Published online Nov 6, 2015. doi: 10.4292/wjgpt.v6.i4.199
Peer-review started: May 6, 2015
First decision: June 2, 2015
Revised: June 22, 2015
Accepted: August 25, 2015
Article in press: August 31, 2015
Published online: November 6, 2015
Processing time: 198 Days and 11.2 Hours
Autoimmune pancreatitis (AIP) is part of a systemic fibrosclerotic process characterized by lymphoplasmacytic infiltrate with immunoglobulin G subtype-4 (IgG4) positive cells. It characteristically presents with biliary obstruction due to mass-like swelling of the pancreas. Frequently AIP is accompanied by extra-pancreatic manifestations including retroperitoneal fibrosis, thyroid disease, and salivary gland involvement. Auto-antibodies, hypergammaglobulemia, and prompt resolution of pancreatic and extrapancreatic findings with steroids signify its autoimmune nature. Refractory cases are responsive to immunomodulators and rituximab. Involvement of the biliary tree, termed IgG4 associated cholangiopathy, mimics primary sclerosing cholangitis and is challenging to manage. High IgG4 levels and swelling of the pancreas with a diminutive pancreatic duct are suggestive of autoimmune pancreatitis. Given similarities in presentation but radical differences in management and outcome, differentiation from pancreatic malignancy is of paramount importance. There is controversy regarding the optimal diagnostic criterion and steroid trials to make the diagnosis. Additionally, the retroperitoneal location of the pancreas and requirement for histologic sampling, makes tissue acquisition challenging. Recently, a second type of autoimmune pancreatitis has been recognized with similar clinical presentation and steroid response though different histology, serologic, and extrapancreatic findings.
Core tip: Autoimmune pancreatitis is a component of a systemic immunoglobulin G subtype-4 mediated disease which also impacts the bile duct, salivary glands, kidney, and numerous other sites. It presents with jaundice and pancreas mass but it responds promptly to steroids and immunomodulators. A careful diagnostic approach is mandatory as autoimmune pancreatitis and its biliary manifestations closely resemble pancreas cancer and primary sclerosing cholangitis, diseases which have a more ominous course.
- Citation: Jani N, Buxbaum J. Autoimmune pancreatitis and cholangitis. World J Gastrointest Pharmacol Ther 2015; 6(4): 199-206
- URL: https://www.wjgnet.com/2150-5349/full/v6/i4/199.htm
- DOI: https://dx.doi.org/10.4292/wjgpt.v6.i4.199
While autoimmune pancreatitis (AIP) has been the subject of intense recent interest, its description has actually been unfolding over the past half century. In 1961, Sarles et al[1] reported a series of patients with chronic inflammatory pancreas sclerosis. In the 1970’s and 1980’s astute clinicians correlated its association with salivary gland and bile duct dysfunction and it was theorized to represent a systemic immunologic dry gland syndrome[2-5]. It came to be known by a number of different monikers including sclerosing pancreatitis, primary sclerosing cholangitis with pancreatitis, and duct narrowing chronic pancreatis. Kawaguchi et al[6] used gross resection specimens to report the characteristic histology of autoimmune pancreatitis. They described a dense lymphocytic infiltrate rich in antibody producing plasma cells (lymphoplasmacytic infiltrate) which surrounded and compressed the pancreatic ducts. Additionally, they observed storiform fibrosis and obliterative phlebitis (but sparring of arterioles) due to the lymphocytic infiltrate[6,7].
Yoshida et al[8] coined the term autoimmune pancreatitis in 1995 when he noticed its clinicopathologic similarities to autoimmune hepatitis. He treated a patient who had undergone exploratory laparotomy and diagnosed (though not confirmed with tissue) with unresectable pancreatic cancer. The patient had waxing and waning jaundice and pancreatic swelling on computed tomography imaging. Yoshida observed markedly elevated immunoglobulin G, antinuclear, anti-thyroglobulin, and anti-microsomal antibodies. He treated the patient with corticosteroids and the swelling, biochemical, and serologic abnormalities all remitted.
Multiple series have further characterized the clinical presentation. Typically, it mimics chronic pancreatitis or pancreatic cancer though acute pancreatitis occurs in 7%-25% of cases[9-11]. Hypergammaglobulinemia and positive serum autoantibodies are seen in three quarter of patients with antinuclear antibody being most frequently elevated. Extra-pancreatic manifestations occur in 50% of cases. Radiographic, serologic, and extrapancreatic manifestations help to further confirm the diagnosis.
