Published online Aug 6, 2013. doi: 10.4292/wjgpt.v4.i3.49
Revised: June 20, 2013
Accepted: June 28, 2013
Published online: August 6, 2013
Processing time: 91 Days and 17.8 Hours
While oral iron supplementation is commonly used throughout many clinical setting, treatment with intravenous (IV) iron has historically been reserved for specific settings, such as chronic kidney disease, gynecologic issues, and anemia associated with cancer and its treatments. However, the use of IV iron has begun to gain popularity in the treatment of iron deficiency anemia (IDA) associated with two conditions that are being seen more frequently than in years past: patients who are status post gastric bypass procedure and those with inflammatory bowel disease (IBD). The Roux-en-Y procedure involves connecting a gastric pouch to the jejunum, creating a blind loop consisting of distal stomach, duodenum, and proximal jejunum that connects to the Roux limb to form a common tract. IDA occurs in 6%-50% of patients who have undergone a gastric bypass, the etiology being multifactorial. The proximal gastric pouch, the primary site of gastric acid secretion, is bypassed, resulting in a decreased ability to metabolize molecular iron. Once metabolized, most iron is absorbed in the duodenum, which is entirely bypassed. After undergoing bypass procedures, most patients significantly limit their intake of red meat, another factor contributing to post-bypass IDA. Chronic anemia occurs in approximately 1/3 of patients who suffer from IBD, and almost half of all IBD patients are iron deficient. IBD leads to IDA through multiple mechanisms, including chronic intestinal blood loss, decreased absorption capabilities of the duodenum secondary to inflammation, and an inability of many IBD patients to tolerate the side effects of oral ferrous sulfate. In this study, we reviewed the charts of all patients who received IV iron at Sylvester Comprehensive Cancer Center/University of Miami Hospital Clinic from January 2007 to May 2012. The most common indications for IV iron were for issues related to cancer and its treatment (21.9%), IBD (20.1%), and gastric bypass (15.0%). Of the 262 patients who received IV iron, 230 received iron sucrose and 36 received iron dextran. While doses of 100, 200, 300, and 400 mg of iron sucrose were given, 100 and 200 mg were by far the most common dosages used, 122 and 120 times, respectively. The number of dosages of iron sucrose given ranged from 1 to 46, with a mean of 5.5 and a median of 4 doses. The average dose of iron dextran given was 870.5 mg, with 1000 mg being the most common dosage used. Most patients (22 of 36) who received iron dextran only received one dose. While patients with traditional indications for IV iron, such as gynecologic issues and kidney disease, still were represented in this study, we expect to see a continued increase in physicians using IV iron for emerging gastrointestinal indications, especially considering the increased safety of new low-molecular formulations.
Core tip: Decreased absorption of oral iron leading to iron deficiency is a significant cause of anemia in several patient groups, including those status post gastric bypass surgery and those with inflammatory bowel disease. In these patients, oral iron supplementation is unlikely to correct the deficiency. Intravenous iron is a safe, effective treatment strategy for overcoming the iron deficit seen in these patients, resulting in better outcomes and improved quality of life.
- Citation: Warsch S, Byrnes J. Emerging causes of iron deficiency anemia refractory to oral iron supplementation. World J Gastrointest Pharmacol Ther 2013; 4(3): 49-53
- URL: https://www.wjgnet.com/2150-5349/full/v4/i3/49.htm
- DOI: https://dx.doi.org/10.4292/wjgpt.v4.i3.49
Intravenous iron has been available for medical use for over 60 years. Traditional indications for its use include medical conditions such as inflammatory bowel disease (IBD), chronic kidney disease (CKD), chronic inflammatory arthritis, congestive heart failure, pregnancy and postpartum state, and cancer, as well as orthopedic, cardiac, colorectal, and gynecologic procedures[1]. The development of recombinant human erythropoietin (EPO) in the late 1980’s led to a renewed interest in its use as combination therapy in the setting of such conditions as CKD and malignancies. In a randomized study of 132 hemodialysis patients, the use of intravenous (IV) iron as an adjunct to EPO led to a greater increase in hemoglobin (Hgb) levels, the need for fewer doses of EPO, and less adverse effects associated with EPO use[2].
