivudine[18], showing that the latter was more cost-effective. Studies evaluating the use of nucleoside analogues as treatments for hepatitis B in Brazil began to be published in 2012[19], evaluating the cost-effectiveness of entecavir and tenofovir. Only one of those studies was a cost-utility analysis, the findings of which were favorable to tenofovir[20]. However, a cost-effectiveness analysis, published in the same year, showed that lamivudine was the more cost-effective option[21].
Of the 11 economic evaluations related to hepatitis C in Brazil, the first, published in 2008, analyzed the cost-effectiveness of diagnostic methods[22]. The first such article dealing with treatment, published the following year, evaluated the cost-effectiveness of treatment with pegylated interferon (peginterferon) alpha-2b in comparison with that of ribavirin[23]. There were a number of subsequent economic evaluations related to the treatment of hepatitis C in Brazil, all of which were based on the use of peginterferon[24-29]. In all of those studies, treatment with peginterferon proved cost-effective. A budget impact analysis, published in 2011, evaluated the treatment of hepatitis C in patients who are candidates for kidney transplantation[25]. In 2012, Blatt et al[27] published a study evaluating the use of ribavirin in combination with interferon alpha, peginterferon alpha-2a or peginterferon alpha-2b, comparing the three combinations in terms of the microeconomics of their use in the treatment of hepatitis C[27]. In addition, there were two studies that analyzed the cost-effectiveness of including hepatitis C patients who are slow responders to treatment with peginterferon, both studies showing that to be a cost-effective strategy for the public health care system in Brazil[28,29].
The first economic evaluations related to gastroenterology in Brazil, published in the 1980s, dealt with therapeutic approaches to epigastric hernia[30] and peptic ulcers[31,32]. More recently, there were three economic evaluations that dealt with liver transplantation[33-35]. Of those three studies, two were cost-description studies based on retrospective analyses of medical records; and one was a cost analysis comparing the costs of living donor liver transplantation with those of deceased donor liver transplantation[34]. The remaining studies were related to a variety of topics: Inflammatory bowel disease[36,37]; colonic diseases[38,39]; the equipment used in patients undergoing colostomy or ileostomy[40]; appendicitis[41]; and peritonitis[42]. There was only one study comparing the costs of surgical procedures-laparoscopic vs conventional appendectomy[41]. As would be expected, the authors found that the minimally invasive (laparoscopic) procedure had a higher cost. One study, comparing the costs of nutritional therapy provided in the hospital with those of that provided in the home environment[43], showed that the home therapy model allowed a savings of US$3132 per patient.
In various countries, economic evaluation has become an integral part of the decision-making processes related to the incorporation of and reimbursement for the use of new technologies, primarily new medications. In gastroenterology, the medications with the highest costs are chemotherapy agents used for the treatment of gastrointestinal cancer, antiviral agents used for the treatment of hepatitis and immunosuppressants used in solid organ transplant recipients. Therefore, it is expected that these technologies would give rise to further studies seeking evidence to support the incorporation of these high-cost medications into the public health care system and to justify their use in the private system.
In Brazil, the reported prevalence of hepatitis C in the general population is 0.28%-1.42%[44], and 75%-85% of all cases progress to the chronic form. The successful treatment of hepatitis C (achieving a sustained virological response) is associated with a better prognosis, reducing the rate of evolution to end-stage liver disease and consequently the need for liver transplantation[29]. Initially, the treatments available for hepatitis C were based on the use of conventional interferon in combination with ribavirin. With the introduction of peginterferon and the advent of studies demonstrating its greater efficacy[27]. Because it is an expensive medication, petitions were filed with the judicial system in Brazil, seeking public funding for its use. In 2006, peginterferon alpha-2 was the fifth most requested medication through such petitions[45]. Soon thereafter, the use of peginterferon alpha-2 was incorporated into the standard practices of the SUS. Although economic evaluations of treatment with peginterferon alpha-2 in Brazil were published only after its incorporation into the standard practices of the public health care system, those studies have shown that it is cost-effective at the national level.
Treatment of hepatitis C with the protease inhibitors telaprevir and boceprevir has now also been incorporated into the standard practices of the SUS. Triple therapy-the association of one of those protease inhibitors with peginterferon and ribavirin-is becoming the standard treatment for viral hepatitis, further increasing the cost of treatment. To our knowledge, there have as yet been no analyses of the cost-effectiveness of this new treatment option in Brazil.
