Published online Aug 6, 2014. doi: 10.4292/wjgpt.v5.i3.113
Revised: April 10, 2014
Accepted: June 20, 2014
Published online: August 6, 2014
Processing time: 184 Days and 4.5 Hours
Methotrexate has been used an immunomodulator in many autoimmune diseases, including inflammatory bowel disease. However, many physicians are unfamiliar or uncomfortable with its use in the management of inflammatory bowel disease. We summarize the data for use of methotrexate in common clinical scenarios: (1) steroid dependant Crohn’s disease (CD); (2) maintenance of remission in steroid free CD; (3) azathioprine failures in CD; (4) in combination therapy with Anti-TNF agents in CD; (5) decreasing antibody formation to Anti-TNF therapy in CD; (6) management of fistulizing disease in CD; and (7) as well as induction and maintenance of remission in ulcerative colitis. An easy to use algorithm is provided for the busy clinician to access and safely prescribe methotrexate for their inflammatory bowel disease patients.
Core tip: Methotrexate can a be a useful adjunct to the treatment of inflammatory bowel disease, but many practitioners are unfamiliar with it’s use. Here, we have provided a succinct summary of the data behind the use of methotrexate and a short “user’s guide” and algorithm to allow for the busy clinician to become quickly familiar with the drug and information to help prescribe it safely.
- Citation: Swaminath A, Taunk R, Lawlor G. Use of methotrexate in inflammatory bowel disease in 2014: A User’s Guide. World J Gastrointest Pharmacol Ther 2014; 5(3): 113-121
- URL: https://www.wjgnet.com/2150-5349/full/v5/i3/113.htm
- DOI: https://dx.doi.org/10.4292/wjgpt.v5.i3.113
Methotrexate (MTX) has a long history for effectively treating rheumatological conditions such as rheumatoid arthritis (RA), systemic lupus erythematosus (SLE) and sarcoidosis[1-3]. Over the past 25 years there have been numerous studies that evaluated its efficacy in Inflammatory Bowel Disease with varied results. It has to date remained in treatment algorithms as a salvage therapy for patients who have failed, or become intolerant of, azathioprine. The goal of our paper is to summarize the data behind methotrexate for common clinical situations and to provide a quick access guide on prescribing the drug.
The landmark studies demonstrating efficacy of MTX in Crohn’s disease (CD) have utilized sq or im at 25 mg/wk. Smaller non-randomized studies in both CD and UC patients have offered conflicting data and, to an extent demonstrate, the relative ineffectiveness with low dose po regimens for induction or maintenance of remission (Table 1)[4,5]. Jundt demonstrated similar bioavailability between po vs sq vs im MTX in RA patients[6]. The bioavailability of po as compared to im was 0.85.
| Study | Dose MTX | Route ofadmin | n | Study design | Patients | Duration follow up (wk) | MTX response | MTX remission | Placebo or (Comparator) Response | AE MTX | AE Placebo | |
| Kozarek | 25 mg/wk | sq | 14 | Non-Randomized-open Label | CD | 12 | 79% | |||||
| Feagan | 25 mg/wk | im | 141 | Double-blind Placebo controlled multi center | Steroid dependent CD | 16 | 39.4%1 | 19.1% | 1% | 2% | ||
| Oren | 12.5 mg/wk | po | 84 | Randomized Double-Blind Placebo Controlled | Active CD | 36 | 38% | 46% | ||||
| Arora | 22.5 mg/wk | po | 33 | Randomized Double Blind Placebo Controlled | Steroid Dependent CD | 52 | 54% | 20% | 23% | 0 | ||
| Feagan | 15 mg/wk | im | 76 | Double Blind Placebo Controlled Multi-Center | CD Maintenance | 40 | 65%1 | 39% | 1% | 2% | ||
| Mate- Jimenez | 15 mg/wk | po | 38 | Randomized Single Center | Steroid Dependent CD | 76 | 80%1 Induction 66.6%1 Maintenance | 14% Induction 0 Maintenance | 11.5% | 0 | ||
| Lemann | 25 mg/wk | im | 49 | Retrospective | Active CD | 84% | 49% | |||||
| Fraser | 20 mg/wk (10-25) | po/im | 48 | Retrospective | Active CD-Maintenance | 62% | 27% | |||||
| Ardizzone | 25 mg/wk | iv | 54 | Investigator Blind, randomized | Active CD | 24 | 56% | 63% AZA | 11% | |||
| Mahadevan | 25 mg/wk | im | 16 | Retrospective case series | Fistulizing CD | 56% | 6% | |||||
| Wahed | 25 mg/wk Induction 15 mg/wk Maintenance | im/po-Induction po-Maintenance | 99 | Retrospective | AZA Intolerance/ AZA non-responders | 62% | 8.3% | |||||
| Feagan | Wk0-10 mg/wk Wk3-20 mg/wk Wk5-25 mg/wk | sq | 126 | Double Blind Placebo Controlled Multi-center | Active CD | 50 | IFX + MTX 56% | IFX + PCBO 57% |
Kurnik et al[7] studied the bioavailability of MTX in adult patients with stable Crohn’s disease. The patients were administered their weekly doses either orally or sq and the MTX levels were measured over the next 24 h. No information on extent of small bowel inflammation was provided. They found that oral bioavailability averages 73% (95%CI: 62%-86%) of that of subcutaneous administration[7]. Hoekstra demonstrated that the bioavailability of po MTX can be boosted by split dosing. RA patients were studied after single dosing of MTX by either sq or po method. Then the same patient underwent a second measurement after split dosing of MTX (50% of the dose taken 8 h later). The bioavailability of the split dose was 28% higher compared to the single dose (P = 0.007) and was statistically significant. The mean bioavailability after single-dose and split-dose MTX was 0.76 and 0.90, respectively, compared to subcutaneous administration[8].
