INTRODUCTION
Perturbation of the intestinal homeostasis might be derived from any cause of breach of the active[1] and passive[2] barrier defense mechanisms of the lining epithelial cells, allowing contact of gut luminal contents with the overly reactive lymphoid tissue underneath. Despite the accumulating results of years of research in the field, the tenet of the need for a “sealed” epithelium as a primary means to restrain undue gut mucosal inflammation is still informing most of the currently active research. Basically, a perturbed mucosal permeability is thought to represent one of the shared features of the two officially recognized phenotypes of the inflammatory bowel diseases (IBDs), Crohn’s disease (CD) and Ulcerative colitis (UC)[3], even though significant elements of difference between the two phenotypes are emerging from modern investigation, as discussed below.
Indeed, currently available evidence seems to best be interpreted by seeing the IBDs not as a dichotomy, but rather as a collection of discrete entities that, though sharing several similarities, do appear distinct enough as to call for a conceptual process of “splitting” over one of “lumping up”. The conception of a genotype-serotype-phenotype axis might serve as the spine for such a way of thinking, as illustrated in a few examples that have recently been mastered in an authoritative review[4]. Polymorphisms of the nucleotide-oligomerization domain 2/caspase activation recruitment domain 15 secondary to mutation of their coding loci (NOD2/CARD15) (see below) would preferentially be expressed in ileal CD[5]; the HLADRB1*0103 allele has been found to correlate with the severity of extra-intestinal disease in UC[6]; a whole range of anti-glycan antibodies including the widely used anti-saccharomyces cerevisiae antibody (ASCA) have been correlated with different CD behaviors in widespread geographical areas[7]; finally, racial factors conditioning for example the onset of peri-anal forms of CD have been described[8]. In the lines to follow, this non-exhaustive list shall be implemented by our own attempt to introduce a concept of the IBDs as centrally conditioned syndromes, insofar as being often diagnosed as co-morbidities of variegated bone marrow anomalies, often affecting the innate arm of the immune response. We shall further discuss that this “splitting drift”, now somewhat prevailing in IBD nosography, can by contrast be mitigated by the breakthrough observation that the IBDs may be intertwined not only between themselves but also with other (apparently unrelated) disorders of remote systems (skin and respiratory organs) insofar as the gut, skin, and lungs are all lining territories between the outer and the inner environments (barrier organ diseases) (please see text below). We shall conclude by presenting evidence that this is not merely an academic exercise, but it can influence medical policies, and our decision making at patient’s bedside.
AN UPDATED REAPPRAISAL OF THE PATHOPHYSIOLOGY OF IBD: CD
For several years, at the end of the last century, CD has been thought of as being derived from an unbalanced secretion of the pro-inflammatory cytokines tumor necrosis factor-α (TNF-α) and interferon (IFN)-γ, hence a Th-1 mediated disorder[9]. However, clinical and genetic data accrued early following 2000, have contributed to place the emphasis on phenomena of defective innate immunity, originating the novel concept of CD as an immune deficiency state. Central to this hypothesis were the observations of defective neutrophil accumulation in CD patients[10], with an impaired clearance of bacteria from tissues. The underlying problem appeared to be a defective macrophage which, though fully capable to form pro-inflammatory cytokines at the translational levels, erroneously trafficked them to premature lysosomal degradation[11]. Some 5 years before these clinical findings, a keystone genetic insight into the underlying innate immunity defects of CD had been released. Basically, some 40% of CD patients of a Western origin in both the European and American continents were found to carry at least one of three disease-associated variants (L1007fsinsC, G908R, R702W) of CARD15, consistently identified as a major susceptibility gene for CD in the caspase recruitment domain[12]. The intact CARD15 encodes the NOD2 receptor, a member of a family of intracellular and host-specific cytosolic pattern-recognition receptors, containing a NOD and a leucine-rich repeat. Through this sequence, NOD2/CARD15 recognizes muramyl-dipeptide (MDP) a component of bacterial cell wall. The ensuing signal transduction cascade then culminates in the serine phosphorylation of the cytoplasmic inhibitor IκB (which then becomes ubiquitinated and tagged for proteosomal degradation) with following release and activation of NF-κB (previously inactive as sequestered in the cytoplasmic dimer). Translocated to the nucleus, NF-κB essentially de-represses the synthesis of several pro-inflammatory cytokines. CD-specific mutations of the CARD gene family hampers initial NOD-mediated recognition of MDPs, blunting the beginning of the response cascade[13]. Whether such genetic faults in fact lead to loss or gain of function has long been debated, with any interpretation being conditioned by the need to account for the excess (not the diminution as would be expected) of inflammation found in CD patients. This question mark seems to be partly met by the hypothesis that TLR-2 driven inhibition of Th-1 responses seems to be lost in CARD-mutated CD patients[14]. NOD2 genes are also highly expressed in Paneth cells, where their mutations would cause decreased expression of antimicrobial peptides, including alpha-defensins[15]. Not less importantly, the Familial Mediterranean Fever genes (MEFV) have been shown to co-localize with NODs on chromosome 16, sharing with the NODs the same protein superfamily that regulates apoptosis, cytokine processing and inflammation[16]. This might warrant envisaging a kind of a continuum between CD and autoinflammatory syndromes, providing arguments for the discussion below[17]. The NODs have most recently been shown to act in synergy with cell autophagy systems, a highly conserved chain that normally effects disposal of cell debris and intracellular bacteria[18]. Wild-type NOD proteins have been shown to serve as nucleating factors for the initiation of bacteria-induced autophagy, by recruiting the ATG16-L1 gene product to coalesce on cell membrane at the point of bacterial contact. Cell bactericidal activity may be hampered both by a polymorphic NOD becoming incapable to accrue the autophagy proteins, and by mutations of the ATG16-L1 products themselves[19].