Iron dextran can lead to both local and systemic side effects. The most commonly reported local reactions include pruritus, pain, phlebitis, and muscle necrosis[3]. A retrospective study of patients who received IV iron for CKD compared the safety of the dextran to the sucrose preparation[4]. A total of 979 doses of dextran and 504 of sucrose were given, with 3 minor adverse events (AEs) occurring in the dextran group and 1 in the sucrose group. No serious AEs or anaphylactic reactions were reported in either group. Another retrospective study examined 619 patients who had received IV iron over a 2 year period[5]. Overall, there were 32 reported AEs, but no serious AEs or anaphylactic reactions. Larger retrospective studies have shown the rate of serious AEs with iron dextran to range from 0.0002% to 0.032%, with rates of serious AEs due to iron sucrose much lower[6]. The primary reason for the increased safety of iron sucrose is likely due to the fact that iron sucrose induces less sensitivity reactions that iron dextran does. A large safety review showed that sucrose induced 3.3 allergy episodes per million doses, while dextran led to 8.7 allergy events per million doses[7].
The Roux-en-Y procedure, the most commonly used method of gastric bypass surgeries, involves connecting a gastric pouch to the jejunum, creating a blind loop consisting of distal stomach, duodenum, and proximal jejunum that is connected to the Roux limb to form a common tract. A retrospective study of 150 patients who received gastric bypass found that 36.8% developed anemia[8]. The mean time from operation to the development of anemia was 20 mo. Almost 50% had a low serum iron concentration. A more recent prospective study followed 348 patients who had undergone gastric bypass for a 10 year period and found that 54% developed anemia, while 47% were iron deficient, with iron deficiency being much more common in women than in men[9].
While the etiology of iron deficiency anemia (IDA) in this population is often multifactorial, there are three causes that are cited most commonly: avoidance of red meat, diminished gastric acid secretion, and exclusion of the duodenum[10]. Red meat is the primary source of iron in North America, with heme accounting for two-thirds of total body iron, while molecular iron accounts for the other third[11]. Studies, as well as common experience, have shown that after patients undergo gastric bypass they are less able to tolerate the intake of red meat. One study of 69 patients found that 39% experienced emesis as a result of eating high fiber meats[12]. Molecular iron must be solubilized in an acidic environment before it can be absorbed[11]. In bypass procedures, the proximal gastric pouch, the primary site of gastric acid secretion, is bypassed. In a prospective study of eight patients who underwent a gastric bypass procedure, Behrns et al[13] demonstrated a marked decreased in gastric acid secretion in the stomachs of patients after they had undergone bypass, compared to pre-procedure levels. As a result of this lack of parietal cells, molecular iron is unable to get optimally metabolized. Once metabolized, most iron is absorbed at the duodenal brush border after it has been reduced from its ferric to ferrous form by ferric reductase[14]. However, in standard Roux-en-Y procedures, the duodenum is entirely bypassed, leading to marked decreased ability to absorb iron.
Other factors that may contribute to iron deficiency include gastritis involving the gastric pouch, esophagitis, and gastric ulcers[15]. While patients are recommended to take multivitamin supplements after undergoing gastric bypass to prevent nutritional deficiencies, patients may still be at risk for the development of IDA. In a randomized, blinded, prospective study of 56 menstruating women who had recently undergone gastric bypass, Brolin et al[16] found that twice daily ferrous sulfate, at a dose of 320 mg, was able to prevent iron deficiency. However, oral iron tablets are often difficult to tolerate, especially in patients who have undergone gastric bypass procedures, and there is still no consensus on the most effective method to limit the development of iron deficiency in this population.
Current guidelines recommend that patients who have undergone a malabsorptive procedure take 40-65 mg of oral iron daily to prevent the development of iron deficiency[17]. However, these guidelines acknowledge that patients may have difficulty tolerating oral supplementation and do not account for the fact that many patients may be iron deficient prior to undergoing the procedure. The guidelines also state that once iron deficiency has developed, patients may be refractory to oral iron, requiring IV iron as a means to replenish their iron stores.
Anemia occurs in approximately 1/3 of patients who suffer from IBD, and almost half of all IBD patients are iron deficient[18]. Anemia in IBD is due to a combination of chronic intestinal blood loss, decreased absorption capabilities of the duodenum secondary to inflammation, the underlying inflammatory conditions that lead to anemia of chronic disease (ACD), and an inability of many IBD patients to tolerate the side effects of oral ferrous sulfate[18]. When patients are in an active inflammatory state secondary to their IBD, successfully treating anemia in IBD is significantly more difficult, making control of IBD paramount to the management of anemia in IBD[19].