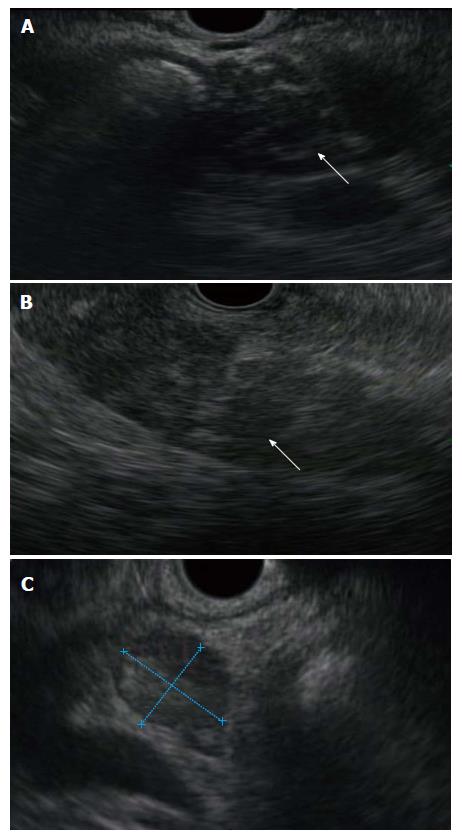
In AIP the pancreas exhibits diffuse gland enlargement with loss of clefts and a peripheral rim of altered enhancement (the halo sign) on cross sectional imaging[12,13]. In 20%-30% of patients, there is mass-like enlargement of the head with tail atrophy. Peripancreatic lymphadenopathy is seen in 25%. Endoscopic and magnetic resonance cholangiopancreatography reveals an irregular, diminutive pancreatic duct[14,15]. Endoscopic ultrasound demonstrates a diffuse altered (hypoechoic echotexture) gland enlargement (Figure 1). It typically lacks the hyperechoic foci and strands seen in chronic pancreatitis. Cross sectional imaging is also useful in detecting extrapancreatic manifestations (see below) and assess response to therapy[14].
Immunoglobulin G subtype-4 (IgG4) is elevated in number of autoimmune diseases including pemphigus vulgaris and membranous nephropathy. In a breakthrough paper in 2001 Hamano et al[16] demonstrated that it was markedly elevated in 20 patients with classic findings of autoimmune pancreatitis but not in normal patients. While it was modestly elevated in a number of pancreatico-biliary processes such as chronic pancreatitis and primary sclerosing cholangitis (PSC), IG4 was much more specific for AIP.
However, subsequent series have revealed that when > 140 mg/dL is defined as abnormal, the specificity of IgG4 is only 93%[9]. As AIP has a low prevalence, even in referral centers, most abnormal levels in patients with nonspecific gastrointestinal symptoms are false positives. Additionally, approximately 10% of pancreas cancer patients and 9% of patients with PSC have elevated levels of IgG4[9,17]. Increasing the cutoff to 280 mg/dL decreases sensitivity but improves specificity to 99%. Similarly, IgG4+ plasma cells may be seen in the periductal and stromal tissue. A small number of IgG4+ cells may be seen in chronic pancreatitis and pancreas cancer whereas dense infiltration with IgG4+ cells is specific for AIP[18]. Additionally, IgG4 staining in cancer does not exhibit the periductal pattern seen in autoimmune pancreatitis.
Concomitant salivary gland and biliary involvement were reported even in the initial reports of autoimmune pancreatitis. Shortly after the acceptance of AIP as a true autoimmune entity a number of authors demonstrated that it was associated with a form of retroperitoneal fibrosis which promptly resolved with steroid therapy[19,20]. AIP patients also may present with concomitant thyroiditis, fibrotic pseudotumor of the orbit, and nodules of the renal cortex and collecting system, all of which demonstrate an IgG4 positive lymphoplasmacytic infiltrate on histology and respond to steroids[21-24]. Lung fibrosis, cervical and mediastinal lymphadenopathy are also associated with AIP[25].
Asymptomatic lymphoplasmacytic infiltration of the gallbladder, duodenum, and stomach with IG4 positive cells has been demonstrated on surgical specimen (cases misinterpreted as pancreas cancer) further suggesting the systemic nature of this disease[21]. Hypothyroidism is seen in 26.8% of patient with autoimmune pancreatis and antithyroglobulin Ab in 7.3%. compared to 0% and 7.3% of those with chronic pancreatitis[26]. In Western populations, there is a correlation of AIP with inflammatory bowel disease[25]. Thus, it is increasingly recognized that IgG4 mediated disease is a systemic, multifocal fibrosclerotic process[22,27].
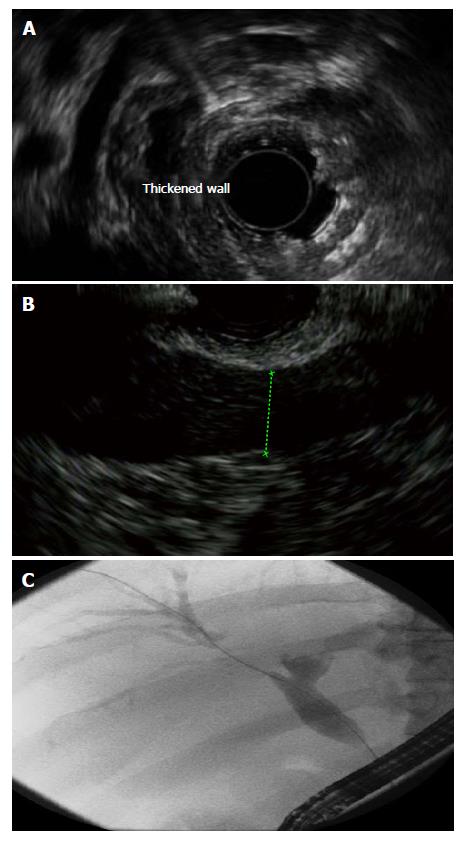
Biliary involvement may be present at the time of AIP diagnosis but more frequently follows diagnosis[28]. Histologically, it is characterized by a peri-bile duct lymphoplasmacytic infiltrate similar to AIP and imaging reveals bile duct thickening and enhancement (Figure 2)[28]. Intraductal ultrasound may demonstrate inflammation before it is clinically significant. IgG4 Associated Cholangiopathy (IAC) often simultaneously involve both the intrahepatic and extrahepatic biliary tree.