AN UPDATED VIEW ON THE PATHOPHYSIOLOGY OF UC
The demonstration of a role of innate immunity or of any of the immune effector limbs has been less immediate in the case of UC, lending limited help to the unraveling of its pathogenesis. A recent review[20] of the cytokine profile of UC has come to list a vast array of cytokines spanning from TNF-α and interleukin (IL)-6 up to IL-33; the lymphocyte subsets Th5 and Th17 seem to be the prevalent functional targets of this cytokine plethora. Of note, a rather robust experimental evidence indicates that the UC epithelial lesions could be derived from the pore-forming effects of IL-13, a secretory product of NK-T cells; in coherence with this tenet, recent experimental evidence has shown that in biopsy specimens of pediatric patients with early UC, STAT6, a transduction element that responds to IL-13, is in a prevalently phosphorylated state[21]. An interpretation of UC as a disease of increased permeability could be derived from the complex of these premises. If there is no convincing evidence for a role of innate immunity, UC could be most easily interpreted as a T-mediated disorder, considering its preferential response to the T cell modulatory drug cyclosporine[22].
IBD AND SOME (IMMUNE) DISORDERS SHARE GENETIC POLYMORPHISMS: THE BASIS TO PERCEIVE IBD AS A SYSTEMIC “SYNDROME”
If defects of the innate immune response have convincingly been described in CD, they have also been shown to be under a bi-directional influence: CD-like pictures have been found to often accompany disorders of innate immunity, and, the specific immune abnormalities that are present in CD have been described in other unrelated disorders. The literature on such topics is rather exhaustive. Among the examples of the former instance, chronic granulomatous disease[23] and glycogen storage disease type-1b[24] have been mostly characterized. Both disorders share an impairment of the respiratory burst, preventing neutrophil-mediated killing of bacteria and fungi. The underlying biochemical fault is the absence/malfunction of the nicotinamide adenine dinucleotide phosphate-oxidase oxidase enzyme complex. In-depth clinical and pathologic characterization of a subset of CGD patients has notably highlighted that their gut disease meets all of the criteria to make it indistinguishable from “idiopathic” CD[25]. CARD15 gene mutations have been found in some cases of early-onset sarcoidosis, and, more strikingly, have been found to be responsible for the Blau syndrome, characterized by a granulomatous inflammation expressing mainly as arthritis, uveitis, and skin rash; the role of such mutations in infectious disease has been more controversial, but an association in patients developing gastric lymphoma during Helicobacter pylori infection has been suspected[26]; perturbed neutrophils but not mutated CARD15 have been associated with the clinical expression of tuberculosis.
APPROACHING THE CONCEPTS: IBD-ALIKES AND IBD-SYNDROMES
These premises may generate the concept of an IBD-like picture, or of IBD-look-alikes, namely those conditions that mimic IBD, yet are still to be distinguished from so-called “idiopathic” IBD. The modes of this distinction must be conceived as being subject to the power of our diagnostic means, to the parameters being used, whether clinical, biochemical, endoscopic, histologic, and finally genetic; at which of these escalating grounds we decide to limit our escalation, and how high the bar is set within each ground.