In the field of oncology in Brazil, we observed a predominance of publications related to chemotherapy in patients with metastatic colorectal cancer, as was expected, given the prevalence of the disease. Colorectal cancer is the second most prevalent type of cancer worldwide. In Brazil, approximately 30000 new cases are diagnosed each year, and 24% of those cases are already metastatic at diagnosis[46]. The use of the various chemotherapy regimens, all aimed at increasing survival in such patients, often results in a significant increase in costs. Oncology studies have attempted to determine the most cost-effective options for the treatment of metastatic colorectal cancer to be incorporated into the standard practices of the SUS.
In Brazil, chemotherapy is provided at hospitals and accredited oncology clinics, which provide the medications to the patients and are subsequently reimbursed by the SUS. Beginning in 2010, the reimbursement values for the treatment of metastatic colorectal cancer were revised and new therapeutic regimens were incorporated[13]. It then became possible to prescribe regimens such as FOLFOX and the combination of folinic acid, 5-fluorouracil and irinotecan performed were after that date, comparing the new arrangements with existing ones. It is unlikely that the decision to incorporate the new treatments was based on local cost-effectiveness analyses, given that there were no economic evaluations of such treatments published before 2010.
The studies on the costs of liver transplantation in Brazil identified here were partial economic evaluations. Transplantation is the only therapeutic option for the treatment of end-stage liver disease. It is highly complex procedure, associated with high hospital costs, implying high costs to the public health care system. The economic evaluations performed were the basis for setting the reimbursement values for this procedure.
The remaining studies were heterogeneous in their focus and reflect the evolution of technologies, from the initial studies for the treatment of peptic ulcer to more recent studies evaluating minimally invasive therapies.
Although there have been a significant number of economic evaluations related to gastroenterology in Brazil, most have focused on the analysis of medications for the treatment of viral hepatitis. In addition, a temporal evaluation of the implementation of the studies showed that these were published after the incorporation of those technologies into the public health care system, which should, ideally, be based on prior economic evaluation.
In the field of gastroenterology, there are currently a number of new technologies that merit evaluation in order to shape the direction of future investments. Minimally invasive surgery is a rapidly expanding area and involves the use of expensive, increasingly specialized, equipment, including the use of robotics. With increasing frequency, such equipment is employed in simple procedures such as cholecystectomy and hernia surgery, although its use is advancing at a more rapid pace in procedures that are more complex and costly, such as cancer and liver surgery.
Another area in which there is a lack of economic evaluations is that of endoscopy. Endoscopic procedures are no longer used solely for diagnosis, having a variety of therapeutic applications, such as the resection of polyps and the palliative treatment of cancer. To our knowledge, there have been no economic evaluations is the field of endoscopy in Brazil.
Therefore, it is evident that, although there have been a number of economic evaluations related to gastroenterology in Brazil, there should be a push for further studies of this kind, so that expenditures on health care in Brazil are made as fairly and efficiently as possible.
Economic evaluations in gastroenterology evaluate or compare medications, surgical techniques, diagnostic tests or procedures. The volume and scope of economic evaluations relating to gastroenterology in Brazil remain unknown.
New technologies in the field of gastroenterology, such as minimally invasive surgery (including the use of robotics), merit detailed evaluation in terms of their cost-effectiveness in simple procedures, such as cholecystectomy and hernia surgery, as well as in those that are more complex and costly, such as surgical procedures used in the treatment of cancer and liver disease.
The authors identified certain trends among economic evaluations related to gastroenterology in Brazil, most of which have focused on the analysis of medications for the treatment of viral hepatitis. In addition, a temporal evaluation of the implementation of the studies showed that many were published after the incorporation of those technologies into the public health care system, which should, ideally, be based on prior economic evaluation. Furthermore, studies on the costs of liver transplantation in Brazil appear to have been predominantly partial, rather than full, economic evaluations. Moreover, although triple therapy-the combination of a protease inhibitor with peginterferon and ribavirin-is becoming the standard treatment for viral hepatitis in Brazil, there have as yet been no analyses of the cost-effectiveness of this new treatment option in the country.
The data should prompt researchers in Brazil to conduct additional economic evaluations related to gastroenterology, attempting to bridge the knowledge gaps that exist at the present time.
The authors considered two broad categories of economic evaluations, partial and full. Partial economic evaluations were designated “cost description studies” (those that examined only costs), “cost-of-illness studies” (those that described the costs of a particular disease to society), “cost-outcome description studies” (those that described the costs and consequences of a single service or program) or “cost analyses” (those that compared two or more interventions only in terms of their costs). Full economic evaluations (those that compared the costs and consequences of two or more health care interventions or alternatives) were designated “cost-consequences analyses”, “cost-minimization analyses”, “cost-effectiveness analyses”, “cost-utility analyses”, or “cost-benefit analyses”.
The manuscript investigates the economic evaluations in gastroenterology in Brazil. The authors tried to bring a reference to the decision-making processes in gastroenterology by analyses the cost-effectiveness of different treatments.
P- Reviewer: Guo YM S- Editor: Ji FF L- Editor: A E- Editor: Wang CH
| 1. | Drummond MF. Economic evaluation of treatment strategies in gastroenterology. Am J Gastroenterol. 2005;100:2143-2145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 2. | Augustovski F, Iglesias C, Manca A, Drummond M, Rubinstein A, Martí SG. Barriers to generalizability of health economic evaluations in Latin America and the Caribbean region. Pharmacoeconomics. 2009;27:919-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 3. | Kleinman L, McIntosh E, Ryan M, Schmier J, Crawley J, Locke GR, De Lissovoy G. Willingness to pay for complete symptom relief of gastroesophageal reflux disease. Arch Intern Med. 2002;162:1361-1366. [PubMed] |
| 4. | Ladabaum U, Fendrick AM, Glidden D, Scheiman JM. Helicobacter pylori test-and-treat intervention compared to usual care in primary care patients with suspected peptic ulcer disease in the United States. Am J Gastroenterol. 2002;97:3007-3014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Ford AC, Delaney BC, Forman D, Moayyedi P. Eradication therapy in Helicobacter pylori positive peptic ulcer disease: systematic review and economic analysis. Am J Gastroenterol. 2004;99:1833-1855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 115] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 6. | Centre for Reviews and Disseminations. Systematic reviews of economic evaluations. CRD’ guidance for undertaking reviews in health care: Centre for Reviews and Disseminations. University of York 2009; . |
| 7. | Decimoni TC, Leandro R, Soarez PC, Craig D. Systematic Review of Economic Evaluation of Health Technologies Developed In Brazil From 1980-2013. Value Health. 2014;17:A438. |
| 8. | Drummond MF, Sculpher M, Torrance G, O’Brien B. Methods for the Economic Evaluation of Health Care Programmes. Third edition. : Oxford University Press 2005; . |
| 9. | Valachis A, Polyzos NP, Nearchou A, Lind P, Mauri D. Financial relationships in economic analyses of targeted therapies in oncology. J Clin Oncol. 2012;30:1316-1320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Hourneaux G, de Moura E, Sakai P, Cecconello I, Ishioka S. [Palliative treatment of advanced esophageal cancer. Comparative study: auto-expandable metal stent and isoperistaltic esophagogastric bypass]. Acta Gastroenterol Latinoam. 2001;31:13-22. [PubMed] |
| 11. | Torres Udos S, Almeida TE, Netinho JG. Increasing hospital admission rates and economic burden for colorectal cancer in Brazil, 1996-2008. Rev Panam Salud Publica. 2010;28:244-248. [PubMed] |
| 12. | Caponero R, Ribeiro Rde A, Santos E, Cirrincione A, Saggia M. Medical resource use and cost of different first-line treatments for metastatic colorectal cancer in Brazil. J Med Econ. 2008;11:311-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | de Carvalho AC, Sasse EC, Sasse AD. Cost-effectiveness analysis of FOLFOX followed by FOLFIRI versus 5-FU/LV followed by irinotecan in patients with metastatic colorectal cancer within the Unified Health Care System of Brazil. In Portuguese. J Bras Econ Saúde. 2012;4:413-419. |