Wilson et al[9] updated the Kurnik study using a more sensitive assay. They compared the pharmacokinetic profile of po and subcutaneous MTX (25 mg) in 11 CD patients. The bioavailability of po MTX compared with sq was found to be 0.86 (90%CI: 0.79-0.92). Of note, the 90%CI to meet definition of bioequivalency proposed by the FDA was not met, (lower end of the 90%CI would have had to be 0.80 rather than 0.79), and so this study could not claim true bioequivalency of the oral and sq routes of administration.
Although these are small studies and many patient factors were not provided (i.e., extent and severity of bowel disease), the po route of administration does appear to be less bioavailable than sq dosing.
Although Kozarek et al[10] (NEJM 1980) had demonstrated the efficacy of 6-mercaptopurine in the induction of remission of Crohn’s disease, the authors noted the response to be delayed and incomplete. The first report of successful induction with methotrexate was reported by Kozarek et al[10] in 1989. This non-randomized, open-label pilot study included 14 patients with Crohn’s disease with an unidentified fraction described as failing immunomodulators. Eleven patients (79%) demonstrated a clinical response to 25 mg/wk im methotrexate as measured by objective decreases in CDAI, and 5 patients (36%) demonstrated endoscopic mucosal healing. Although this study lacked a control arm, it suggested MTX may have value in inducing remission in patients with Crohns’ disease.
Feagan completed a prospective double-blind, placebo-controlled Canadian multicenter study of weekly im injections of methotrexate in patients who had chronically active Crohn’s disease despite a minimum of 3 mo of prednisone therapy with the primary outcome being the induction of clinical remission[11]. A total of 141 patients assigned in a 2:1 ratio of MTX to placebo were included in the trial and 37 (39.4%) achieved clinical remission in the methotrexate group compared with 9 (19.1%) in the placebo group (P = 0.025). The response among patients requiring high dose prednisone (> 20 mg/d) was equally good as those requiring low doses at study initiation. Prednisone dose was appreciably lower by week 4 in the MTX group and demonstrated the largest difference from week 12 through 16. A greater number of patients withdrew from the treatment arm due to adverse events (17% vs 2%). The withdrawals from the MTX arm were due to asymptomatic elevation of serum aminotransferase concentrations (7), nausea (6), skin rash (1), atypical pneumonia (1), and optic neuritis (1).
Oren et al[5] conducted a prospective randomized, double blind, placebo-controlled Israeli multi-center trial to evaluate the effectiveness of oral methotrexate in patients who had required steroids or immunomodulators for at least 4 mo out of the year prior to enrollment. Although it would be difficult to characterize these patients as steroid dependant, they had active ongoing disease as measured by Harvey Bradshaw Index. The study randomized 84 patients to 12.5 mg po MTX/week vs 6-MP 50 mg/daily vs placebo. The lower dose of oral MTX (compared to 25 mg/wk im in the Feagan study) was based on reported efficacy in the rheumatoid arthritis literature. Remission rates were 39% and 41% in the MTX and 6-MP groups respectively. However, the rate of remission in the placebo group was 46%, thereby inferring no benefit for either the MTX or 6 MP treatment arm. Criticisms of this study included presumed underdosing of MTX and 6 MP. Also, no standard steroid tapering regimen was described in this study, although reduction in steroid dose was described as an outcome measure. Although improvement was seen based on intra-patient evaluation (each patient used as their own control), this was not a pre-specified analysis. Hence, these results should be viewed with caution.
A cohort of 38 patients with steroid dependant CD was evaluated by Mate-Jimenez, but the requirement to separate these patients into 3 arms (1.5 mg/kg per day 6MP, 15 mg/wk po MTX, or 5-ASA) resulted in a small number of patients in each arm[12]. However, the large differences in outcomes for induction of remission in both treatment arms (93.7% 6MP, 80%MTX) compared to placebo (14%) was statistically significant. Interestingly, these findings show a degree of benefit that has not been reproduced for either the 6MP or MTX treatment arms. Arora et al[13] evaluated 28 steroid-dependant Crohn’s disease patients who received 15 mg/wk po MTX vs placebo. Dose escalation to 22.5 mg/wk was allowed at the discretion of the clinician. The primary endpoint was clinical exacerbation of Crohn’s disease. Although fewer patients in the MTX group (6/13, 46%) experienced exacerbation of CD vs placebo (12/15, 80%), the findings did not reach statistical significance. Despite the 43% relative risk reduction in flare frequency between the treatment and placebo, this study was underpowered to find this difference to be significant.