To gain more insight into the issue, we searched the PubMed database mainly using IBD-likes, IBD-look-alikes, and IBD mimics as key words; the references of the articles that were retrieved were further manually scanned. This search disclosed an initial paper published in 1984[27], enumerating and recommending the pathologic features to distinguish true IBDs from so-called “impostors”. This report was followed in 2006[28] by a rather extensive discussion of the IBD-like pictures that may accompany diverticular disease, the so-called segmental colitis associated with diverticular disease (SCAD). The authors emphasized that the contents of the several relevant articles were heterogeneous, with some of them finding morphologic features indistinguishable from CD or UC in a high proportion of the cases, others finding only a minority of such “IBD-alikes”, and others denouncing a plethora of non specific lesions. As it is evident from the above paragraphs, a considerable literature has accumulated on this topic, and we have now attempted to identify a few common mechanisms in the genesis of these IBD-like diseases, gathering them in clusters.
We aimed to identify three main cluster mechanisms that are likely to generate an IBD-like picture: errors (often of pediatric relevance) of either of the two arms of the immune response; involvement of the gut from acquired hematologic disorders or vasculitides; polymorphism of specific genes involved in the immune response, leading, through variegated pathways though, to the final common inflammatory imbalance that marks an IBD. We like to draw particular attention to the role attributed to the polymorphisms of pyrin, a protein domain coded for by chromosome 16 that, together with the TIR and CARD domains, is assumed to belong to the gene superfamily (often ill-defined as CATERPILLER)[29] of innate immune detection of micro-organisms in mammals. It has been shown that carriers of some pyrin mutations may develop not only familial Mediterranean fever, but, notably, a UC-like disease that turns out to resist conventional treatment, whilst showing response to colchicines[30].
We believe that from the above presented arguments it stems clear that a plethora of pictures that fit the requirements to be labeled as IBD may be derived from a vast array of factors; to signify this, the Greek-rooted term “syndrome” should be used to progressively replace the term “disease” and the acronym IBD. This change should adequately reflect the tapering of the list of the so-called “idiopathic” IBDs that is likely to occur as our causative knowledge becomes progressively refined[31].
RE-POSITIONING IBD INTO A BROAD CATEGORY: THE BARRIER ORGAN DEFECTS
If CARD gene sequences turn out to be embedded into a highly conserved domain complex that govern the response of mammalian cells to micro-organisms (The TIR, CARD and PYRIN triad)[32], it seems justified to expect that the clinical expressions of their eventual polymorphisms (e.g., CD) will best be interpreted if contextualized.
Barrier organs and systems
The skin, gastrointestinal tract, and the respiratory epithelia constitute the main components of the barrier systems in the human body. Their structure mainly comprises an epithelial surface or a mucosa extensively interfacing the polluted environment, a basement membrane which, together with a wide array of cell-junction devices, serves to upgrade the sealing state of the system, and, typically, a highly reactive lymphoid tissue underneath, composed of antigen-presenting cells, often classified as dendritic cells, and various lymphocyte subsets capable to react with release of pro-inflammatory cytokines mostly including IL-1, TNF-α and IL-17. The surface of the barrier systems further harbors a huge variety of microbial species, which, at least in the case of the gastrointestinal tract, outweighs the number of somatic cells: this second cell universe within the body is sometimes called “metagenoma”[33]. When working properly, for instance at the gut level, the sensor machinery that was labeled as CATERPILLER above, serves to maintain the balanced co-existence between the local immune system at the barrier organ and the bacterial flora (with genetically-based mutations leading to undue inflammation up to the CD phenotype as discussed at the beginning); on the other hand, in the case of a full-blown outer infection, the CATERPILLERS will be called on the battleground to restrain a pathogen that has perforated the epithelial seals.
Two main phenotypes of barrier organ disease correlated with IBD
Representing an extended surface between the body and the outer world, being endowed with an overly reactive immune tissue, and harboring a large microbe metagenome, the skin shows a number of commonalities with the gut archetype. The development of its main disorder, psoriasis, is thought to initiate with a genetic/environmental breach of the sealing epithelium, causing substance loss and psoriatic plaques; this ends up with activating keratinocytes and inducing dendritic cells to produce pro-inflammatory mediators, fueled by degraded RNAs and DNAs as debris of massive cell wreck; in the presence of favoring genetic prerequisites and external factors (stress) the process may easily become chronic with the typical waxing-and-waning course that recalls IBD. Several observations of clinical and genetic orders suggest a link between IBD and psoriasis. Psoriatic skin lesions seem to occur seven times more frequently in CD patients than controls[34]; ten percent of patients with CD had a first-degree relative with psoriasis[35]; both disorders do respond well to the T-lymphocyte inhibitor drug cyclosporine. On genetic grounds, a susceptibility area for psoriasis, named PSORS8 has been identified, in proximity with the CATERPILLER domain, on chromosome 16q21[36].