Several randomized trials have evaluated the efficacy of iron versus oral supplementation in anemic patients with IBD. Lindgren et al[20] randomized 91 patients with IBD and anemia to receive oral iron sulfate or IV iron sucrose for 20 wk. The IV iron group tolerated the treatment better and saw a greater amount of patients increase their Hgb by > 2 g/dL (66% to 47%), have a resolution of their anemia (16% to 41%), and reach their reference Hgb level (42% to 22%). Another study randomized 200 patients with anemia and IBD to receive IV or oral iron in a 2:1 ratio[21]. The study met its primary endpoint, which was to prove non-inferiority of IV iron in increasing Hgb levels over a 12 week course. Of note, that rate of discontinuation of therapy due to AEs was 7.9% in the oral group compared to 1.5% in the IV group.
New guidelines recommend IV iron as first line therapy for IDA in patients with IBD. Absolute indications for the use of IV iron include a hemoglobin < 10 g/dL, intolerance or inappropriate response to oral iron supplementation, severe disease activity, use of EPO, and patient preference[22]. IV iron leads to a more rapid and prolonged response compared to oral therapy, and is better tolerated and leads to an improved quality of life. Furthermore, recent evidence has shown that oral iron can actually have a deleterious effect in patients with IBD, including an increase in oxidative stress, disease activity, and intestinal inflammation, as well as increasing the risk of colorectal cancer, as seen in animal models[22]. IV iron is beneficial even in cases where the anemia is attributable to ACD, which is defined as ferritin > 100 μg/L and transferrin saturation < 16% in the setting of anemia[23].
The combination of EPO plus IV iron has been shown to be an effective method to reduce the need for blood transfusion in patients with cancer who suffer from chemotherapy-induced anemia, as well as ACD. European Organization for Research and Treatment of Cancer (EORTC) guidelines recommend that patients receiving chemotherapy and/or radiotherapy who develop Hgb levels between 9-11 g/dL and display symptoms of anemia be considered for EPO treatment. Patients with Hgb < 9 g/dL will likely need blood transfusions, at least as initial treatment[24].
In a randomized trial of 477 women with IDA secondary to heavy uterine bleeding, patients were randomized to receive either weekly IV iron or oral ferrous sulfate 325 mg three times a day for six weeks. Compared to those in the oral repletion group, more patients who received IV iron achieved a > 2 g/dL increase in Hgb (82% to 62%), a > 3 g/dL increase in Hgb (53% to 36%), and a correction of Hgb levels, defined as Hgb > 12 g/dL (73% to 50%), with no serious adverse effects reported in either group[25].
After obtaining approval through the University Institutional Review Board, we searched Intellidose, the electronic program that records medication administration, for all instances in which intravenous iron was administered at Sylvester Comprehensive Cancer Center/University of Miami Hospital Clinic from January 2007 to May 2012. We documented the type of iron used, number of administrations, and dosages. We then searched UChart, an electronic medical record used by the university, to ascertain the indications for IV iron based on the patients’ known diagnoses.
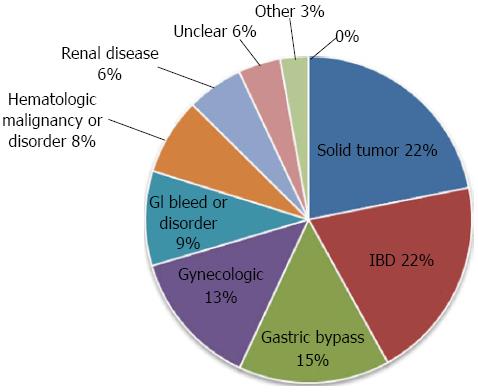
A total of 262 patients received IV iron. Several patients had multiple indications for IV iron. The most common indications for IV iron were for issues related to cancer and its treatment (21.9%), IBD (20.1%), and gastric bypass (15.0%). Other indications included gynecologic issues (13%), a gastrointestinal bleed or disorder other than IBD (9%), and hematologic malignancies or disorders (8%) (Figure 1 and Table 1).
