Published online Nov 6, 2013. doi: 10.4292/wjgpt.v4.i4.113
Revised: August 9, 2013
Accepted: September 4, 2013
Published online: November 6, 2013
Processing time: 143 Days and 21.6 Hours
AIM: To validate methods for determining mast cell density, extracellular major basic protein content, and presence of fibrosis in esophageal eosinophilia.
METHODS: Twenty specimens with > 20 eosinophils/high-power field (hpf) classified as high eosinophil density (HE) and 20 specimens with < 5 eosinophils/hpf classified as low esophageal density (LE) were identified. All 40 specimens underwent immunohistochemical staining and trichrome staining. Mast cell density, extracellular major basic protein (MBP) density, and presence of subepithelial fibrosis were assessed in a standardized manner. All specimens were evaluated by two separate observers and by a single observer on two separate occasions to evaluate reproducibility of the methods.
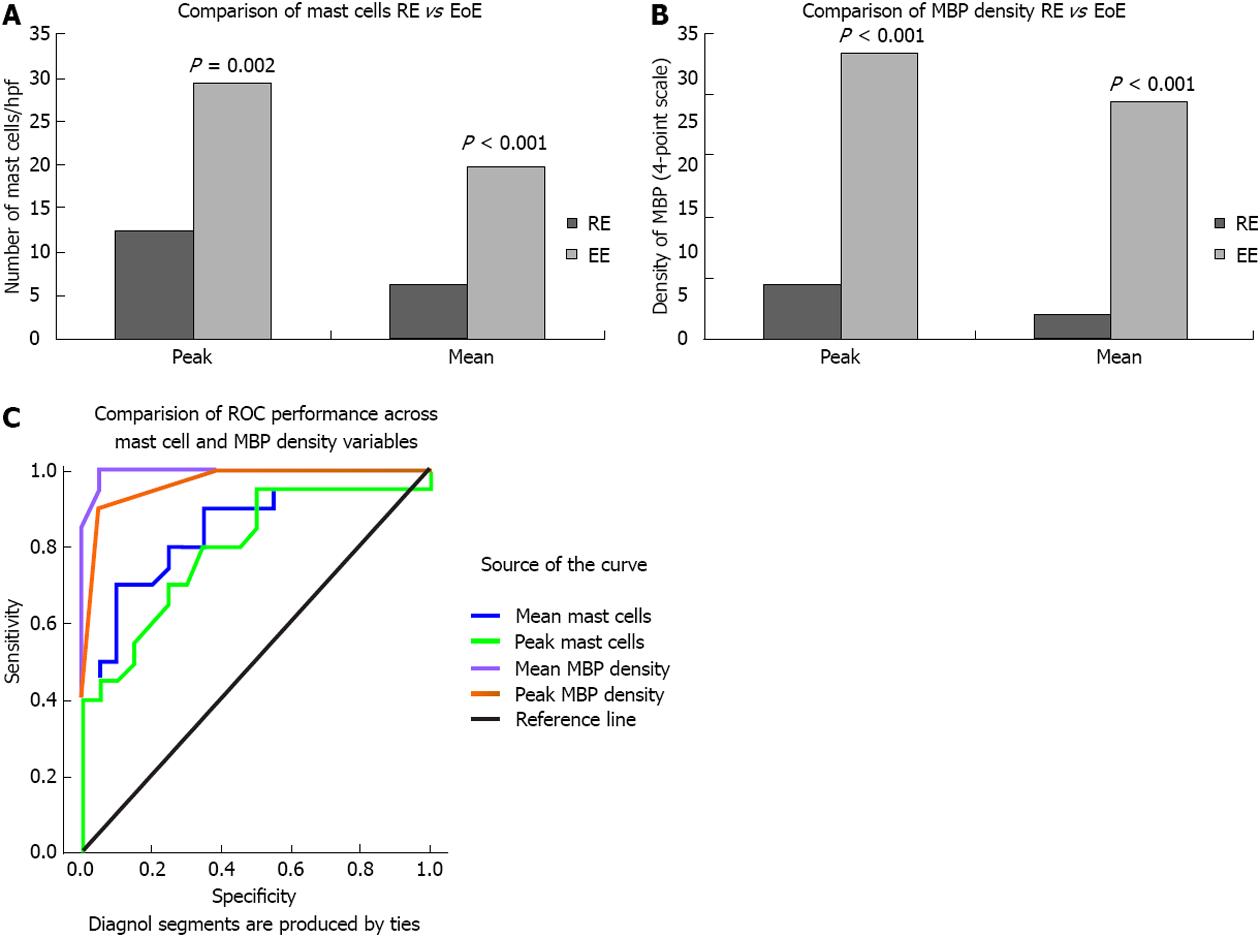
RESULTS: A strong inter-observer correlation was noted for both peak and mean mast cell counts (r = 0.725, P < 0.0001 and r = 0.823, P < 0.0001). A strong intra-observer correlation also was noted for both peak and mean mast cell counts (r = 0.752, P < 0.0001 and r = 0.878, P < 0.0001). A very strong inter-observer correlation was noted for both peak (τ = 0.867, P < 0.0001) and mean extracellular MBP densities (r = 0.925, P < 0.0001). A very strong intra-observer correlation was noted for both peak (τ = 0.875; P < 0.0001) and mean extracellular MBP densities (r = 0.956, P < 0.0001). Excellent inter-rater reliability was found for fibrosis (κ = 0.887). Mast cell and MBP densities, as well as presence of fibrosis, were significantly increased in HE vs LE. The HE group had significantly higher intraepithelial mast cell peak (29.35 ± 21.61 vs 12.45 ± 8.26, P = 0.002) and mean (19.84 ± 15.81 vs 6.35 ± 4.5, P = 0.001) densities than the LE group. The HE group had significantly higher peak extracellular MBP (2.35 ± 0.67 vs 0.45 ± 0.61, P < 0.001) and mean extracellular MBP (1.95 ± 0.76 vs 0.20 ± 0.29, P < 0.0001) densities than the LE group. Seventy-three percent of patients with HE (11/15) had fibrosis, whereas only 10% of patients with LE (1/10) had fibrosis (P < 0.01). MBP performed the best in predicting classification of HE vs LE, with mean MBP demonstrating 100% sensitivity and 95% specificity at the optimal cut point.
CONCLUSION: This study provides methodology and proof-of-concept for future evaluation of these biomarkers for differentiating esophageal eosinophilic diseases such as reflux esophagitis and eosinophilic esophagitis.
Core tip: Esophageal mucosal eosinophilia challenges many clinicians and researchers. Biomarkers have been proposed to help clarify and better characterize eosinophil driven disease. This study provides validation of methods used previously to differentiate low eosinophil density from high eosinophil density. Eosinophilic major basic protein appears to be the best predictor for classification of eosinophilia at both extremes. This lays the ground work for future studies to examine varying degrees of eosinophilia using biomarkers.