Ardizzone evaluated the efficacy of iv MTX in comparison to AZA[4]. This randomized investigator-blind study enrolled 54 steroid-dependent active (CDAI > 200) CD patients on > 10 mg/d of steroid therapy. Patients were randomized to 25 mg iv/wk of MTX vs po AZA 2 mg/kg per day for 3 mo, after which MTX dosing was changed to 25 mg/wk po for an additional 3 mo follow up. The primary outcome considered was the proportion of patients entering steroid-free remission after 3 and 6 mo of therapy. No statistically significant difference was found between the two treatment regimens with respect to remission rate after 3 mo (methotrexate 44%, azathioprine 33%, P = 0.28, (95%CI: 0.369-0.147), and 6 mo (methotrexate 56%, azathioprine 63%, P = 0.39, 95%CI: 0.187-0.335), respectively. MTX and AZA demonstrated similar rates of adverse events leading to medication withdrawal. While there appeared to no additional benefit to providing MTX via the IV route, MTX at 25 mg/wk appeared to have similar efficacy as weight based azathioprine in inducing and maintaining remission in active Crohn’s disease.
A 2011 meta-analysis of MTX in active Crohn’s did not include either the Mate-Jiminez or Ardizzone studies (no placebo arm) or Arora studies (categorized the study patients as quiescent)[14]. Their conclusion that MTX was not better than placebo in active Crohn’s was based only on the inclusion of Feagan’s positive trial (25 mg/wk im MTX) and the negative orally administered MTX (12.5 mg/wk po) Oren trial. The Cochrane collaboration reached similar conclusions a year later, but understood the limitations of the data on oral MTX and suggested further study[15].
Feagan demonstrated the use of MTX in Crohn’s disease for maintenance of remission in a large double-blind, placebo controlled multi-center study with 76 patients in 2000[16]. Some of these patients were enrolled from Feagan’s trial for induction of remission using 25 mg im/wk MTX in 1995 and others from an open label trial of 25 mg/wk im MTX. The patients were randomized to 15 mg im MTX/weekly vs placebo and followed for 40 wk. Impressively, no other therapy for Crohn’s disease was permitted. At the completion of the trial 65% (26/40) of the MTX group maintained remission compared to 39% (14/36) of the placebo group (P = 0.04). A majority (55%) of the relapsers could be re-induced with 25 mg/wk im MTX. Adverse events were minimal as only 1 patient discontinued MTX therapy for nausea and vomiting.
The efficacy of oral MTX (10-20 mg po) for maintenance of remission in Crohn’s and ulcerative colitis was evaluated by a retrospective review by Fraser. Although 1 year remission rates approached 90%, the data for Crohn’s and UC were combined and the clinical definition of remission was vague[17].
Given the dearth of high quality studies of MTX in maintaining remission in Crohn’s, the only maintenance study used in the Kahn meta-analysis was Feagan’s (15 mg im/wk MTX) suggesting benefit with a number needed to treat (NNT) of 4[14]. Interestingly, the Cochrane meta-analysis of MTX for maintenance of remission, included both the Mata-Jimenez study and Oren studies as part their analysis[18]. Their main conclusions track the benefit shown by the Feagan’s 15 mg/wk im MTX and suggest that lower oral doses do not benefit maintenance of remission.
Despite the widespread use of thiopurines, approximately one third do not respond and another 10% cannot tolerate the drugs[19]. In the United States, MTX is often reserved for AZA intolerance or failure and fewer physicians are comfortable prescribing it[20]. AZA Intolerance can include bone marrow suppression, upper GI symptoms, pancreatic dysfunction, abnormal LFT’s and nonspecific symptoms including joint aches, hair loss, rash and flu like illness.
A study by Lemann in 2000 evaluated the durability of MTX for maintenance of remission in a population of patients who had (mostly) failed or were intolerant to AZA and had already been treated with MTX for period of at least 6 mo were followed for an additional 18 mo[21]. Out of 49 patients, 42 had previously failed AZA (85%). Out of the 41 achieving remission, 36 had previously failed AZA (87%). Most of the patients were administered 25 mg/wk im MTX, but some physicians changed the dose to oral administration and some were even able to taper it. Despite some patients with oral MTX dosing and despite a heavy proportion of AZA failures in the study population, 71% of the study population remained in remission for 1 year and up to 52% remained in remission after 3 years. Among patients who initially do well on MTX after AZA failure, they are likely to remain well on that therapy over the next several years.
Wahed et al[22] evaluated clinical response of 99 CD patients retrospectively who were placed on MTX due to AZA intolerance or nonresponse. The study suffers from a non-homogenous doses and method of administration of MTX for induction and maintenance. The range of induction dose of MTX was 2.5-25 mg/wk and administration varied as either im or po. Improvement was based on multiple variables as available from the charts, but was not standardized. With these caveats, clinical response occurred in 18 of 29 patients (62%) refractory to AZA/MP and 42 of 70 patients (60%) intolerant to AZA/MP. This suggests that MTX is effective in CD patients previously treated with AZA who experienced failure or non-response.
At present, there are no high quality trials (prospective, identical induction doses and method of administration, presence of control groups) on which to confidently choose to use MTX specifically in a population of AZA/6MP failures, but it would not be unreasonable to attempt MTX.