It is challenging to differentiate IAC from PSC. Cholangiographic features of segmental narrowing with pre-stenotic bile duct dilation suggests IgG4 IAC compared to beading, pruning, and diverticuli which are more consistent with PSC[29]. Additionally IAC is more likely to involve the distal bile duct than PSC[17,30]. Clinically, IAC manifests with the sudden onset of jaundice while a slow rise in cholestatic enzymes is seen in PSC. At comparable bilirubin levels, IAC tends to demonstrate much less advanced histology than PSC and there is a predominance of lymphocytes[30].
Cholangiocarcinoma complicates PSC but typically not IAC though an inflammatory pseudotumor due to a dense IG4-lymphoplasmytic infiltrate may mimic an intrahepatic bile duct tumor[31]. High levels of IgG4 (4 times upper limit of normal) positive cells in brushings helps to distinguish IAC from cholangiocarcinoma[32]. Retrospective assessment of IgG4 in patients who underwent transplant for PSC revealed that those who were positive had much higher bilirubin levels, Mayo risk scores, and progression to transplant. It is plausible that they might have actually had IAC. These findings emphasize the importance of making this diagnosis as IAC is responsive to steroids[17].
Steroid response was one of the initial features which suggested that AIP was an autoimmune process and corticosteroids remain the primary treatment modality. A large multicenter trial demonstrated that corticosteroid treatment induces remission in 98%[33]. The recommended starting dose of prednisone is 0.6 mg/kg per day (30-40 mg) followed by a slow taper over 3-6 mo[33-35]. Maintenance treatment with prednisone 2.5-10 mg for an additional 3-6 mo decreased relapse rates to 24%, which is significantly lower than those who stop taking steroids early, 34%, or are not treated 42%[33].
IgG4 levels may help determine disease activity especially following treatment with steroids and absence of improvement in the first 2-4 wk may suggest underlying malignancy[36,37]. As long term steroid therapy in this patient population has been associated with major complications including avascular necrosis, steroid cessation or transition to an immunomodulator is recommended within 1 year[33].
There are several predictors of relapse including extrapancreatic manifestations, diffuse pancreas swelling, and concomitant IAC[38,39]. While steroids are effective in 95% of cases of relapse, immunomodulators including azathioprine, 6-mercaptopurine, and mycophenolate mofetil should be considered for patients with multiple relapses or steroid refractory disease. Failure or inability to tolerate immunomodulators occurs in a significant percentage of those with AIP[40]. In these refractory cases the monoclonal antibody rituximab (anti-CD20), which depletes B cells, is successful in most (> 80%) of cases[40,41]. Rituximab’s efficacy may lie in its ability to modulate the Th2 process implicated in AIP[42].
The clinical and pathologic manifestations of AIP with IAC are also responsive to corticosteroid therapy[28]. However, these patients have a 30% higher rate of relapse than those without biliary involvement[40]. Relapse is particularly common in proximal (65%) vs distal (23%) biliary disease[33,39]. Immunomodulators are required in relapsing and refractory IAC[39]. Rituximab is an important option for refractory IAC and its use in biliary IgG4 mediated disease preceded its use in AIP without IAC[41]. In addition, a biliary stent is temporarily required in > 70% of cases to alleviate jaundice but typically may be removed following medical therapy[40].
The diagnosis of autoimmune pancreatitis must be made with caution as pancreatic cancer has a similar presentation but is much more prevalent. In an early series, Kamisawa et al[21] reported that > 50% of patients with AIP may have findings which are highly suggestive of pancreas cancer including elevated CEA, CA 19-9, portal vein narrowing, and bile duct stenosis. Additionally 10% of pancreas cancer patients will have significantly elevated levels of IgG4[9]. The Japanese Pancreas Society proposed the first diagnostic criterion for AIP which required diffuse enlargement of the pancreas with irregular pancreatic duct narrowing and histologic anomalies or elevated IgG4 levels[43,44].
Chari et al[45] found that the Japanese criterion would diagnose < 30% of patients in a Western population with histologic confirmed autoimmune pancreatitis. Additionally, routine diagnostic endoscopic pancreatography is not feasible in the litigious culture of the United States. Chari et al[45] proposed 3 potential methods to diagnose AIP: (1) classic histology; (2) typical imaging and elevated serum IgG4; and (3) pancreatic disease or extrapancreatic disease which responds to steroids[46]. These histology imaging serology, other organ involvement, response to treatment were adopted in most Western centers to diagnose autoimmune pancreatitis[45].