- Citation: Colombo JM, Neilan NA, Schurman JV, Friesen CA. Validation of methods to assess potential biomarkers in pediatric patients with esophageal eosinophilia. World J Gastrointest Pharmacol Ther 2013; 4(4): 113-119
- URL: https://www.wjgnet.com/2150-5349/full/v4/i4/113.htm
- DOI: https://dx.doi.org/10.4292/wjgpt.v4.i4.113
It is well known that the presence of eosinophils in esophageal mucosa denotes pathology; however, the basis for eosinophilic infiltration is not always clear and has been a topic of numerous studies in both children and adults[1]. Mucosal eosinophils are increased in both reflux esophagitis (RE) and eosinophilic esophagitis (EoE) as well as Candidal esophagitis, viral esophagitis and Crohn’s esophagitis to name a few. The two most common causes of increased eosinophils without other disease features are reflux esophagitis and eosinophilic esophagitis. Determining the density of eosinophils (i.e., cells per high-power field, hpf) present on biopsy specimens is one factor considered in distinguishing between these two diagnoses[1-6]. Most studies suggest that a significantly higher number of eosinophils are found in patients with EoE; however, the appropriate number needed to make a diagnosis of EoE is unclear[1]. This is problematic as the two forms of esophagitis have markedly different treatments and prognosis. Thus, finding additional biomarkers to aid in diagnosis is warranted.
Examination of the pathway through which eosinophils exert their influence in EoE may shed light on potential biomarkers. First, eosinophils, which are normally absent in the esophagus, may infiltrate the esophageal mucosa and become activated. Once activated, eosinophils release their granule proteins causing inflammation and tissue damage. Granule proteins, such as major basic protein (MBP), eosinophilic cationic protein, and eosinophilic peroxidase, have cytotoxic effects on esophageal epithelium[7-9]. Their pro-inflammatory properties continue the cycle of inflammation. Eosinophils elaborate fibrogenic growth factors and induce fibrogenesis through secretion of granule proteins such as MBP. MBP also triggers degranulation of mast cells which, in turn, release inflammatory mediators such as cytokines and histamine[7,8].
Mucosal mast cells may have a role as a biomarker in that they are increased in pediatric patients with EoE as compared to RE, or gastroesophageal reflux disease (GERD), which is often used interchangeably with RE[9]. Mast cells are present at higher densities in GERD patients exhibiting > 7 eosinophils/hpf[10]. Moreover, no intraepithelial esophageal mast cells are present in controls[10]. Further, mast cell density has been documented to decrease with treatment of EoE[11].