The landmark SONIC study demonstrated that patients with moderate-to-severe Crohn’s disease who were treated with combination infliximab plus azathioprine were more likely to have a corticosteroid-free clinical remission than those receiving azathioprine or infliximab monotherapy[23]. Concomitant immunosuppressive therapy also reduces the magnitude of the immunogenic response of infliximab[24]. It follows that methotrexate, as part of combination therapy with anti-TNF agents, may provide similar benefits.
Feagan et al[25] studied this hypothesis in the COMMIT trial. They performed a 50-wk double-blind, placebo-controlled trial of MTX + IFX vs IFX monotherapy in Crohn’s patients who had started prednisone therapy within the preceding 6 wk. Patients were not permitted to use any other therapy with the exception of antibiotics for 14 d in the case of active perianal disease. Patients were initiated on IFX 5 mg/wk and 10 mg sq MTX/week (escalating to 25 mg/wk by week 5) or IFX 5 mg/wk and placebo injections. Prednisone was force tapered in all patients by week 14. The primary outcome evaluated steroid free-remission by week 14 or maintenance of remission by week 50. Steroid-free remission at week 14 was 76% (48/63) in combination therapy compared to 78%(49/63) with IFX mono therapy (P = 0.83). At week 50, 56%(35/63) vs 57%(36/63) maintained remission in the combination arm vs monotherapy arm. Mean methotrexate doses at week 50 in the treatment arm was 22.3 mg/wk. This study found that combination therapy with IFX and MTX had no more benefit than IFX alone.
Based on the strongest current body of evidence (SONIC, COMMIT), it seems reasonable to prefer combination therapy using AZA/6MP rather than MTX in those Crohn’s patients able to tolerate it.
A prospective study by Vermeire evaluated the development of antibodies to infliximab (ATI) when combined with AZA, MTX, or placebo[26]. The concomitant use of immunosuppressive therapy (MTX or AZA) was associated with a lower incidence of antibodies to IFX (53/115, 46%) compared with patients not receiving concomitant immunosuppressive therapy (43/59, 73%; P < 0.0001). Furthermore, the incidence of antibody formation was not different between the MTX and AZA groups, 44% compared to 48% respectively. Patients not taking IS therapy had lower IFX levels (median 2.42 mcg/mL) 4 wk after any follow-up infusion than patients taking concomitant IS therapy (median 6.45 mcg/mL) (P = 0.065), but there was no difference between MTX or AZA. Sokol et al[27] confirm that patients using co-treatment with immunosuppressives experienced less IBD activity and less need to switch Anti-TNF therapy due to secondary loss of response. In fact, their data suggest efficacy of AZA over MTX, though their patient population included both CD and UC patients, and it is not clear whether any of the UC patients were treated with MTX and included in the analysis.
Although the COMMIT study did not show an improvement in 50 wk outcomes using combination therapy (IFX + MTX vs IFX alone), the MTX combination group did achieve statistically significant lower antibody levels (4% compared with 20%, P = 0.01) and demonstrated higher median serum trough levels of IFX (6.35 µg/mL vs 3.75 µg/mL, P = 0.08), similar to what is seen with azathioprine combination therapy[25]. Whether this would result in fewer instances of infusion reactions or secondary non-response to IFX beyond 50 wk remains to be seen.
Absah retrospectively evaluated 14 pediatric patients with moderate to severe (CD) eventually failing anti-TNF-α therapy (13 ADA and 1 IFX) who then received concomitant methotrexate (median dose 17.5 mg sq/wk)[28]. Most (12/14) patients had also previously failed AZA therapy (though it is not made clear whether this was as part of combination with biologic). Clinical remission was achieved in 7/14 (50%) of patients on average of 6 wk after MTX initiation with no additional improvement in the other 7 patients during 10 mo of follow up. Unfortunately, no levels of biologic or antibody to biologic were measured in this study, so the mechanism of improvement remains unknown. Further research focusing on the adult population along with mechanism of action would serve to direct therapy in this refractory population often seen in tertiary centers.
To date, only small retrospective series are available to evaluate the efficacy of MTX monotherapy in fistulizing Crohn’s disease. A research conducted a retrospective chart review of all Crohn’s disease receiving methotrexate 15-25 mg im MTX/weekly. This group of patients that had failed or were intolerant to 6MP and were made up of perianal fistulae (9), abdominal wall (3), rectovaginal (1), bladder (1), perianal + rectovaginal (2). Overall, 4/16 (25%) experienced complete fistula closure and 5/16 (31%) had partial fistula closure. Fourteen of sixteen patients received full dose 25 mg im/wk of MTX for 3 mo and were switched to po for maintenance. The time to response could not be determined in half of the patients, but ranged from 4-13 wk in the other half. Another study found that 8/18 (44%) patients with Crohn’s-related fistulas achieved partial or complete response using MTX for 6 mo, but information about success and failure based on oral or im administration was not provided[29]. A pilot study of 12 patients using combination infliximab and MTX found 7 patients had total or partial response to fistula, but there was no MTX only arm and the data seem similar to the benefit achieved with IFX monotherapy[30,31].