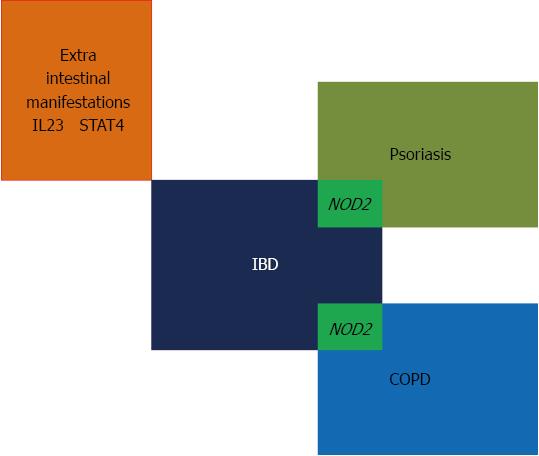
The mucosae of the airways represent a second example of obvious analogy, in terms of contact with outer antigens, and functional anatomy of their reactive tissue. To this end, chronic obstructive pulmonary disease (COPD) has received most of the attention[37]. Available studies have shown that COPD patients might be at an increased risk of developing CD[38], whereas IBD patients are notoriously prone to exhibit respiratory manifestations[39]. At a partial contrast with the generally shared opinion that smoking plays a crucial role in causing COPD, clinics do advise concern over several inconsistencies: (1) COPD severity varies widely irrespective of the number of pack years of smoking; and (2) full-blown COPD is found to develop only in 10%-20% of smokers[40]. Such observations have advised some investigators to focus on genetic studies of COPD, and their efforts have not remained fruitless. Recently, a loss-of-function conformational mutation of NOD2 has been described in COPD patients[41]. In this paper, non-COPD smokers were appropriately used as controls, in order to exclude a role of smoking per se in the distribution of the NOD2 variant (Figure 1).
Figure 1 Morbid inflammatory bowel diseases galaxy.
As barrier organ dysfunctions (see text) psoriasis and chronic obstructive pulmonary disease are supposed to overlap with inflammatory bowel disease (IBD) insofar as sharing mutations of nucleotide-oligomerization domain 2 (NOD2) genes; the so-called extra-intestinal manifestations of IBD might be mediated by interference with polymorphisms of the cytokine interleukin (IL)-23 or signal transduction systems 4 (STAT4); being not a topic in the present text, they are not further commented.
CONCLUSION AND CAVEATS
We are excited and interested by seeing that part of our conclusive thoughts might coincide with those of other barrier disease researchers e.g., psoriasis[36], in reckoning that intensifying attempts at modulating surface bacterial flora and unifying research on shared mediators of inflammation can be successful and cost-effective.
Conceiving IBD as a polyfactorial condition may make us more conducive to perceive the disorder hiding behind a given IBD-like picture; the possibly dramatic clinical counterpart of this changed mindset might be the identification of the patient’s personalized treatment, or even the only causative treatment (as for example in the case of pseudo-UC incited by the mutated pyrin gene) that can save lives and financial resources.
Caveats
The above data and statements must still be interpreted with caution: it may still take a while before genetic profiling reaches its wide-scale clinical application; as of today, the described anomalies of innate immunity do more readily apply to CD than UC; CD is an extraordinarily heterogeneous affection, as shown for example by failure to detect NOD mutations in the disease subtypes that have been characterized in the Far East[42]. The latter observation has stimulated lively debate in the last several years. An interesting analysis of 2005[43] has hypothesized that in populations traditionally exposed to highly contaminated environments, defects of the innate immune response may confer a disadvantage and get selected out. However, progressive environment decontamination and use of “aseptic” industrial distribution food enriched in chemical additives yet virtually “sterile”, may favor loosening of tolerance originating from continuous stimulation of sensors, thus allowing emergence of unchecked gut inflammation. Thus, one may cast the hypothesis that lifestyle rather than key genetic defects might drive the defective innate immune responses in Asians with IBD. With their accelerated pace of development, the Far East communities still represent a seminal area of interest and investigation, perhaps allowing to identify causative IBD factors and take preventive measures that in Western countries were not taken on time and got outdated.
P- Reviewers Cui GL, Palmer AM S- Editor Zhai HH L- Editor A E- Editor Ma S