Extracellular MBP also shows promise as a biomarker for EoE. Chehade et al[12] classified eosinophil degranulation as either absent/mild or extensive based on the pattern of extracellular MBP. Extensive degranulation was more prevalent in EoE patients and the extent of degranulation was unrelated to eosinophil density. Mueller et al[5] studied EoE in adults, evaluating MBP by a semi-quantitative method, and found degranulation in 72%.
Fibrosis also may be a potential biomarker, as indicated by the increased frequency of esophageal stricture in EoE[1]. In children, esophageal subepithelial fibrosis has been demonstrated in 89% of EoE patients as compared to 37.5% of GERD patients[13]. Although fibrosis was found in a significant portion of the GERD patients, the pattern was clearly different than with EoE, as the fibrosis was associated with lymphoid tissue in GERD. Chehade et al[12] documented fibrosis in 57% of EoE pediatric patients and in no patients with GERD. Moreover, the presence of fibrosis in the EoE group was associated with a higher proportion of patients demonstrating extensive eosinophil degranulation[12].
Evaluation of mast cell density, extracellular MBP, and/or fibrosis may be useful in making a diagnosis of EoE, particularly in patients with eosinophil counts placing in the mid-range between typical reflux-associated esophagitis and EoE on biopsy. While mast cell density, extracellular MBP, and fibrosis all appear to be potential markers, the methods for histologic evaluation of these have varied and, further, reproducibility and reliability of these markers in differentiating RE and EoE has not yet been established.
The current study was performed in two steps. In part one, the aim was to establish reproducible methods for determining mast cell density, extracellular MBP content, and presence of fibrosis, respectively. In part two, the primary aim was to determine whether the reproducible markers validated in the first step would reliably differentiate between patients with high esophageal density and patients with low esophageal density. A secondary aim was to explore relationships between eosinophil density and the potential biomarkers.
The study was approved by the institutional review board at Children’s Mercy Hospital, Kansas City, Missouri.
Archived distal esophageal specimens from patients previously undergoing endoscopy by a pediatric gastroenterologist during a one year period were identified from the pathology database at Children’s Mercy Hospital. No medical chart review was performed for this methodology study. Specimens with esophageal eosinophilia were reviewed. Twenty cases classified as low esophageal density (LE; 1-4 eosinophils/hpf) and twenty cases classified as high esophageal density (HE; > 20 eosinophils/hpf) were chosen. All biopsy specimens, which were previously fixed in formalin, embedded in paraffin, sliced (5-μm sections), and stained with haematoxylin and eosin, were independently evaluated by a pediatric gastroenterologist who confirmed the number of eosinophils/hpf prior to inclusion of the specimen in this study. The entire specimen was scanned to identify the subjective area of greatest eosinophil density. Eosinophils were counted in five consecutive hpfs. Peak and mean (the average of the 5 hpfs) eosinophil counts were recorded.
Mast cells: The archival tissue blocks of esophageal mucosa were evaluated by immunohistochemical (IHC) staining using the labeled streptavidin-biotin (LSAB) method. The paraffin-embedded tissue specimens were de-paraffinized using a xylenes-substitute followed by 100% ethanol then rehydrated in aqueous buffer. No antigen retrieval was performed on tissue for anti-tryptase staining. The primary antibody fixation occurred using mouse monoclonal anti-human mast-cell tryptase (Clone AA1) diluted to 1:1000. Tissues were incubated with anti-tryptase for 1 h at room temperature (20 °C-25 °C). After washing in aqueous buffer, a secondary antibody using biotin-anti immunoglobulin G (IgG) was incubated for 20 min at room temperature. The specimens again were washed in aqueous buffer. Specimens were incubated with streptavidin-horseradish peroxidase at room temperature for 20 min, washed in aqueous buffer, followed by the addition of diaminobenzidine (DAB) hydrogen peroxide solution for 5 min at room temperature. Specimens were washed in running tap water for 5 min. All specimens were counterstained with haematoxylin followed by bluing solution and then mounted for microscopic exam.