Approximately 10% of peri-anal and abdominal fistulas in Crohn’s heal spontaneously[31]. Given a closure rate well above the spontaneous closure rate, we consider MTX a potentially useful adjunct in management of Crohn’s fistulas.
Evidence pertaining to the utility of methotrexate in induction of remission for ulcerative colitis is conflicting (Table 2). Disparate results reflect disagreement over appropriate dosing and route of administration. To date, only one prospective, randomized placebo-controlled trial examining the efficacy of methotrexate in the treatment of ulcerative colitis exists; Oren et al[5] in 1996 compared 12.5 mg oral methotrexate to placebo in the induction of remission of 67 patients with moderate/severe UC[5,14]. All patients had active disease with a Mayo score of >7, and were taking steroids for at least 4 mo in the preceding year. The results were disappointing, with clinical remission rates of 46.7% (14/30) in the methotrexate arm in comparison to 48.6% (18/37) for the placebo arm, a non-significant difference. Of those who entered clinical remission, 64.3% of patients in the methotrexate arm had a relapse requiring steroid induction compared to 44.4% of placebo patients, again, an insignificant difference.
| Study | Dose (mean) | Route | No. of patients | Study design | Follow-up (wk) | MTX response | MTX remission | Placebo response |
| Kozarek | 25 mg | im | 7 | Open label | 12 | 5/7 (71.40%) | N/A | |
| Baron | 15 mg | Oral | 8 | Open label | 18 | 3/8 (37.5%) | 0 | N/A |
| Oren | 12.5 mg | Oral | 67 | Placebo control | 36 | 14/30 (46.7%) | 18/37 (48.6%) | |
| Egan | 15 mg 25 mg | scsc | 18 12 | Open label | 16 | 7/18 (39%) 4/12 (33%) | 3/18 (17%) 2/12 (17%) | N/A N/A |
| Mate-Jimenez | 15 mg | Oral | 34 | 6-MP control | 30 | 7/12 (58.30%) | 11/14 (78.6%) | |
| Paoluzi | 12.5 mg | im | 10 thiopurine resistant/intolerant | Open label | 26 | 10/10 (100%) | 6/10 (60%) | N/A |
| Cummings | 19.9 mg mean | Oral | 11 AZA failure 31 AZA intolerant | Retrospective | 30 | 3/11 (27%) 18/31 (58%) | 14/31 | N/A |
| Nathan | 20-25 mg | sc/ oral | 23 | Retrospective | N/A | 11/23 (48%) | N/A | |
| Wahed | 10-25 mg | Oral, sc | 9 thiopurine ineffective 23 thiopurine intolerant | Retrospective | 26 | 7/9 (78%) 15/23 (65%) | N/A | N/A |
| Manosa | 25 mg | Oral sc | 7 33 | Retrospective | 26 | 24/40 (60%) remission | N/A | |
| Saibeni | 20 mg | Oral/ sc/im | 23 | Retrospective | N/A | 11/23 (47.8%) | N/A | |
| Khan | 14 mg 25 mg | Oral sc/im | 68 23 | Retrospective | 60 | 25/68 (37%) 7/23 (30%) | N/A |
Overall, a low remission rate relative to placebo, long time to remission, and a high relapse rate in Oren’s study all suggest a lack of efficacy for methotrexate in either the induction or maintenance of remission in ulcerative colitis. Of course, important criticism may be directed at the relatively low dose of MTX used and the oral route of administration.
Otherwise, a number of small open-label and larger retrospective analyses have been conflicting, not least due to differing definitions of response, length of follow up (12 wk-2 years), dose of MTX (7.5-25 mg/wk), and route administered (po vs im). None of these studies were considered of sufficient quality to be included in the meta-analysis by Khan et al[14].
The most comprehensive of these was published last year by Khan et al[32], presenting retrospective data regarding experience with methotrexate in the Veterans Affairs (VA) system. A total of 91 patients with ulcerative colitis who were steroid dependent or refractory were commenced on oral (mean 14 mg) or parenteral (mean 25 mg) methotrexate. In the oral MTX cohort, 37% (25/68) were able to successfully wean from steroid therapy, compared to 30% (7/23) of the parenteral cohort.
Overall, looking specifically at induction of remission in ulcerative colitis, response to methotrexate ranged from 27%-100%, and remission rates ranged from 0%-63%. Considering the retrospective nature of most studies, it is impossible to determine the true impact of dose or route of administration. In prospective, open label or randomized controlled trials, response rates similarly ranged from 33%-100%, with remission rates ranging 17%-60%. There are no clear signals regarding the impact of dose, route of administration, or indication for step-up in therapy on remission or response rates in UC.