However, following application of these criteria, core biopsy, surgery, or steroid trials are required in 30% to differentiate AIP from cancer[46]. Experts from Asia and Western Countries recently proposed the International Consensus Diagnostic Criteria, which include 3 out of 4 characteristic histologic criterion: (1) lymphoplasmacytic infiltrate; (2) obliterative phlebitis; (3) storiform fibrosis; and (4) > 10 IgG4 positive cells/high power field or a combination of characteristic imaging and either 2/4 pathologic criterion, elevated IgG4, or extrapancreatic manifestations[47]. The consensus guidelines discourage the use of diagnostic steroid trials to diagnose AIP as pancreatitis and IgG4 elevations related to pancreas cancer may improve and give a false diagnosis of autoimmune pancreatitis.
In difficult cases, surgical evaluation is a reasonable approach given the difficulty of differentiating AIP from malignancy. A combined series from Johns Hopkins Medical Center and the Mayo Clinic included 37 patients with autoimmune pancreatitis among 1648 who had undergone pancreaticoduodenectomy[48]. While blood loss in AIP was higher than for other indications for Whipple resection, the quality of life in 68% was improved and none had recurrent jaundice at a median follow up of 33 mo.
Histology, which enables architectural assessment is needed to confirm the diagnosis in challenging cases. While endoscopic ultrasound guided aspiration methods offer the most direct access to the pancreas, this technique is more amenable to providing cytology specimens, which are less useful in AIP. A core endoscopic ultrasound (EUS) needle equipped with a spring loaded cutting sheath was used in a small series of patients with autoimmune pancreatitis[49]. However, the torque on the device required to access the pancreas head and uncinate from the duodenum prevented it from firing[50]. A new reverse bevel core needle has shown promise in the acquisition of tissue for histology and is the topic of an ongoing randomized trial for AIP tissue acquisition. Larger 19 gauge standard EUS needles have been used with meticulous specimen preparation techniques to yield histologic samples in lymphoma[51]. However, the results of this approach were disappointing in autoimmune pancreatitis as histologic confirmation was only achieved in 43% of patients[52]. Even with acquisition of histology, the patchy involvement of autoimmune pancreatitis limits EUS sampling and tissue acquisition remains a challenge. Surgery may be required for definite diagnosis.
Surgical specimens from AIP resection (cases thought to be cancer) reveal IgG4 infiltration of the stomach, duodenum, and other surrounding structures which provides another avenue for diagnosis. Endoscopically, AIP demonstrate papillary swelling in 41% of those with AIP and biopsies in cases of papillary swelling may reveal dense IgG4 cell (> 10/high power field) infiltration[53,54]. Pale thickened regions of mucosa of the stomach, duodenum and colon on biopsy also reveals focal infiltrates of IG4 (> 10/high powerfield) positive plasma cells[53,55]. Mucosal biopsies represent a minimally invasive adjunct to other sampling methods[53,56].
In some cases, AIP disease is progressive. Relapse is associated with chronic pancreatic injury. Between 33%-55% of those with recurrence develop pancreatic duct stones, though 4% without recurrence also develop evidence of chronic disease[35,40,57]. While steroids improve both the exocrine and endocrine abnormalities seen in autoimmune pancreatitis, abnormalities are only partially reversible with some patients developing chronic diabetes mellitus and steatorrhea[58]. In addition to the pancreatic dysfunction, chronic salivary gland dysfunction has also been shown to occur[58]. In some cases IAC results in progression to cirrhosis and portal hypertension[39]. AIP is also associated with increased risk of malignancy; the most common cancers were gastric, lung and prostate[59].
Recently a distinct variant of autoimmune pancreatitis, type II, has been described.
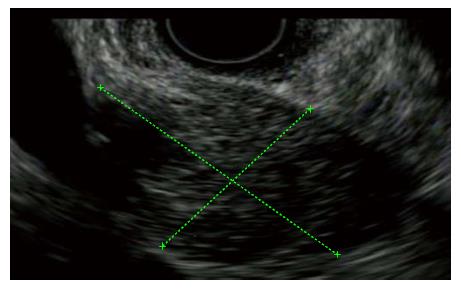
Similar to Type I AIP it presents with jaundice and mass-like changes in the gland (Figure 3). However, Type II AIP presents with a completely different histological pattern, an idiopathic duct-centric chronic pancreatitis with increased intraluminal and epithelial neutrophils[60,61]. It has primarily been described in western nations and is not associated with extrapancreatic manifestations with the exception of inflammatory bowel disease[60]. In addition serologies and IgG4 are typically negative[40,61]. Given the absence of potential corroborative markers, definitive diagnosis requires histology[62]. Type II is found predominantly in much younger patients than those with type I AIP[61]. The pediatric entity of idiopathic fibrosing pancreatitis in which children (often with concomitant IBD) present with jaundice and pancreas mass is likely the same disease as type II AIP[63]. Type II AIP responds to steroids in 92% and is much less likely to relapse than Type I AIP[40,61]. However, given that it is a recently described entity, studies of long-term sequelae and clinical course are lacking.