Major basic protein: The archival tissue blocks of esophageal mucosa were evaluated by IHC staining using the LSAB method. The paraffin-embedded tissue specimens were de-paraffinized using a xylenes-substitute followed by 100% ethanol then rehydrated in aqueous buffer. Tissue was digested with 0.5% pepsin for 30 min at 37 °C for antigen retrieval. Peroxidase activity was inactivated using 3% hydrogen peroxide solution for 5 min at room temperature. To reduce background staining, normal goat serum block (5% goat serum in distilled water) and avidin/biotin blocking systems were used. The primary antibody fixation occurred using mouse monoclonal anti-human e-MBP (Clone BMK13) diluted to 1:30. Tissues were incubated with anti-MBP for 24 h at 4 °C. After washing in aqueous buffer, a secondary antibody using biotin-anti IgG was incubated for 20 min at room temperature. The specimens were washed in aqueous buffer. Specimens were incubated with streptavidin-horseradish peroxidase at room temperature for 20 min, washed in aqueous buffer, followed by the addition of DAB hydrogen peroxide solution for 5 min at room temperature. Specimens were washed in running tap water for 5 min. All specimens were counterstained with haematoxylin followed by bluing solution and then mounted for microscopic exam.
Fibrosis: The archival tissue blocks of esophageal mucosa were subjected to trichrome staining using Gomori’s one-step method. The paraffin-embedded tissue specimens were de-paraffinized using xylenes and then rehydrated in distilled water. Slides were pretreated with hot Bouin’s solution for 1 h. After washing well in running water to remove all yellow color, slides were placed in Gill’s Haematoxylin for 5 min. Slides were washed in tap water then stained with Gomori’s trichrome for 10 min. They were rinsed briefly in 1% acetic acid solution then rinsed quickly in distilled water. The tissue specimens were dehydrated in 100% ethyl alcohol and then mounted for microscopic exam.
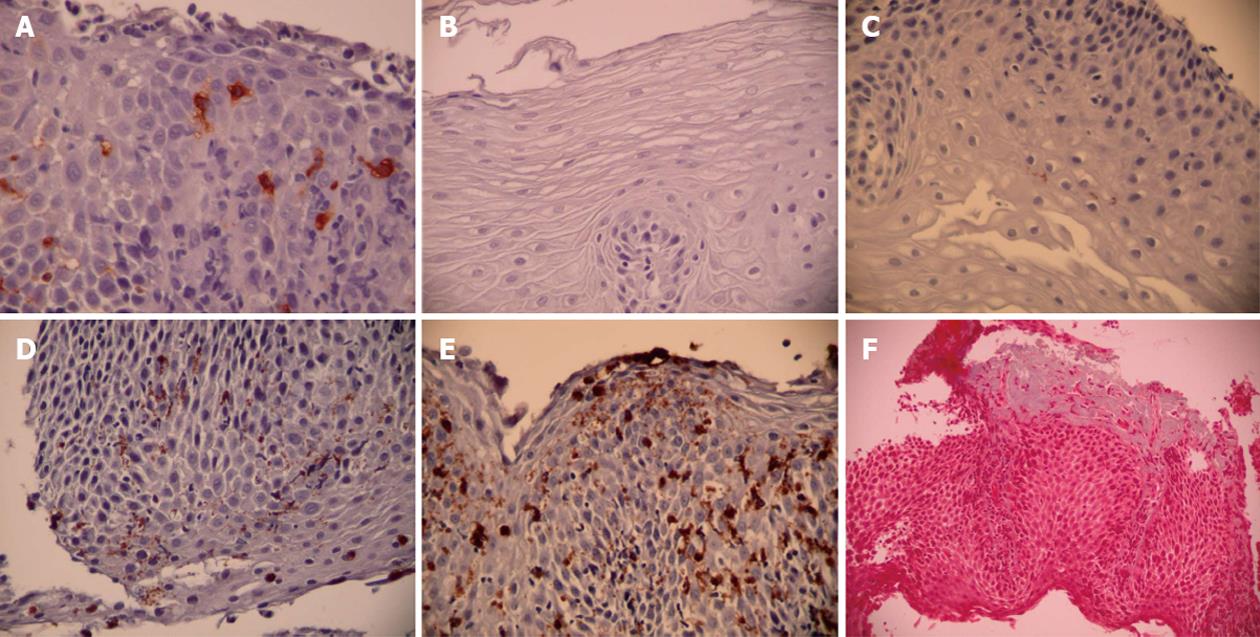
Mast cell density: Mast cell density was assessed by subjectively identifying the area of greatest involvement after scanning the entire specimen and then counting tryptase-positive cells in 5 consecutive hpfs (Figure 1A). Mast cell enumeration was performed by 2 blinded observers and by one of the observers (Observer 1) on 2 separate occasions separated by at least one week to establish inter-rater and intra-rater reliability for the method. Peak and mean mast cell densities were recorded.
Major basic protein: Specimens initially were subjectively evaluated in a blinded fashion by a pathologist and two observers to determine a stratification strategy for extracellular MBP. MBP has been previously been evaluated by semi-quantitative methods with classification into either 2 or 4 categories[5,12]. A decision was made to employ a 4-point scale as follows: 0, none; 1, mild (< 5% involvement of MBP granules); 2, moderate (5%-25% involvement of MBP granules); and 3, severe (> 25% involvement of MBP granules) (Figure 1B-E). We elected to evaluate with 4 categories because our pre-decision specimen evaluation seemed to indicate that this was a feasible solution and because 4 categories gave us the possibility of a more robust method if reproducible. Extracellular MBP density was assessed by subjectively identifying the area of greatest involvement after scanning the entire specimen and then rating MBP density on the 4 point scale in 5 consecutive hpfs. MBP evaluation was performed by the same 2 blinded observers and by one of the observers (Observer 1) on 2 separate occasions separated by at least one week to establish inter-rater and intra-rater reliability for the method. Peak and mean densities of extracellular MBP were recorded.
Fibrosis: Subepithelial fibrosis was assessed by the trichrome stain and rated as normal (no fibrosis seen) or abnormal (increased collagen deposition) (Figure 1F). The entire specimen was scanned to determine the presence of collagen deposition. Specimens were evaluated by the same 2 blinded observers and by one of the observers (Observer 1) on 2 separate occasions separated by at least one week to establish inter-rater and intra-rater reliability for the method.
Measures of agreement were calculated to establish reliability between the two observers for each of the three markers. Measures of agreement also were calculated for mast cell density and extracellular MBP to establish reproducibility on the two separate evaluations by Observer 1; no reproducibility evaluation was conducted for fibrosis given the simple dichotomous nature of this variable. Pearson’s correlation was employed for mast cell density and mean extracellular MBP, while Kendall’s tau was used for peak extracellular MBP and kappa was used for fibrosis.
Once reproducible methods were obtained, differences in mast cell density, extracellular MBP, and presence of subepithelial fibrosis were compared between HE and LE by a combination of Student’s t test and χ2. Receiver operating characteristic (ROC) curves were used to determine the sensitivity and specificity of different mast cell and extracellular MBP densities, respectively, in predicting classification group membership (i.e., HE vs LE) based on eosinophil counts completed at the time of biopsy. Correlations between eosinophil, mast cell, and extracellular MBP densities were evaluated by Pearson’s correlation. Statistical analysis was performed with SPSS version 16.0. A P value of 0.05 was considered significant.
The HE group had significantly more eosinophils/hpf (peak: 96.45 ± 45.6; mean: 63.07 ± 27.99) than the LE group (peak: 2.10 ± 1.07; mean: 0.86 ± 0.61, P < 0.0001). Peak eosinophil density ranged from 39-201/hpf in the HE patients and from 1-4/hpf in the LE group. In all cases, the original classification was confirmed and served as the gold standard for group assignment (HE vs LE).
Mast cell density: A strong inter-observer correlation was noted for both peak and mean mast cell counts (r = 0.725, P < 0.0001 and r = 0.823, P < 0.0001). A strong intraobserver correlation also was noted for both peak and mean mast cell counts (r = 0.752, P < 0.0001 and r = 0.878, P < 0.0001).
Major basic protein: A very strong inter-observer correlation was noted for both peak (τ = 0.867, P < 0.0001) and mean extracellular MBP densities (r = 0.925, P < 0.0001). A very strong intra-observer correlation was noted for both peak (τ = 0.875, P < 0.0001) and mean extracellular MBP densities (r = 0.956, P < 0.0001).
Fibrosis: Excellent inter-rater reliability was found for fibrosis (κ = 0.887).
Mast cell density: The HE group had significantly higher intraepithelial mast cell peak (29.35 ± 21.61 vs 12.45 ± 8.26, P = 0.002) and mean (19.84 ± 15.81 vs 6.35 ± 4.5, P = 0.001) densities than the LE group (Figure 2A). Peak mast cell density ranged from 3-89 in the HE group and from 4-32 in the LE group. Mean mast cell density ranged from 1.4-65.0 in the HE group and from 2.0-17.8 in the LE group. ROC curve analysis indicated that both mean (AUC = 0.839, P < 0.0001) and peak (AUC = 0.795, P < 0.001) mast cell density differentiate between HE and LE, but no specific cut point could be identified with adequate sensitivity and specificity. The best performer in accurately classifying HE was a cut-off 11.2 mast cells/hpf (i.e., average across 5 consecutive hpfs in the area deemed to have greatest involvement after visual scan), which detected a true positive classification for HE 70% of the time and a false positive classification for HE 10% of the time.
Major basic protein: The HE group had significantly higher peak extracellular MBP (2.35 ± 0.67 vs 0.45 ± 0.61, P < 0.001) and mean extracellular MBP (1.95 ± 0.76 vs 0.20 ± 0.29, P < 0.0001) densities than the LE group (Figure 2B). Ninety percent of patients with HE (18/20) had moderate to severe peak staining with extracellular MBP, whereas 95% of patients with LE (19/20) had none to mild peak staining for extracellular MBP. ROC curve analysis indicated that both mean (AUC = 0.995, P < 0.0001) and peak (AUC = 0.966, P < 0.0001) extracellular MBP density differentiate between HE and LE. The best performer in accurately classifying HE was a cut-off of 0.7 for mean extracellular MBP (i.e., average across 5 consecutive hpfs in the area deemed to have greatest involvement after visual scan), which detected a true positive classification for HE 100% of the time and a false positive classification for HE only 5% of the time (Figure 2C).
Fibrosis: Fifteen specimens were excluded because of the lack of lamina propria. Seventy-three percent of patients with HE (11/15) had fibrosis, whereas only 10% of patients with LE (1/10) had fibrosis (P < 0.01).
Relationships between markers in HE patients: Mean eosinophil density was correlated with both peak (r = 0.495, P = 0.03) and mean (r = 0.610, P = 0.004) extracellular MBP density, while peak eosinophil density was correlated with mean (r = 0.539, P = 0.01), but not peak, extracellular MBP density. There was no correlation between eosinophil density and mast cell density or between mast cell density and extracellular MBP density, respectively. There was no relationship between the presence of fibrosis and densities of eosinophils, mast cells, or extracellular MBP, respectively.
When attempting to establish clinically meaningful biomarkers, the process includes several challenging steps. Establishing reproducible and valid methods for measuring or identifying the markers, demonstrating that the markers differ, noting adequate sensitivity and specificity of the markers, and demonstrating that the marker prospectively predicts treatment response, disease progression, and/or prognosis are all necessary components. The current study was undertaken to evaluate the first of these steps for three potential biomarkers supported by previous studies as differing in patients with HE vs LE, namely: mast cell density, extracellular MBP density, and the presence of fibrosis. The esophageal specimens in this study were chosen at extremes (LE < 5 eosinophils/hpf and HE > 20 eosinophils/hpf) in an attempt to validate each of the biomarkers individually. A future direction would apply this validated methodology to varying degrees of eosinophilia and diagnostic dilemmas.
The methodology of immunohistochemical staining to differentiate extreme degrees of eosinophilia is valid based on our study. Applying this method to future studies with less extreme degrees of eosinophilia is warranted. If this method can accurately distinguish varying degrees of eosinophilia on initial biopsies, then follow-up biopsies which are often necessary to clarify diagnoses can be eliminated. For example, distinguishing reflux esophagitis from eosinophilic esophagitis has important implications for the patient, as the treatment and natural history vary greatly for each disorder. Currently, an initial biopsy with determination of the number of eosinophils is performed on patients with clinical symptoms of dysphagia, vomiting, acid reflux, and/or abdominal pain. If a patient is not taking proton pump inhibitor, a trial of high dose proton pump inhibitor is usually followed by a second endoscopy with biopsy to clarify or confirm the diagnosis and hence distinguish between severe RE or GERD and EoE.
Basal cell hyperplasia, papillary elongation, and eosinophilic infiltration are all non-specific findings of esophagitis, even though they tend to be more prominent in patients with EoE[3,5,6,13-15]. The actual number of eosinophils per hpf is the only current histologic parameter used, in part, to establish the diagnosis of EoE. This might be simple enough for the most mild cases of RE and the most severe cases of EoE; however, clear cut-offs are not established and in the subtle, indeterminate cases, it is clearly not enough. Identifying biomarkers capable of differentiating EoE and RE has the potential to decrease the diagnostic burden of additional tests, the costs of evaluation, and sequelae associated with treatment delay in EoE.
We also sought to provide proof-of-concept for the second step by determining whether the markers differed between HE and LE. We were able to demonstrate excellent intra-and inter-observer reproducibility for the methodologies employed, with reliabilities above 0.80 for all methods except peak mast cell density counts, which fell only slightly below this threshold (0.73-0.75).
After demonstrating reproducibility, we undertook the next step of evaluating whether the particular markers could predict group assignment for HE vs LE. Mast cell density, density of extracellular MBP, and presence of fibrosis were all significantly greater in the HE group when compared to the LE group. These findings are consistent with previous studies evaluating histological differences between RE and EoE in both children and adults[5,10,12,16,17]. However, no particular cut point could be identified for mast cell density which demonstrated both good sensitivity and specificity. The presence of fibrosis appeared to be more useful in differentiating between EoE and RE. Fibrosis was present in 73% of HE patients and 10% of LE patients. However, the biggest challenge with using fibrosis as a marker is having biopsies deep enough to be evaluable. We had to exclude 38% of our specimens because we did not have adequate lamina propria. This difficulty has been previously reported[2]. In the current study, quantifying extracellular MBP appeared to be the most promising method for differentiating HE and LE (and potentially EoE from RE), with mean MBP density, in particular, yielding excellent sensitivity and specificity in the diagnostic classification of HE.