Regarding the maintenance of remission, the results are equally confusing - maintenance of remission rates range from 14%-75% (Table 3). Unfortunately, two open-labeled studies suggesting successful maintenance rates > 60%[10,33] using parenteral methotrexate did not include a placebo arm as comparison[10,33]. Oren et al[5] and Mate-Jimenez et al[12] included control arms, but provided disappointing results for the efficacy of oral methotrexate. Whether the route is a factor for better response rates remains to be seen.
| Study | Dose (mean) | Route | No. of pts | Study design | Follow-upperiod (mo) | MTX responsemaintained? | Controlresponse | Significantlyeffective? |
| Kozarek | > 7.5 mg | sc | 5 | Open label | 24 | 3/5 (60%) | N/A | N/A |
| Oren | 12.5 mg | oral | 32 | Placebo-controlled | 9 | 5/14 (36%) | 10/18 (56%) | No |
| Mate-Jimenez | 15 mg | oral | 12 | 6-MP control | 18 | 1/7 (14%) | 7/11 (64%) | No |
| Paoluzi | 12.5 mg | im | 10 | Open label | 24 | 6/8 (75%) | N/A | N/A |
| Manosa | 25 mg | Oral/ | 7 | Retrospective | 24 | 35% | N/A | |
| sc | 33 |
There has been no data to date investigating the utility of combining methotrexate with biologic therapy in UC. Increasing interest in using methotrexate as a “synergistic enhancer” - to augment and prolong biologic efficacy - may help define its role in this disease.
Injectable MTX is available in 50 mg/2 mL vials. We prescribe one vial (2 loading dose equivalents) as well as a supply of “tuberculin” 1 mL syringes with 27 guage, 1/2” needles. The patient draws 25 mg weekly from the vial and injects subcutaneously in either lower quadrant of the abdomen or inner thighs as their preference. After 12 wk, if they have a response, they can be transitioned to oral methotrexate maintenance. A patient friendly resource on injecting MTX is available via the Canadian rheumatology association (http://rheuminfo.com/wp-content/uploads/2011/04/METHOTREXATE_INJECTION_SHEET.pdf).
Oral methotrexate is available in 10 and 15 mg strengths as Trexall™. If using oral methotrexate in the induction of remission of IBD, we would recommend starting with 25 mg weekly, reverting to the subcutaneous route in non-responders and those who develop nausea attributed to the oral route.
All patients should be prescribed folic acid 1mg daily as it significantly reduces hepatic toxicity, an infrequent occurrence, and gastrointestinal toxicity associated with MTX[34,35]. At present, our target population for MTX are CD patients who are unable to tolerate azathioprine or 6Mercaptopurine due to adverse events, homozygous TMPT mutations, or inefficacy. In the event that methotrexate is required in a woman of child bearing age, we counsel regarding the need for effective contraception (i.e., IUD) and recommend a discussion with their obstetric physician. We advocate obtaining routine blood labs (complete blood count, basic chemistry panel, hepatic function panel) 1 wk after initiation as well as every 8-12 wk subsequently.
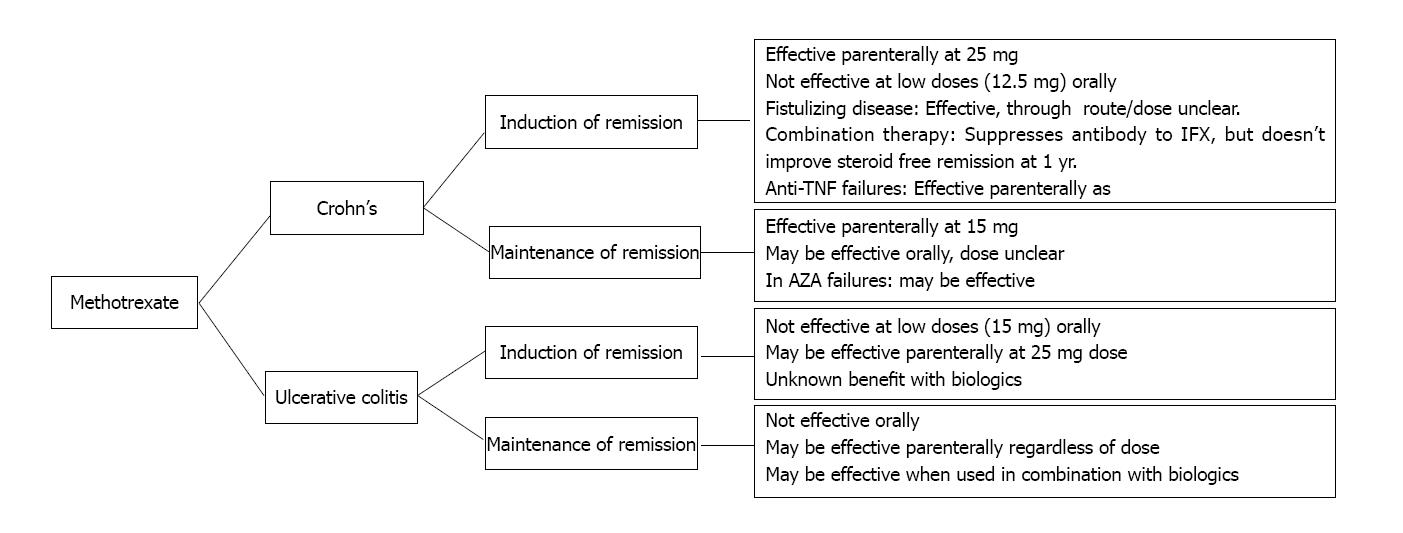
Given the current evidence an algorithm for MTX can be elucidated (Figure 1). Providers should no longer shy away from using MTX due to concerns of hepatotoxicity and intolerance. Methotrexate demonstrates a similar rate of drug withdrawal as AZA, and may be considered favorable in young males in whom practitioners are reluctant to use AZA (due to concerns of hepato-splenic T-cell lymphoma risk). Determining the optimal dose and route of administration in the various indications for use in IBD is the current priority. MTX is largely used as a second line therapy after AZA failure. It may be useful in combination with Anti-TNF therapy to reduce the risk of immunogenicity and subsequent secondary loss of response to anti-TNF therapy. We eagerly await the results of two studies that will shed further light; the METEOR trial and MERIT-UC, both randomized, controlled trials of parenteral MTX 25 mg weekly in the induction and maintenance of remission in steroid dependent or refractory ulcerative colitis.