| 14. | Mak M. Modified FLOX as first-line chemotherapy for metastatic colorectal cancer patients in the public health system in Brazil: Effectiveness and cost-utility analysis. Mol Clin Oncol. 2013;1:175-179. [PubMed] |
| 15. | Castelo A, Pessôa MG, Barreto TC, Alves MR, Araújo DV. [Cost estimates of chronic hepatitis B virus for the Brazilian unified health system in 2005]. Rev Assoc Med Bras. 2007;53:486-491. [PubMed] |
| 16. | Costa AM, L ‘italien G, Nita ME, Araujo ES. Cost-effectiveness of entecavir versus lamivudine for the suppression of viral replication in chronic hepatitis B patients in Brazil. Braz J Infect Dis. 2008;12:368-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Almeida AM, da Silva AL, Cherchiglia ML, Andrade EI, de Oliveira GL, Acurcio Fde A. Chronic hepatitis B treatment: the cost-effectiveness of interferon compared to lamivudine. Value Health. 2011;14:S24-S28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Wiens A, Venson R, Correr CJ, Pontarolo R. Cost-effectiveness of telbivudine versus lamivudine for chronic hepatitis B. Braz J Infect Dis. 2011;15:225-230. [PubMed] |
| 19. | Almeida AM, Silva AL, Brandão CM, Cherchiglia ML, Andrade EI, Araújo de Oliveira GL, Carmo RA, Acurcio Fde A. [Cost-effectiveness of nucleoside/nucleotide analogues in chronic hepatitis B]. Rev Saude Publica. 2012;46:942-949. [PubMed] |
| 20. | Wiens A, Lenzi L, Venson R, Pedroso ML, Correr CJ, Pontarolo R. Economic evaluation of treatments for chronic hepatitis B. Braz J Infect Dis. 2013;17:418-426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Mosegui G, Silva V, Mello MD. Assessment of Interferon, Peginterferon, Tenofovir, Entecavir, Lamivudine and Adefovir for the Treatment of Chronic Hepatitis B in HBEAG-Positive Patients Without Cirrhosis. Int J Pharm Pharm Sc. 2013;5:208-212. |
| 22. | Barreto AM, Takei K, E C S, Bellesa MA, Salles NA, Barreto CC, Nishiya AS, Chamone DF. Cost-effective analysis of different algorithms for the diagnosis of hepatitis C virus infection. Braz J Med Biol Res. 2008;41:126-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 23. | Fonseca MC, Araújo GT, Araújo DV. Cost effectiveness of peginterferon alfa-2B combined with ribavirin for the treatment of chronic hepatitis C in Brazil. Braz J Infect Dis. 2009;13:191-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 24. | Massad E, Coutinho FA, Chaib E, Burattini MN. Cost-effectiveness analysis of a hypothetical hepatitis C vaccine compared to antiviral therapy. Epidemiol Infect. 2009;137:241-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Silva F, Peregrino A, Costa-Couto MH. Treatment of hepatitis C infection in renal transplant candidates undergoing dialysis: budget impact on the SUS (Unified Health Care System of Brazil). In Portuguese. Physis RSC. 2011;21:437-448. |
| 26. | Mosegui G, Vianna C, Rodrigues M. Peginterferon alpha-2a and ribavirin versus peginterferon alpha-2b and ribavirin: cost-effectiveness and budget impact of the treatment of chronic hepatitis C, genotype 1. In Portuguese. Physis RSC. 2011;21:377-393. |
| 27. | Blatt CR, da Cunha Bernardo NLM, Rosa JA, Bagatini F, Alexandre RF, Neto GB, Siebert U, Farias MR. An Estimate of the Cost of Hepatitis C Treatment for the Brazilian Health System. Value in Health Regional Issues. 2012;1:129-135. [RCA] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | de Mello Vianna CM, Gonzalez Mosegui GB, Costa e Silva FV, Freitas Peregrino AA, da Silva Rodrigues MP, Nagib Jardim F. Economic evaluation of pegylated interferon alpha-2a in combination with ribavirin for the treatment of chronic infection with hepatitis C, genotype 1: comparison of 48 weeks of treatment and extended therapy. Value in Health Regional Issues. 2013;2:342-346. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 29. | Barros FM, Cheinquer H, Tsuchiya CT, Santos EA. Cost-effectiveness analysis of treatment with peginterferon-alfa-2a versus peginterferon-alfa-2b for patients with chronic hepatitis C under the public payer perspective in Brazil. Cost Eff Resour Alloc. 2013;11:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 30. | Pimenta LG, Silva AL. [Epigastric hernia (xipho-umbilical lipoma): classification, frequency, repercussion on work leave time and operating costs. Valorization of the outpatient surgical system]. In Portuguese. Rev Assoc Med Bras. 1983;29:162-165. |