Extracellular MBP is a marker of eosinophil activation and degranulation. Previous electron microscopic studies of esophageal eosinophils in esophagitis have demonstrated eosinophil activation indicated by inversion of core-to-matrix densities and lucency of core protein[18]. This core lucency corresponds to the release of MBP. From a practical standpoint, the evaluation of extracellular MBP can be performed on the routine biopsies obtained during endoscopy but does require immunohistochemical staining, as MBP or extracellular granules are not sufficiently detected by routine staining[5]. The cost and feasibility of IHC staining for extracellular MBP granules appear reasonable; however, this would need to be confirmed with future prospective studies. Although there was moderate correlation between eosinophil density and MBP density, eosinophil enumeration alone does not predict MBP density; thus, while related, these two measures appear non-redundant and may add unique information helpful in the diagnosis of EoE.
We have identified reproducible methodologies for evaluating three potential biomarkers in differentiating LE from HE. Of the three, semi-quantitative assessment of extracellular MBP appears to be the most promising, with both mean and peak values performing well in terms of sensitivity and specificity on a group of patients with HE based on ROC curve analysis. It remains to be seen whether extracellular MBP can add to diagnostic differentiation in prospective studies, particularly histologically indeterminate cases, but this is certainly an important direction for future research. This is a very important topic for future studies. It would be necessary to correlate a sensitive and specific biomarker with clinical symptoms, disease activity, pathology findings, and treatment response. This initial validation of methodology study provides evidence for future studies. Future work in this area will help establish whether extracellular MBP, or other potential biomarkers for EoE, will be able to predict responses to treatment or prognosis in a prospective fashion to reduce diagnostic burden, evaluation costs, and sequelae associated with treatment delay in EoE.
The authors thank Vivekenend Singh, MD, Pediatric Pathology, Children’s Mercy Hospitals and Clinics for providing the images of the histopathology slides.
Inflammation in the esophagus can cause clinical symptoms of pain in the chest or upper abdomen, difficulty swallowing, vomiting or regurgitation. The most common type of inflammation in the esophagus is due to eosinophilic inflammation. While a number of disorders can cause eosinophilic inflammation, the two most common are reflux esophagitis and eosinophilic esophagitis. These two disorders have similar clinical complaints and are challenging to differentiate. These disorders have different treatment pathways so it is important to make not only an accurate, but also a timely diagnosis. Using biomarkers to help distinguish these two disease entities will allow for more efficient and accurate diagnoses. There have not been any established or validated biomarkers for these diseases to date.
Identifying biomarkers for diagnosis and management of eosinophilic disorders of the esophagus is a current hotspot for research. A biomarker that is reproducible, inexpensive, non-invasive, and corresponds to the severity of disease would be a major advantage in the treatment of children with eosinophilic disorders of the esophagus.
To date, biomarkers for eosinophilic disease of the esophagus have been described; however, not validated for their reliability or reproducibility. This study is the first step in validation of methods to assess for potential biomarkers. Being able to differentiate between low eosinophil density vs high eosinophil density will aid in timely diagnosis of disease and decrease the need for multiple biopsies on separate occasions to better characterize the disorder.
This study demonstrated that extracellular major basic protein is the best predictor of determining the degree of esophageal eosinophilia at two extremes. This was both reliable and reproducible. This provides a basis for future studies to examine varying degrees of eosinophilia using extracellular major basic protein as a biomarker for eosinophilic disease of the esophagus.
Eosinophils: Eosinophils are white blood cells that are normally produced in the body in response to allergic or parasitic conditions; Major basic protein: Major basic protein (MBP) is a granule protein released by the activated eosinophil. MBP also triggers degranulation of mast cells which, in turn, release inflammatory mediators such as cytokines and histamine. Immunohistochemical staining: Immunohistochemical staining is a process for detecting proteins by using antibodies that bind to specific antigens.
The present study is an interesting study for validation of methods for assessing potential biomarkers for eosinophilic disease of the esophagus. Future work should include correlation of the validated methods with symptoms, disease severity and treatment response.