In summary, autoimmune pancreatitis presents with jaundice and pancreas mass mimicking pancreatic cancer. However, prompt resolution with steroids, autoantibodies, and hypergammaglobulinemia led to its recognition as an autoimmune process. It is frequently accompanied by diverse steroid responsive, IgG4 mediated abnormalities including cholangiopathy, retroperitoneal fibrosis, and sialadenitis. Challenges include the appropriate use of IgG4 and various criterion for its diagnosis, better methods to obtain tissue, and treatment of steroid refractory cases. Type II AIP has a similar clinical presentation and treatment, but distinct histology, serology, and extrapancreatic manifestations.
P- Reviewer: Hauser G S- Editor: Ma YJ
L- Editor: A E- Editor: Li D
| 1. | Sarles H, Sarles JC, Muratore R, Guien C. Chronic inflammatory sclerosis of the pancreas--an autonomous pancreatic disease. Am J Dig Dis. 1961;6:688-698. [PubMed] |
| 2. | Waldram R, Kopelman H, Tsantoulas D, Williams R. Chronic pancreatitis, sclerosing cholangitis, and sicca complex in two siblings. Lancet. 1975;1:550-552. [PubMed] |
| 3. | Sjögren I, Wengle B, Korsgren M. Primary sclerosing cholangitis associated with fibrosis of the submandibular glands and the pancreas. Acta Med Scand. 1979;205:139-141. [PubMed] |
| 4. | Epstein O, Chapman RW, Lake-Bakaar G, Foo AY, Rosalki SB, Sherlock S. The pancreas in primary biliary cirrhosis and primary sclerosing cholangitis. Gastroenterology. 1982;83:1177-1182. [PubMed] |
| 5. | Montefusco PP, Geiss AC, Bronzo RL, Randall S, Kahn E, McKinley MJ. Sclerosing cholangitis, chronic pancreatitis, and Sjogren’s syndrome: a syndrome complex. Am J Surg. 1984;147:822-826. [PubMed] |
| 6. | Kawaguchi K, Koike M, Tsuruta K, Okamoto A, Tabata I, Fujita N. Lymphoplasmacytic sclerosing pancreatitis with cholangitis: a variant of primary sclerosing cholangitis extensively involving pancreas. Hum Pathol. 1991;22:387-395. [PubMed] |
| 7. | Deshpande V, Zen Y, Chan JK, Yi EE, Sato Y, Yoshino T, Klöppel G, Heathcote JG, Khosroshahi A, Ferry JA. Consensus statement on the pathology of IgG4-related disease. Mod Pathol. 2012;25:1181-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1714] [Cited by in RCA: 1774] [Article Influence: 136.5] [Reference Citation Analysis (0)] |
| 8. | Yoshida K, Toki F, Takeuchi T, Watanabe S, Shiratori K, Hayashi N. Chronic pancreatitis caused by an autoimmune abnormality. Proposal of the concept of autoimmune pancreatitis. Dig Dis Sci. 1995;40:1561-1568. [PubMed] |
| 9. | Ghazale A, Chari ST, Smyrk TC, Levy MJ, Topazian MD, Takahashi N, Clain JE, Pearson RK, Pelaez-Luna M, Petersen BT. Value of serum IgG4 in the diagnosis of autoimmune pancreatitis and in distinguishing it from pancreatic cancer. Am J Gastroenterol. 2007;102:1646-1653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 434] [Cited by in RCA: 362] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 10. | Sah RP, Pannala R, Chari ST, Sugumar A, Clain JE, Levy MJ, Pearson RK, Smyrk TC, Petersen BT, Topazian MD. Prevalence, diagnosis, and profile of autoimmune pancreatitis presenting with features of acute or chronic pancreatitis. Clin Gastroenterol Hepatol. 2010;8:91-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Uchida K, Okazaki K, Konishi Y, Ohana M, Takakuwa H, Hajiro K, Chiba T. Clinical analysis of autoimmune-related pancreatitis. Am J Gastroenterol. 2000;95:2788-2794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 138] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 12. | Farrell JJ, Garber J, Sahani D, Brugge WR. EUS findings in patients with autoimmune pancreatitis. Gastrointest Endosc. 2004;60:927-936. [PubMed] |
| 13. | Sahani DV, Kalva SP, Farrell J, Maher MM, Saini S, Mueller PR, Lauwers GY, Fernandez CD, Warshaw AL, Simeone JF. Autoimmune pancreatitis: imaging features. Radiology. 2004;233:345-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 258] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 14. | Manfredi R, Frulloni L, Mantovani W, Bonatti M, Graziani R, Pozzi Mucelli R. Autoimmune pancreatitis: pancreatic and extrapancreatic MR imaging-MR cholangiopancreatography findings at diagnosis, after steroid therapy, and at recurrence. Radiology. 2011;260:428-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 15. | Moon SH, Kim MH. The role of endoscopy in the diagnosis of autoimmune pancreatitis. Gastrointest Endosc. 2012;76:645-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Hamano H, Kawa S, Horiuchi A, Unno H, Furuya N, Akamatsu T, Fukushima M, Nikaido T, Nakayama K, Usuda N. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med. 2001;344:732-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2026] [Cited by in RCA: 1878] [Article Influence: 78.3] [Reference Citation Analysis (0)] |