| Gastric bypass | 43 |
| Inflammatory bowel disease (Crohn’s disease, Ulcerative colitis) | 58 |
| Gastrointestinal bleed (ulcers, arteriovenous malformation, hemorrhoids, diverticulosis, Cronkhite-Canada syndrome) | 16 |
| Gastrectomy (secondary to gastric cancer, Mucosa-associated lymphoid tissue lymphoma) | 5 |
| Celiac disease | 3 |
| Pernicious anemia | 1 |
| Colectomy secondary to Familial adenomatous polyposis | 1 |
| Bulimia | 1 |
| Small bowel resection- reason unclear | 1 |
| Solid tumor (secondary to chemotherapy, due to disease infiltration/progression) | 63 |
| Gynecologic (menorrhagia, fibroids, endometriosis) | 39 |
| Hematologic malignancy (Hodgkin’s, Myelodysplastic Syndrome, Post-transplant lymphoproliferative disorder, myelofibrosis, paraproteinemia, follicular lymphoma, Monoclonal gammopathy of undetermined significance, Acute myeloid leukemia, Chronic myeloid leukemia) | 11 |
| Lupus Anti-Coagulant on anti-coagulation, Antiphospholipid syndrome on anti-coagulation | 4 |
| Systemic lupus erythematosus | 3 |
| Anemia of chronic disease | 1 |
| Thallassemia | 1 |
| Sickle cell trait | 1 |
| Autoimmune hemolytic anemia | 1 |
| Jehovah’s witness | 4 |
| Renal disease (polycystic kidney disease , chronic kidney disease end-stage renal disease ) | 16 |
| Pregnancy | 1 |
| Rheumatoid arthritis | 1 |
| Skin wounds | 1 |
| Other indication, also on warfarin | 5 |
| Unclear on review of records (some have iron deficiency anemia as a diagnosis) | 12 |
Of the 262 patients who received IV iron, 230 received iron sucrose and 36 received iron dextran. While doses of 100, 200, 300 and 400 mg of iron sucrose were given, 100 and 200 mg were by far the most common dosages used, 122 and 120 times, respectively. The number of dosages of iron sucrose given ranged from 1 to 46, with a mean of 5.5 and a median of 4 doses. The average dose of iron dextran given was 870.5 mg, with 1000 mg being the most common dosage used. Most patients (22 of 36) who received iron dextran only received one dose.
While we expect IV iron to continue to be used for traditional indications, such as CKD and conditions associated with malignancies, we also expect to see a rise in its use for emerging indications, such as in patients status post gastric bypass procedures and in patients with IBD. Our study supports this claim, as 35% of the patients who received IV iron at our institution received it for one of these two emerging indications. Large studies have demonstrated the safety of iron dextran, and iron sucrose appears to be an even safer alternative. IV iron avoids many of the downsides of oral supplementation, such as decreased GI tolerance, absorption issues, and the ability to correct the deficiency with a short course of treatments, as opposed to long-term oral repletion. IV iron in combination with EPO has also been shown to decrease the need for blood transfusions. While oral iron remains front-line therapy for IDA, we expect to see IV iron used sooner in the course of treatment for GI-related deficiencies. This issue is likely to become more important in the future, as increasing numbers of patients undergo gastric bypass procedures and the prevalence of IBD continues to rise[26].
P- Reviewer Muhammad A S- Editor Song XX L- Editor A E- Editor Lu YJ
| 1. | Muñoz M, Breymann C, García-Erce JA, Gómez-Ramírez S, Comin J, Bisbe E. Efficacy and safety of intravenous iron therapy as an alternative/adjunct to allogeneic blood transfusion. Vox Sang. 2008;94:172-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 71] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 2. | Kapoian T, O’Mara NB, Singh AK, Moran J, Rizkala AR, Geronemus R, Kopelman RC, Dahl NV, Coyne DW. Ferric gluconate reduces epoetin requirements in hemodialysis patients with elevated ferritin. J Am Soc Nephrol. 2008;19:372-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 128] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 3. | Fishbane S, Ungureanu VD, Maesaka JK, Kaupke CJ, Lim V, Wish J. The safety of intravenous iron dextran in hemodialysis patients. Am J Kidney Dis. 1996;28:529-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 185] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 4. | Sinha S, Chiu DY, Peebles G, Kolakkat S, Lamerton E, Fenwick S, Kalra PA. Comparison of intravenous iron sucrose versus low-molecular-weight iron dextran in chronic kidney disease. J Ren Care. 2009;35:67-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Okam MM, Mandell E, Hevelone N, Wentz R, Ross A, Abel GA. Comparative rates of adverse events with different formulations of intravenous iron. Am J Hematol. 2012;87:E123-E124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Wysowski DK, Swartz L, Borders-Hemphill BV, Goulding MR, Dormitzer C. Use of parenteral iron products and serious anaphylactic-type reactions. Am J Hematol. 2010;85:650-654. [PubMed] |
| 7. | Faich G, Strobos J. Sodium ferric gluconate complex in sucrose: safer intravenous iron therapy than iron dextrans. Am J Kidney Dis. 1999;33:464-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 124] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 8. | Amaral JF, Thompson WR, Caldwell MD, Martin HF, Randall HT. Prospective hematologic evaluation of gastric exclusion surgery for morbid obesity. Ann Surg. 1985;201:186-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 113] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Brolin RE, Gorman JH, Gorman RC, Petschenik AJ, Bradley LJ, Kenler HA, Cody RP. Are vitamin B12 and folate deficiency clinically important after roux-en-Y gastric bypass. J Gastrointest Surg. 1998;2:436-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 175] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 10. | Love AL, Billett HH. Obesity, bariatric surgery, and iron deficiency: true, true, true and related. Am J Hematol. 2008;83:403-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 104] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 11. | Conrad ME, Umbreit JN. Iron absorption and transport-an update. Am J Hematol. 2000;64:287-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 12. | Halverson JD, Zuckerman GR, Koehler RE, Gentry K, Michael HE, DeSchryver-Kecskemeti K. Gastric bypass for morbid obesity: a medical--surgical assessment. Ann Surg. 1981;194:152-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 82] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Behrns KE, Smith CD, Sarr MG. Prospective evaluation of gastric acid secretion and cobalamin absorption following gastric bypass for clinically severe obesity. Dig Dis Sci. 1994;39:315-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 100] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 14. | Fleming RE, Bacon BR. Orchestration of iron homeostasis. N Engl J Med. 2005;352:1741-1744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 125] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 15. | Marinella MA. Anemia following Roux-en-Y surgery for morbid obesity: a review. South Med J. 2008;101:1024-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Brolin RE, Gorman JH, Gorman RC, Petschenik AJ, Bradley LB, Kenler HA, Cody RP. Prophylactic iron supplementation after Roux-en-Y gastric bypass: a prospective, double-blind, randomized study. Arch Surg. 1998;133:740-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 89] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Mechanick JI, Kushner RF, Sugerman HJ, Gonzalez-Campoy JM, Collazo-Clavell ML, Guven S, Spitz AF, Apovian CM, Livingston EH, Brolin R. Executive summary of the recommendations of the American Association of Clinical Endocrinologists, the Obesity Society, and American Society for Metabolic & amp; Bariatric Surgery medical guidelines for clinical practice for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient. Endocr Pract. 2008;14:318-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Muñoz M, Gómez-Ramírez S, García-Erce JA. Intravenous iron in inflammatory bowel disease. World J Gastroenterol. 2009;15:4666-4674. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 38] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 19. | de la Morena F, Gisbert JP. [Anemia and inflammatory bowel disease]. Rev Esp Enferm Dig. 2008;100:285-293. [PubMed] |
| 20. | Lindgren S, Wikman O, Befrits R, Blom H, Eriksson A, Grännö C, Ung KA, Hjortswang H, Lindgren A, Unge P. Intravenous iron sucrose is superior to oral iron sulphate for correcting anaemia and restoring iron stores in IBD patients: A randomized, controlled, evaluator-blind, multicentre study. Scand J Gastroenterol. 2009;44:838-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 128] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 21. | Kulnigg S, Stoinov S, Simanenkov V, Dudar LV, Karnafel W, Garcia LC, Sambuelli AM, D’Haens G, Gasche C. A novel intravenous iron formulation for treatment of anemia in inflammatory bowel disease: the ferric carboxymaltose (FERINJECT) randomized controlled trial. Am J Gastroenterol. 2008;103:1182-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 252] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 22. | Gasche C, Berstad A, Befrits R, Beglinger C, Dignass A, Erichsen K, Gomollon F, Hjortswang H, Koutroubakis I, Kulnigg S. Guidelines on the diagnosis and management of iron deficiency and anemia in inflammatory bowel diseases. Inflamm Bowel Dis. 2007;13:1545-1553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 313] [Article Influence: 17.4] [Reference Citation Analysis (3)] |
| 23. | Auerbach M, Coyne D, Ballard H. Intravenous iron: from anathema to standard of care. Am J Hematol. 2008;83:580-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 72] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 24. | Bokemeyer C, Aapro MS, Courdi A, Foubert J, Link H, Osterborg A, Repetto L, Soubeyran P. EORTC guidelines for the use of erythropoietic proteins in anaemic patients with cancer: 2006 update. Eur J Cancer. 2007;43:258-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 260] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 25. | Van Wyck DB, Mangione A, Morrison J, Hadley PE, Jehle JA, Goodnough LT. Large-dose intravenous ferric carboxymaltose injection for iron deficiency anemia in heavy uterine bleeding: a randomized, controlled trial. Transfusion. 2009;49:2719-2728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 157] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 26. | Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46-54. e42; quiz e30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3789] [Cited by in RCA: 3527] [Article Influence: 271.3] [Reference Citation Analysis (5)] |