| 31. | Castro LP, Souza ZP, Ribeiro TC. Financial costs of treating peptic ulcers. In Portuguese. Rev Assoc Med. 1986;37:26-29. |
| 32. | Verceze AV, Troncon L. Analysis of buffering power and concentration of sodium, and the cost of some antacids. In Portuguese. Medicina (Ribeirao Preto). 1982;15:211-216. |
| 33. | Coelho JCU, Weiderkehr M, Campos AC, Matias J, Campos G. Cost of liver transplantation at the Clinical Hospital of the (Brazilian) Federal University of Paraná. In Portuguese. Rev Assoc Med Bras. 2000;43:1-5. |
| 34. | Coelho JC, Trubian PS, Freitas AC, Parolin MB, Schulz GJ, Martins EL. Cost comparison of cadaveric liver transplantation with living-donor transplantation. Rev Assoc Med Bras. 2005;51:158-163. [PubMed] |
| 35. | Portela MP, Neri ED, Fonteles MM, Garcia JH, Fernandes ME. [The cost of liver transplantation at a university hospital of Brazil]. Rev Assoc Med Bras. 2010;56:322-326. [PubMed] |
| 36. | Kotze PG, Albuquerque IC, Moraes AC, Vieira A. Cost-minimization analysis of infliximab (IFX) versus adalimumab (ADA) in the treatment of Crohn’s disease (CD). In Portuguese. Rev Bras Coloproct. 2009;29:158-162. |
| 37. | Nishikawa AM, Paladini L, Delfini R, Kotze PG, Clark O. Decision tree construction and cost-effectiveness analysis of treatment of ulcerative colitis with pentasa® mesalazine 2 g sachet. Arq Gastroenterol. 2013;50:297-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 38. | Takemoto ML, Fernandes RA, Almeida GR, Monteiro RD, Colombini-Neto M, Bertola-Neto A. Health care resource use and costs in opioid-treated patients with and without constipation in Brazil. Value Health. 2011;14:S78-S81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 39. | Britto M, Fillmann L, Seabra MK. Comparative study of mannitol and polyethylene glycol in bowel preparation for colonoscopy. In Portuguese. J Coloproctol (Rio J). 2009;29:226-230. |
| 40. | Santos VL, de Paula CA, Secoli SR. [Adult ostomy patients in the city of São Paulo: a study of specialized equipment costs]. Rev Esc Enferm USP. 2008;42:249-255. [PubMed] |
| 41. | Bresciani C, Perez RO, Habr-Gama A, Jacob CE, Ozaki A, Batagello C, Proscurshim I, Gama-Rodrigues J. Laparoscopic versus standard appendectomy outcomes and cost comparisons in the private sector. J Gastrointest Surg. 2005;9:1174-1180; discussion 1180-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 42. | de Araujo A, de Barros Lopes A, Trucollo Michalczuk M, Stifft J, Nardelli E, Escobar G, Rossi G, Alvares-da-Silva MR. Is there yet any place for reagent strips in diagnosing spontaneous bacterial peritonitis in cirrhotic patients? An accuracy and cost-effectiveness study in Brazil. J Gastroenterol Hepatol. 2008;23:1895-1900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 43. | Baxter YC, Dias MC, Maculevicius J, Cecconello I, Cotteleng B, Waitzberg DL. Economic study in surgical patients of a new model of nutrition therapy integrating hospital and home vs the conventional hospital model. JPEN J Parenter Enteral Nutr. 2005;29:S96-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 44. | Ribeiro JE. Clinical protocol and treatment guidelines for treating viral hepatitis C and co-infections. Portuguese: Brasília DF 2011; . |
| 45. | Chieffi AL, Barata RCB. Litigation: the pharmaceutical industry’s strategy for introducing new drugs. In Portuguese. Rev Saude Publica. 2010;44:1-9. |
| 46. | INCA - Instituto Nacional do Câncer [Internet]. 2014. Available from: http://www2.inca.gov.br/wps/wcm/connect/inca/portal/home. |