| 17. | Mendes FD, Jorgensen R, Keach J, Katzmann JA, Smyrk T, Donlinger J, Chari S, Lindor KD. Elevated serum IgG4 concentration in patients with primary sclerosing cholangitis. Am J Gastroenterol. 2006;101:2070-2075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 231] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 18. | Zhang L, Notohara K, Levy MJ, Chari ST, Smyrk TC. IgG4-positive plasma cell infiltration in the diagnosis of autoimmune pancreatitis. Mod Pathol. 2007;20:23-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 215] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 19. | Chutaputti A, Burrell MI, Boyer JL. Pseudotumor of the pancreas associated with retroperitoneal fibrosis: a dramatic response to corticosteroid therapy. Am J Gastroenterol. 1995;90:1155-1158. [PubMed] |
| 20. | Hamano H, Kawa S, Ochi Y, Unno H, Shiba N, Wajiki M, Nakazawa K, Shimojo H, Kiyosawa K. Hydronephrosis associated with retroperitoneal fibrosis and sclerosing pancreatitis. Lancet. 2002;359:1403-1404. [PubMed] |
| 21. | Kamisawa T, Egawa N, Nakajima H. Autoimmune pancreatitis is a systemic autoimmune disease. Am J Gastroenterol. 2003;98:2811-2812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 152] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 22. | Kamisawa T, Funata N, Hayashi Y, Tsuruta K, Okamoto A, Amemiya K, Egawa N, Nakajima H. Close relationship between autoimmune pancreatitis and multifocal fibrosclerosis. Gut. 2003;52:683-687. [PubMed] |
| 23. | Takahashi N, Kawashima A, Fletcher JG, Chari ST. Renal involvement in patients with autoimmune pancreatitis: CT and MR imaging findings. Radiology. 2007;242:791-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 185] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 24. | Umehara H, Okazaki K, Masaki Y, Kawano M, Yamamoto M, Saeki T, Matsui S, Sumida T, Mimori T, Tanaka Y. A novel clinical entity, IgG4-related disease (IgG4RD): general concept and details. Mod Rheumatol. 2012;22:1-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 291] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 25. | Ohara H, Nakazawa T, Sano H, Ando T, Okamoto T, Takada H, Hayashi K, Kitajima Y, Nakao H, Joh T. Systemic extrapancreatic lesions associated with autoimmune pancreatitis. Pancreas. 2005;31:232-237. [PubMed] |
| 26. | Komatsu K, Hamano H, Ochi Y, Takayama M, Muraki T, Yoshizawa K, Sakurai A, Ota M, Kawa S. High prevalence of hypothyroidism in patients with autoimmune pancreatitis. Dig Dis Sci. 2005;50:1052-1057. [PubMed] |
| 27. | Stone JH, Zen Y, Deshpande V. IgG4-related disease. N Engl J Med. 2012;366:539-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1856] [Cited by in RCA: 1871] [Article Influence: 143.9] [Reference Citation Analysis (83)] |
| 28. | Hirano K, Shiratori Y, Komatsu Y, Yamamoto N, Sasahira N, Toda N, Isayama H, Tada M, Tsujino T, Nakata R. Involvement of the biliary system in autoimmune pancreatitis: a follow-up study. Clin Gastroenterol Hepatol. 2003;1:453-464. [PubMed] |
| 29. | Nakazawa T, Ohara H, Sano H, Aoki S, Kobayashi S, Okamoto T, Imai H, Nomura T, Joh T, Itoh M. Cholangiography can discriminate sclerosing cholangitis with autoimmune pancreatitis from primary sclerosing cholangitis. Gastrointest Endosc. 2004;60:937-944. [PubMed] |
| 30. | Nakazawa T, Ohara H, Sano H, Ando T, Aoki S, Kobayashi S, Okamoto T, Nomura T, Joh T, Itoh M. Clinical differences between primary sclerosing cholangitis and sclerosing cholangitis with autoimmune pancreatitis. Pancreas. 2005;30:20-25. [PubMed] |
| 31. | Zen Y, Harada K, Sasaki M, Sato Y, Tsuneyama K, Haratake J, Kurumaya H, Katayanagi K, Masuda S, Niwa H. IgG4-related sclerosing cholangitis with and without hepatic inflammatory pseudotumor, and sclerosing pancreatitis-associated sclerosing cholangitis: do they belong to a spectrum of sclerosing pancreatitis. Am J Surg Pathol. 2004;28:1193-1203. [PubMed] |
| 32. | Oseini AM, Chaiteerakij R, Shire AM, Ghazale A, Kaiya J, Moser CD, Aderca I, Mettler TA, Therneau TM, Zhang L. Utility of serum immunoglobulin G4 in distinguishing immunoglobulin G4-associated cholangitis from cholangiocarcinoma. Hepatology. 2011;54:940-948. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 135] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 33. | Kamisawa T, Shimosegawa T, Okazaki K, Nishino T, Watanabe H, Kanno A, Okumura F, Nishikawa T, Kobayashi K, Ichiya T. Standard steroid treatment for autoimmune pancreatitis. Gut. 2009;58:1504-1507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 500] [Cited by in RCA: 486] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 34. | Joshi D, Webster GJ. Immunoglobulin G4-related sclerosing cholangitis. Hepatology. 2015;61:1432-1434. [PubMed] |