P- Reviewer: Iacobellis F, Sung J S- Editor: Wen LL L- Editor: A E- Editor: Lu YJ
| 1. | Lower EE, Baughman RP. Prolonged use of methotrexate for sarcoidosis. Arch Intern Med. 1995;155:846-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 141] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 2. | Suarez-Almazor ME, Belseck E, Shea B, Wells G, Tugwell P. Methotrexate for rheumatoid arthritis. Cochrane Database Syst Rev. 2000;CD000957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Wenzel J. Methotrexate in systemic lupus erythematosus. Lupus. 2005;14:569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 4. | Ardizzone S, Bollani S, Manzionna G, Imbesi V, Colombo E, Bianchi Porro G. Comparison between methotrexate and azathioprine in the treatment of chronic active Crohn’s disease: a randomised, investigator-blind study. Dig Liver Dis. 2003;35:619-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 104] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 5. | Oren R, Arber N, Odes S, Moshkowitz M, Keter D, Pomeranz I, Ron Y, Reisfeld I, Broide E, Lavy A. Methotrexate in chronic active ulcerative colitis: a double-blind, randomized, Israeli multicenter trial. Gastroenterology. 1996;110:1416-1421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 188] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 6. | Jundt JW, Browne BA, Fiocco GP, Steele AD, Mock D. A comparison of low dose methotrexate bioavailability: oral solution, oral tablet, subcutaneous and intramuscular dosing. J Rheumatol. 1993;20:1845-1849. [PubMed] |
| 7. | Kurnik D, Loebstein R, Fishbein E, Almog S, Halkin H, Bar-Meir S, Chowers Y. Bioavailability of oral vs. subcutaneous low-dose methotrexate in patients with Crohn’s disease. Aliment Pharmacol Ther. 2003;18:57-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 69] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Hoekstra M, Haagsma C, Neef C, Proost J, Knuif A, van de Laar M. Splitting high-dose oral methotrexate improves bioavailability: a pharmacokinetic study in patients with rheumatoid arthritis. J Rheumatol. 2006;33:481-485. [PubMed] |
| 9. | Wilson A, Patel V, Chande N, Ponich T, Urquhart B, Asher L, Choi Y, Tirona R, Kim RB, Gregor JC. Pharmacokinetic profiles for oral and subcutaneous methotrexate in patients with Crohn’s disease. Aliment Pharmacol Ther. 2013;37:340-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Kozarek RA, Patterson DJ, Gelfand MD, Botoman VA, Ball TJ, Wilske KR. Methotrexate induces clinical and histologic remission in patients with refractory inflammatory bowel disease. Ann Intern Med. 1989;110:353-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 351] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 11. | Feagan BG, Rochon J, Fedorak RN, Irvine EJ, Wild G, Sutherland L, Steinhart AH, Greenberg GR, Gillies R, Hopkins M. Methotrexate for the treatment of Crohn’s disease. The North American Crohn’s Study Group Investigators. N Engl J Med. 1995;332:292-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 683] [Cited by in RCA: 607] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 12. | Maté-Jiménez J, Hermida C, Cantero-Perona J, Moreno-Otero R. 6-mercaptopurine or methotrexate added to prednisone induces and maintains remission in steroid-dependent inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2000;12:1227-1233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 150] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 13. | Arora S, Katkov W, Cooley J, Kemp JA, Johnston DE, Schapiro RH, Podolsky D. Methotrexate in Crohn’s disease: results of a randomized, double-blind, placebo-controlled trial. Hepatogastroenterology. 1999;46:1724-1729. [PubMed] |
| 14. | Khan KJ, Dubinsky MC, Ford AC, Ullman TA, Talley NJ, Moayyedi P. Efficacy of immunosuppressive therapy for inflammatory bowel disease: a systematic review and meta-analysis. Am J Gastroenterol. 2011;106:630-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 191] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 15. | McDonald JW, Tsoulis DJ, Macdonald JK, Feagan BG. Methotrexate for induction of remission in refractory Crohn’s disease. Cochrane Database Syst Rev. 2012;12:CD003459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Feagan BG, Fedorak RN, Irvine EJ, Wild G, Sutherland L, Steinhart AH, Greenberg GR, Koval J, Wong CJ, Hopkins M. A comparison of methotrexate with placebo for the maintenance of remission in Crohn’s disease. North American Crohn’s Study Group Investigators. N Engl J Med. 2000;342:1627-1632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 554] [Cited by in RCA: 511] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 17. | Fraser AG, Morton D, McGovern D, Travis S, Jewell DP. The efficacy of methotrexate for maintaining remission in inflammatory bowel disease. Aliment Pharmacol Ther. 2002;16:693-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 81] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 18. | Patel V, Macdonald JK, McDonald JW, Chande N. Methotrexate for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev. 2009;CD006884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Krishnareddy S, Swaminath A. When combination therapy isn’t working: emerging therapies for the management of inflammatory bowel disease. World J Gastroenterol. 2014;20:1139-1146. [PubMed] |
| 20. | Swaminath A, Lebwohl B, Capiak KM, Present DH. Practice patterns in the use of anti-tumor necrosis factor alpha agents in the management of Crohn’s disease: a US national practice survey comparing experts and non-experts. Dig Dis Sci. 2011;56:1160-1164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Lémann M, Zenjari T, Bouhnik Y, Cosnes J, Mesnard B, Rambaud JC, Modigliani R, Cortot A, Colombel JF. Methotrexate in Crohn’s disease: long-term efficacy and toxicity. Am J Gastroenterol. 2000;95:1730-1734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Wahed M, Louis-Auguste JR, Baxter LM, Limdi JK, McCartney SA, Lindsay JO, Bloom SL. Efficacy of methotrexate in Crohn’s disease and ulcerative colitis patients unresponsive or intolerant to azathioprine /mercaptopurine. Aliment Pharmacol Ther. 2009;30:614-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 23. | Colombel JF, Sandborn WJ, Reinisch W, Mantzaris GJ, Kornbluth A, Rachmilewitz D, Lichtiger S, D’Haens G, Diamond RH, Broussard DL. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med. 2010;362:1383-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2539] [Cited by in RCA: 2366] [Article Influence: 157.7] [Reference Citation Analysis (1)] |
| 24. | Baert F, Noman M, Vermeire S, Van Assche G, D’ Haens G, Carbonez A, Rutgeerts P. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn’s disease. N Engl J Med. 2003;348:601-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1523] [Cited by in RCA: 1518] [Article Influence: 69.0] [Reference Citation Analysis (0)] |
| 25. | Feagan BG, McDonald JW, Panaccione R, Enns RA, Bernstein CN, Ponich TP, Bourdages R, Macintosh DG, Dallaire C, Cohen A. Methotrexate in combination with infliximab is no more effective than infliximab alone in patients with Crohn’s disease. Gastroenterology. 2014;146:681-688.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 247] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 26. | Vermeire S, Noman M, Van Assche G, Baert F, D’Haens G, Rutgeerts P. Effectiveness of concomitant immunosuppressive therapy in suppressing the formation of antibodies to infliximab in Crohn’s disease. Gut. 2007;56:1226-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 450] [Cited by in RCA: 467] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 27. | Sokol H, Seksik P, Carrat F, Nion-Larmurier I, Vienne A, Beaugerie L, Cosnes J. Usefulness of co-treatment with immunomodulators in patients with inflammatory bowel disease treated with scheduled infliximab maintenance therapy. Gut. 2010;59:1363-1368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 144] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 28. | Absah I, Faubion WA. Concomitant therapy with methotrexate and anti-TNF-α in pediatric patients with refractory crohn’s colitis: a case series. Inflamm Bowel Dis. 2012;18:1488-1492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 29. | Soon SY, Ansari A, Yaneza M, Raoof S, Hirst J, Sanderson JD. Experience with the use of low-dose methotrexate for inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2004;16:921-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 30. | Schröder O, Blumenstein I, Schulte-Bockholt A, Stein J. Combining infliximab and methotrexate in fistulizing Crohn’s disease resistant or intolerant to azathioprine. Aliment Pharmacol Ther. 2004;19:295-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 47] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 31. | Bressler B, Sands BE. Review article: Medical therapy for fistulizing Crohn’s disease. Aliment Pharmacol Ther. 2006;24:1283-1293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | Khan N, Abbas AM, Moehlen M, Balart L. Methotrexate in ulcerative colitis: a nationwide retrospective cohort from the Veterans Affairs Health Care System. Inflamm Bowel Dis. 2013;19:1379-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 33. | Paoluzi OA, Pica R, Marcheggiano A, Crispino P, Iacopini F, Iannoni C, Rivera M, Paoluzi P. Azathioprine or methotrexate in the treatment of patients with steroid-dependent or steroid-resistant ulcerative colitis: results of an open-label study on efficacy and tolerability in inducing and maintaining remission. Aliment Pharmacol Ther. 2002;16:1751-1759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 98] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 34. | Prey S, Paul C. Effect of folic or folinic acid supplementation on methotrexate-associated safety and efficacy in inflammatory disease: a systematic review. Br J Dermatol. 2009;160:622-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 93] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 35. | Te HS, Schiano TD, Kuan SF, Hanauer SB, Conjeevaram HS, Baker AL. Hepatic effects of long-term methotrexate use in the treatment of inflammatory bowel disease. Am J Gastroenterol. 2000;95:3150-3156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 105] [Article Influence: 4.2] [Reference Citation Analysis (0)] |