P- Reviewers: Alshehabi Z, Fouad YM, Hashimoto N, Schwarz AM, Syam AF S- Editor: Gou SX L- Editor: A E- Editor: Wang CH
| 1. | Liacouras CA, Furuta GT, Hirano I, Atkins D, Attwood SE, Bonis PA, Burks AW, Chehade M, Collins MH, Dellon ES. Eosinophilic esophagitis: updated consensus recommendations for children and adults. J Allergy Clin Immunol. 2011;128:3-20.e6; quiz 21-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1605] [Cited by in RCA: 1486] [Article Influence: 106.1] [Reference Citation Analysis (1)] |
| 2. | Parfitt JR, Gregor JC, Suskin NG, Jawa HA, Driman DK. Eosinophilic esophagitis in adults: distinguishing features from gastroesophageal reflux disease: a study of 41 patients. Mod Pathol. 2006;19:90-96. [PubMed] |
| 3. | Steiner SJ, Kernek KM, Fitzgerald JF. Severity of basal cell hyperplasia differs in reflux versus eosinophilic esophagitis. J Pediatr Gastroenterol Nutr. 2006;42:506-509. [PubMed] |
| 4. | Steiner SJ, Gupta SK, Croffie JM, Fitzgerald JF. Correlation between number of eosinophils and reflux index on same day esophageal biopsy and 24 hour esophageal pH monitoring. Am J Gastroenterol. 2004;99:801-805. [PubMed] |
| 5. | Mueller S, Aigner T, Neureiter D, Stolte M. Eosinophil infiltration and degranulation in oesophageal mucosa from adult patients with eosinophilic oesophagitis: a retrospective and comparative study on pathological biopsy. J Clin Pathol. 2006;59:1175-1180. [PubMed] |
| 6. | Dahms BB. Reflux esophagitis: sequelae and differential diagnosis in infants and children including eosinophilic esophagitis. Pediatr Dev Pathol. 2004;7:5-16. [PubMed] |
| 7. | Rothenberg ME. Eosinophilic gastrointestinal disorders (EGID). J Allergy Clin Immunol. 2004;113:11-28; quiz 29. [PubMed] |
| 8. | Blanchard C, Rothenberg ME. Basic pathogenesis of eosinophilic esophagitis. Gastrointest Endosc Clin N Am. 2008;18:133-143; x. [PubMed] |
| 9. | Aceves SS, Furuta GT, Spechler SJ. Integrated approach to treatment of children and adults with eosinophilic esophagitis. Gastrointest Endosc Clin N Am. 2008;18:195-217. [PubMed] |
| 10. | Kirsch R, Bokhary R, Marcon MA, Cutz E. Activated mucosal mast cells differentiate eosinophilic (allergic) esophagitis from gastroesophageal reflux disease. J Pediatr Gastroenterol Nutr. 2007;44:20-26. [PubMed] |
| 11. | Lucendo AJ, Bellón T, Lucendo B. The role of mast cells in eosinophilic esophagitis. Pediatr Allergy Immunol. 2009;20:512-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Chehade M, Sampson HA, Morotti RA, Magid MS. Esophageal subepithelial fibrosis in children with eosinophilic esophagitis. J Pediatr Gastroenterol Nutr. 2007;45:319-328. [PubMed] |
| 13. | Li-Kim-Moy JP, Tobias V, Day AS, Leach S, Lemberg DA. Esophageal subepithelial fibrosis and hyalinization are features of eosinophilic esophagitis. J Pediatr Gastroenterol Nutr. 2011;52:147-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Rodrigo S, Abboud G, Oh D, DeMeester SR, Hagen J, Lipham J, DeMeester TR, Chandrasoma P. High intraepithelial eosinophil counts in esophageal squamous epithelium are not specific for eosinophilic esophagitis in adults. Am J Gastroenterol. 2008;103:435-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 141] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 15. | Sayej WN, Patel R, Baker RD, Tron E, Baker SS. Treatment with high-dose proton pump inhibitors helps distinguish eosinophilic esophagitis from noneosinophilic esophagitis. J Pediatr Gastroenterol Nutr. 2009;49:393-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 106] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 16. | Lucendo AJ, Navarro M, Comas C, Pascual JM, Burgos E, Santamaría L, Larrauri J. Immunophenotypic characterization and quantification of the epithelial inflammatory infiltrate in eosinophilic esophagitis through stereology: an analysis of the cellular mechanisms of the disease and the immunologic capacity of the esophagus. Am J Surg Pathol. 2007;31:598-606. [PubMed] |
| 17. | Aceves SS, Newbury RO, Dohil R, Bastian JF, Broide DH. Esophageal remodeling in pediatric eosinophilic esophagitis. J Allergy Clin Immunol. 2007;119:206-212. [PubMed] |
| 18. | Justinich CJ, Ricci A, Kalafus DA, Treem WR, Hyams JS, Kreutzer DL. Activated eosinophils in esophagitis in children: a transmission electron microscopic study. J Pediatr Gastroenterol Nutr. 1997;25:194-198. [PubMed] |