| 35. | Takayama M, Hamano H, Ochi Y, Saegusa H, Komatsu K, Muraki T, Arakura N, Imai Y, Hasebe O, Kawa S. Recurrent attacks of autoimmune pancreatitis result in pancreatic stone formation. Am J Gastroenterol. 2004;99:932-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 120] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 36. | Tabata T, Kamisawa T, Takuma K, Egawa N, Setoguchi K, Tsuruta K, Obayashi T, Sasaki T. Serial changes of elevated serum IgG4 levels in IgG4-related systemic disease. Intern Med. 2011;50:69-75. [PubMed] |
| 37. | Moon SH, Kim MH, Park DH, Hwang CY, Park SJ, Lee SS, Seo DW, Lee SK. Is a 2-week steroid trial after initial negative investigation for malignancy useful in differentiating autoimmune pancreatitis from pancreatic cancer A prospective outcome study. Gut. 2008;57:1704-1712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 142] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 38. | Kubota K, Iida H, Fujisawa T, Yoneda M, Inamori M, Abe Y, Kirikoshi H, Saito S, Ohshiro H, Kakuta Y. Clinical factors predictive of spontaneous remission or relapse in cases of autoimmune pancreatitis. Gastrointest Endosc. 2007;66:1142-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 102] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 39. | Ghazale A, Chari ST, Zhang L, Smyrk TC, Takahashi N, Levy MJ, Topazian MD, Clain JE, Pearson RK, Petersen BT. Immunoglobulin G4-associated cholangitis: clinical profile and response to therapy. Gastroenterology. 2008;134:706-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 667] [Cited by in RCA: 585] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 40. | Hart PA, Topazian MD, Witzig TE, Clain JE, Gleeson FC, Klebig RR, Levy MJ, Pearson RK, Petersen BT, Smyrk TC. Treatment of relapsing autoimmune pancreatitis with immunomodulators and rituximab: the Mayo Clinic experience. Gut. 2013;62:1607-1615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 261] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 41. | Topazian M, Witzig TE, Smyrk TC, Pulido JS, Levy MJ, Kamath PS, Chari ST. Rituximab therapy for refractory biliary strictures in immunoglobulin G4-associated cholangitis. Clin Gastroenterol Hepatol. 2008;6:364-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 116] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 42. | Zen Y, Fujii T, Harada K, Kawano M, Yamada K, Takahira M, Nakanuma Y. Th2 and regulatory immune reactions are increased in immunoglobin G4-related sclerosing pancreatitis and cholangitis. Hepatology. 2007;45:1538-1546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 526] [Cited by in RCA: 481] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 43. | Okazaki K, Kawa S, Kamisawa T, Naruse S, Tanaka S, Nishimori I, Ohara H, Ito T, Kiriyama S, Inui K. Clinical diagnostic criteria of autoimmune pancreatitis: revised proposal. J Gastroenterol. 2006;41:626-631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 439] [Cited by in RCA: 418] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 44. | Members of the Criteria Committee for Autoimmune Pancreatitis of the Japan Pancreas Society Diagnostic criteria for autoimmune pancreatitis by the Japan Pancreas Society. J Jpn Pancreas (Suizou). 2002;17:587. |
| 45. | Chari ST, Smyrk TC, Levy MJ, Topazian MD, Takahashi N, Zhang L, Clain JE, Pearson RK, Petersen BT, Vege SS. Diagnosis of autoimmune pancreatitis: the Mayo Clinic experience. Clin Gastroenterol Hepatol. 2006;4:1010-1016; quiz 934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 722] [Cited by in RCA: 657] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 46. | Chari ST, Takahashi N, Levy MJ, Smyrk TC, Clain JE, Pearson RK, Petersen BT, Topazian MA, Vege SS. A diagnostic strategy to distinguish autoimmune pancreatitis from pancreatic cancer. Clin Gastroenterol Hepatol. 2009;7:1097-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 224] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 47. | Shimosegawa T, Chari ST, Frulloni L, Kamisawa T, Kawa S, Mino-Kenudson M, Kim MH, Klöppel G, Lerch MM, Löhr M. International consensus diagnostic criteria for autoimmune pancreatitis: guidelines of the International Association of Pancreatology. Pancreas. 2011;40:352-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1050] [Cited by in RCA: 1058] [Article Influence: 75.6] [Reference Citation Analysis (0)] |
| 48. | Hardacre JM, Iacobuzio-Donahue CA, Sohn TA, Abraham SC, Yeo CJ, Lillemoe KD, Choti MA, Campbell KA, Schulick RD, Hruban RH. Results of pancreaticoduodenectomy for lymphoplasmacytic sclerosing pancreatitis. Ann Surg. 2003;237:853-858; discussion 858-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 129] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 49. | Levy MJ, Reddy RP, Wiersema MJ, Smyrk TC, Clain JE, Harewood GC, Pearson RK, Rajan E, Topazian MD, Yusuf TE. EUS-guided trucut biopsy in establishing autoimmune pancreatitis as the cause of obstructive jaundice. Gastrointest Endosc. 2005;61:467-472. [PubMed] |
| 50. | Sakamoto H, Kitano M, Komaki T, Noda K, Chikugo T, Dote K, Takeyama Y, Das K, Yamao K, Kudo M. Prospective comparative study of the EUS guided 25-gauge FNA needle with the 19-gauge Trucut needle and 22-gauge FNA needle in patients with solid pancreatic masses. J Gastroenterol Hepatol. 2009;24:384-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 172] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 51. | Yasuda I, Goto N, Tsurumi H, Nakashima M, Doi S, Iwashita T, Kanemura N, Kasahara S, Adachi S, Hara T. Endoscopic ultrasound-guided fine needle aspiration biopsy for diagnosis of lymphoproliferative disorders: feasibility of immunohistological, flow cytometric, and cytogenetic assessments. Am J Gastroenterol. 2012;107:397-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 52. | Iwashita T, Yasuda I, Doi S, Ando N, Nakashima M, Adachi S, Hirose Y, Mukai T, Iwata K, Tomita E. Use of samples from endoscopic ultrasound-guided 19-gauge fine-needle aspiration in diagnosis of autoimmune pancreatitis. Clin Gastroenterol Hepatol. 2012;10:316-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 119] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 53. | Kamisawa T, Egawa N, Nakajima H, Tsuruta K, Okamoto A, Hayashi Y, Funata N. Gastrointestinal findings in patients with autoimmune pancreatitis. Endoscopy. 2005;37:1127-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 54. | Unno H, Saegusa H, Fukushima M, Hamano H. Usefulness of endoscopic observation of the main duodenal papilla in the diagnosis of sclerosing pancreatitis. Gastrointest Endosc. 2002;56:880-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 55. | Kamisawa T, Nakajima H, Egawa N, Hayashi Y, Funata N. Autoimmune pancreatitis can be confirmed with gastroscopy. Dig Dis Sci. 2004;49:155-156. [PubMed] |
| 56. | Hirano K, Fukushima N, Tada M, Isayama H, Mizuno S, Yamamoto K, Yashima Y, Yagioka H, Sasaki T, Kogure H. Diagnostic utility of biopsy specimens for autoimmune pancreatitis. J Gastroenterol. 2009;44:765-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 57. | Kawa S, Hamano H, Ozaki Y, Ito T, Kodama R, Chou Y, Takayama M, Arakura N. Long-term follow-up of autoimmune pancreatitis: characteristics of chronic disease and recurrence. Clin Gastroenterol Hepatol. 2009;7:S18-S22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 58. | Kamisawa T, Egawa N, Inokuma S, Tsuruta K, Okamoto A, Kamata N, Nakamura T, Matsukawa M. Pancreatic endocrine and exocrine function and salivary gland function in autoimmune pancreatitis before and after steroid therapy. Pancreas. 2003;27:235-238. [PubMed] |
| 59. | Hart PA, Kamisawa T, Brugge WR, Chung JB, Culver EL, Czakó L, Frulloni L, Go VL, Gress TM, Kim MH. Long-term outcomes of autoimmune pancreatitis: a multicentre, international analysis. Gut. 2013;62:1771-1776. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 461] [Cited by in RCA: 375] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 60. | Kamisawa T, Chari ST, Giday SA, Kim MH, Chung JB, Lee KT, Werner J, Bergmann F, Lerch MM, Mayerle J. Clinical profile of autoimmune pancreatitis and its histological subtypes: an international multicenter survey. Pancreas. 2011;40:809-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 183] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 61. | Sah RP, Chari ST, Pannala R, Sugumar A, Clain JE, Levy MJ, Pearson RK, Smyrk TC, Petersen BT, Topazian MD. Differences in clinical profile and relapse rate of type 1 versus type 2 autoimmune p ancreatitis. Gastroenterology. 2010;139:140-148; quiz e12-e13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 299] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 62. | Kamisawa T, Chari ST, Lerch MM, Kim MH, Gress TM, Shimosegawa T. Republished: recent advances in autoimmune pancreatitis: type 1 and type 2. Postgrad Med J. 2014;90:18-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 63. | Buxbaum JL, Eloubeidi MA, Varadarajulu S. Utility of EUS-guided FNA in the management of children with idiopathic fibrosing pancreatitis. J Pediatr Gastroenterol Nutr. 2011;52:482-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |