Published online Dec 6, 2012. doi: 10.4292/wjgpt.v3.i6.86
Revised: October 31, 2012
Accepted: November 20, 2012
Published online: December 6, 2012
AIM: To evaluate the effect of chronic thrombin inhibition by heparin on experimentally induced chronic liver injury (liver fibrosis) in rats.
METHODS: Chronic liver injury (liver fibrosis) was induced in Wistar rats by oral administration of carbon tetrachloride (CCl4) for 7 wk, an animal model with persistent severe hepatic fibrosis. Intravenous administration of the thrombin antagonist (heparin) started 1 wk after the start of CCl4 intoxication for 6 wk. After completion of treatment (7 wk), markers of hepatic dysfunction were measured and changes evaluated histopathologically.
RESULTS: Higher serum glutamate oxaloacetate transaminase (SGOT), serum glutamate pyruvate transaminase (SGPT), alkaline phosphatase (ALP), total, direct and indirect bilirubin levels, as well as lower fibrinogen levels, were found in CCl4 intoxicated rats. Heparin, silymarin and combination of drug (heparin and silymarin) treatment for 6 wk prevented a rise in SGOT, SGPT, ALP, total, direct and indirect bilirubin levels and improved fibrinogen levels. Deterioration in hepatic function determined by the fibrosis area was retarded, as evident from hepatic histopathology. Total protein levels were not changed in all groups.
CONCLUSION: Heparin, a thrombin antagonist, preserved hepatic function and reduced severity of hepatic dysfunction/fibrogenesis. Combination of heparin and silymarin produced additional benefits on liver fibrosis.
- Citation: Shah B, Shah G. Antifibrotic effect of heparin on liver fibrosis model in rats. World J Gastrointest Pharmacol Ther 2012; 3(6): 86-92
- URL: https://www.wjgnet.com/2150-5349/full/v3/i6/86.htm
- DOI: https://dx.doi.org/10.4292/wjgpt.v3.i6.86
Liver fibrosis is one of the main complications of chronic liver disease. It is insidious in nature as symptoms appear late. Liver fibrosis is characterized by an excessive deposition of extracellular matrix (ECM) components in the liver parenchyma. The treatment of liver fibrosis or cirrhosis is always a problem in clinical practice. To control and stop liver fibrosis towards cirrhosis is of utmost importance. Although liver fibrosis is known to be a serious chronic liver disease, an ideal agent to be used in this disease is still not available and there has been a constant urge for the discovery of newer drugs.
During the past decade, it was understood that activation of the coagulation cascade classically proceeds and eventually leads to cirrhosis. If not detected and treated in time, there is imminent danger of permanent liver failure. Synthesis of ECM components is performed by fibrogenic cells that derive from the activation of precursors, such as hepatic stellate cells and fibroblasts. During the past decade, a lot of attention has been given to the stimuli responsible for fibrogenic cell activation in the liver[1].
The role of the coagulation cascade has been suggested by various observations but has not been formally proved. Activation of the coagulation cascade classically proceeds through the activation of thrombin. Thrombin is responsible for the conversion of fibrinogen to fibrin. Fibrin accumulates in the liver during acute and chronic experimental liver injury[2]. A similar result has been observed in models of pulmonary fibrosis[3]. Clot formation by itself leads to flow disturbance and local hypoxia which is a cofactor for fibrosis[4]. Thrombin signals via specific cellular receptors to regulate functions associated with tissue remodeling[5]. Thrombin receptor protease activated receptors (PAR)-1 is up regulated in human liver during acute and chronic injury[6]. Thrombin stimulates the proliferation of rat and human hepatic stellate cells.
Recently, thrombin inhibitors have attracted attention as a new class of drugs for the treatment of liver fibrosis. Direct inhibition of thrombin with a synthetic antagonist decreased lung collagen accumulation in experimental pulmonary fibrosis[7]. There are no clear data for liver fibrosis. Moreover, heparin could inhibit the growth of the Ito cells effect in vitro, which suggested that heparin does display some thrombin inhibitory activity[8]. A role for thrombin in liver fibrosis has been reported[9]. Hence, chronic thrombin inhibition decreases the rate of thrombin deposition and thus reduces liver injury. There are few reports suggesting beneficial effects of thrombin antagonism in liver fibrosis[10].
However, no attempt has been made to come to a logical conclusion about the relative beneficial effects of such drugs in liver fibrosis. Furthermore, heparin has been reported to inactivate the coagulation system and prevent acute liver injury from a hepatotoxic dose of lipopolysaccharide[10]. Similarly, heparin has been effective in cholestatic liver injury in rats[11]. Moreover, the effects of heparin on liver fibrosis in patients with chronic hepatitis B has been reported[12].
In the light of the above and looking at the beneficial properties of heparin in liver disorders, the present study was performed to investigate the therapeutic potential of heparin by studying its effect in an experimentally induced chronic model of liver fibrosis in rats associated with clinically relevant co-morbidities, eventually leading to progressive hepatic dysfunction.
Male albino rats of the Wistar strain (200-250 g) obtained from the animal house of our institute were used for the study. Rats were housed in a conventional cage, maintained on a 12 h light-dark cycle and had free access to standard rat chow and water ad libitum. Rats (200-250 g) were divided into six groups, 2 controls and 4 treatment groups, of six rats each. The first group received 0.375 mL/kg olive oil (Loba Chemie Pvt Ltd.) po od (n = 6) served as a normal control and carbon tetrachloride CCl4 control group (n = 6) to which a mixture of CCl4 (Loba Chemie Pvt Ltd.) and olive oil (1:5) 0.375 mL/kg po od thrice a week was administered until the study termination at 7 wk. Silymarin (standard) group (Micro Labs Ltd.) 100 mg/kg po od (n = 6), heparin (test) group (Biological E Limited) 2000 IU/kg iv od (n = 6), silymarin 100 mg/kg po od and heparin 2000 IU/kg iv od treated (combination) group (n = 6) was administered for 6 wk (continued with CCl4 injury for 7 wk) and a control treated group received heparin 2000 IU/kg iv od for 6 wk without CCl4 injury.
One day after the last CCl4 administration, blood samples were collected under light anesthesia by the retro-orbital route in anticoagulant free vials. After 30 min, samples were centrifuged at 3500 r/minute for 20 min to separate serum which was used for further biochemical estimations (liver function tests). Fresh venous blood (1.5 mL) was collected in 0.15 mL heparin sodium containing eppendroff. The blood sample was centrifuged at 1500-3000 r/min for 20 min to separate plasma samples which were used to determine the fibrinogen level by the Kjeldahl-Nesslerization method[13]. The rats were sacrificed by an overdose of anesthesia and liver was collected from same set of animals.
Terminal measurement of hepatic function was performed by liver function tests in the same animals. Serum glutamate oxaloacetate transaminase (SGOT) (Merck Limited India), serum glutamate pyruvate transaminase (SGPT) (Span diagnostics Ltd.), alkaline phosphatase (ALP) (Span diagnostics Ltd.), total and direct bilirubin (Span diagnostics Ltd.), total protein levels (Beacon diagnostics Pvt Ltd.) were measured using commercially available rat enzyme kits. Indirect bilirubin was calculated by subtracting direct bilirubin from total bilirubin.
A reagent for fibrinogen estimation, stock Nessler’s reagent, was prepared by mixing of 150 g of potassium iodide and 200 g mercuric iodide in about 1 L of distilled water, the solution was filtered and final volume was made to 2 L (Solution A). In a separate vessel, 10 g of sodium hydroxide pellets were dissolved in 100 mL of distilled water (solution B). At the time of use, 15 mL of solution A was mixed with 70 mL of solution B and 15 mL of distilled water was added and the solution cooled by refrigeration. Stock standard was prepared by dissolving 0.3772 g ammonium sulphate (Finar Lab reagents) in 100 mL of distilled water to get a 0.3772 g/dL concentration. Working standard was prepared by diluting 5 mL of stock standard solution up to 100 mL using distilled water. This solution contains 4 mg/dL nitrogen. Zero point five mL of plasma was mixed with 14 mL of distilled water and 0.5 mL of calcium chloride solution (2.5 g/dL) (Finar Lab reagents) in a porcelain dish. A fine glass rod was placed in a porcelain dish. The porcelain dish was kept at 37 °C overnight so that clot formation was complete. One milliliter of digestion mixture [1 g/dL Selenium dioxide (Loba chemie Pvt Ltd.) in 50% (v/v) sulphuric acid (Thomas baker)] was added to this. The beaker was then heated carefully using a microburner. A drop of 30% w/v H2O2 (Rankem Ltd.) was added. The mixture first darkened and with further heating it became colorless. It was then cooled and distilled water was added to make a 10 mL volume and mixed thoroughly. Two mL from this was mixed with 5 mL cold distilled water and 3 mL of cold working Nessler’s reagent. After mixing thoroughly, absorbance was taken at 440 nm. For the standard solution, 1 mL of working standard solution was mixed with 6 mL cold distilled water and 3 mL of working Nessler’s reagent and absorbance was read at 440 nm. Optical density of the test solution divided by optical density of standard and multiplied by conversion factor 250 gave the concentration of fibrinogen in mg/dL.
Liver was collected from the same set of animals at termination for gross and microscopic histopathological examination from all groups. Sections of the liver lobe a few mm thick were taken from each rat and processed for observation by light microscopy. The process involved fixation of the tissue with 4% formalin (Loba Chemie), embedded in paraffin blocks, sectioned with microtome (0.7 U thick) and finally stained by the hematoxylin and eosin stain technique. Hematoxylin stains the nucleus light blue which turns red in the presence of acid. The cell differentiation is achieved by treating the tissue with acid solution and the counter staining is performed by using eosin which imparts a pink color to the cytoplasm. The sections were scanned and analyzed by a pathologist who was blinded to the different treatments in the experiment.
This work was carried out in accordance with the principles of the committee for the purpose of control and supervision on experiments on animals and guidelines for animal care were followed.
Body weight and liver function test data in text are expressed as mean ± SE of six observations. Differences between any two groups for all data were assessed by one way ANOVA with post test (Turkey). Statistical analysis was performed using a graph pad statistical analysis system. Histopathological changes of varying severity were observed and scored between groups. Percentage area of fibrosis was assessed by t-test. Statistical significance was defined as P < 0.05.
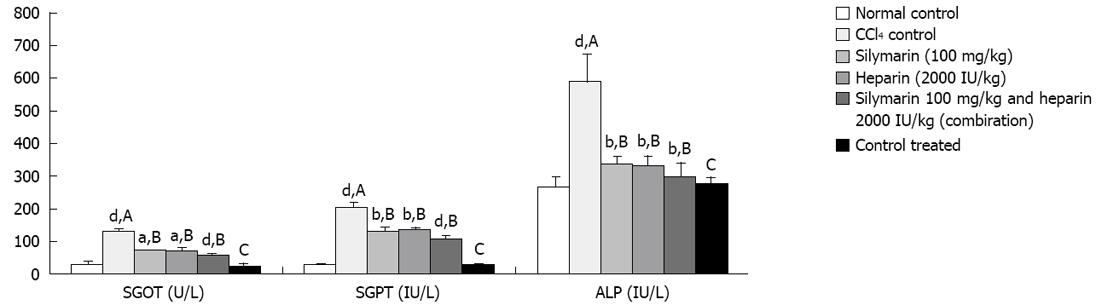
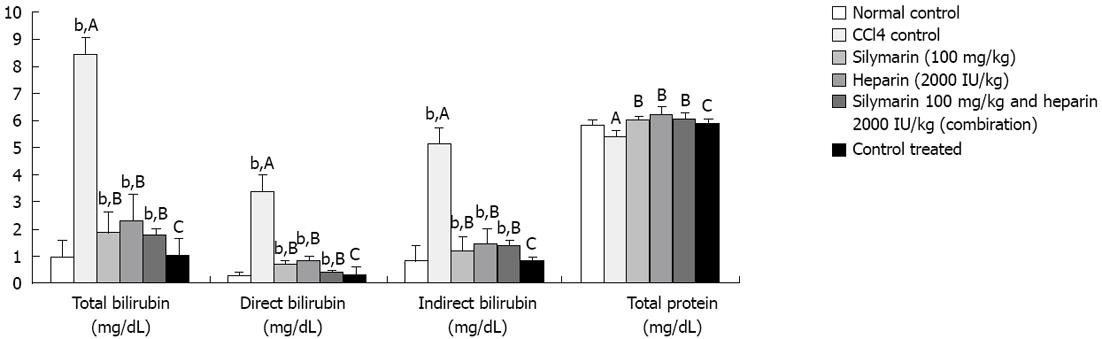
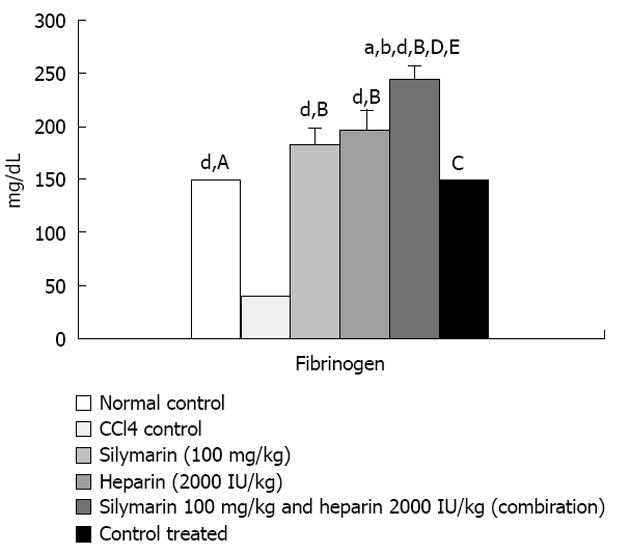
CCl4 administration (0.375 mL/kg) thrice a week for 7 wk resulted into significant elevation in SGOT, SGPT, aspartate aminotransferase (AST), total, direct and indirect bilirubin levels compared to the normal control. However, no significant elevation was observed in total protein levels. Fibrinogen levels were found significantly decreased after CCl4 administration compared to the normal control. Heparin, silymarin and heparin-silymarin combination groups showed distinct improvement in liver functional tests compared to the control. Animals treated with heparin, silymarin and heparin-silymarin combination for 6 wk along with CCl4 administration for 7 wk showed significantly lesser SGOT, SGPT, AST, total, direct and indirect bilirubin levels and slightly higher total protein levels which did not reached a significant level. Heparin, silymarin and heparin-silymarin combination for 6 wk along with CCl4 administration for 7 wk showed significantly higher fibrinogen levels. The combination of heparin and silymarin was seen to be more effective than the single drug. The heparin treatment group without CCl4 injury did not show any significant effect on all measured parameters (Figures 1-3 and Table 1).
| Parameters | Normal control | CCl4 control | Silymarin (100 mg/kg) | Heparin (2000 IU/kg) | Silymarin 100 mg/kg and heparin 2000 IU/kg treated (combination) group | Control treated |
| Food intake (g/rat per day) | 25.87 ± 2.9 | 24.62 ± 3.5 | 24.01 ± 2.5 | 22.56 ± 3.9 | 23.46 ± 1.5 | 21.82 ± 4.0 |
| Water intake (mL/d) | 145.78 ± 5.1 | 148.01 ± 6.2 | 146.71 ± 5.2 | 140.98 ± 5.5 | 142.64 ± 7.2 | 141.72 ± 4.6 |
| SGOT (U/L) | 25.4 ± 13.9 | 124.11 ± 13.4b | 71.81 ± 3.7c | 72.32 ± 5.4c | 56.63 ± 9.2d | 22.6 ± 11.2 |
| SGPT (IU/L) | 27.8 ± 8.3 | 199.99 ± 15.8b | 128.17 ± 14.0d | 132.17 ± 9.4d | 105.62 ± 12.2d | 28.6 ± 8.1 |
| ALP (IU/L) | 266.6 ± 31.4 | 583.46 ± 86.1b | 334.9 ± 24.1d | 330.86 ± 29.7d | 296.11 ± 46.1d | 273.0 ± 25.1 |
| Total bilirubin (mg/dL) | 0.96 ± 0.6 | 8.45 ± 0.5b | 1.88 ± 0.8d | 2.30 ± 1.0d | 1.73 ± 0.3d | 1.03 ± 0.7 |
| Direct bilirubin (mg/dL) | 0.2 ± 0.2 | 3.34 ± 0.5b | 0.73 ± 0.1d | 0.84 ± 0.1d | 0.38 ± 0.1d | 0.35 ± 0.3 |
| Indirect bilirubin (mg/dL) | 0.85 ± 0.6 | 5.12 ± 0.8b | 1.15 ± 0.6d | 1.46 ± 0.6d | 1.35 ± 0.2d | 0.83 ± 0.2 |
| Total protein (gm/dL) | 5.8 ± 0.3 | 5.45 ± 0.2 | 6.04 ± 0.2 | 6.22 ± 0.3 | 6.05 ± 0.2 | 5.9 ± 0.2 |
| Fibrinogen (mg/dL) | 148.89 ± 3.5 | 38.24 ± 2.3b | 183.04 ± 16.7d | 196.99 ± 19.4d | 246.4 ± 9.2dfh | 149.54 ± 0.3 |
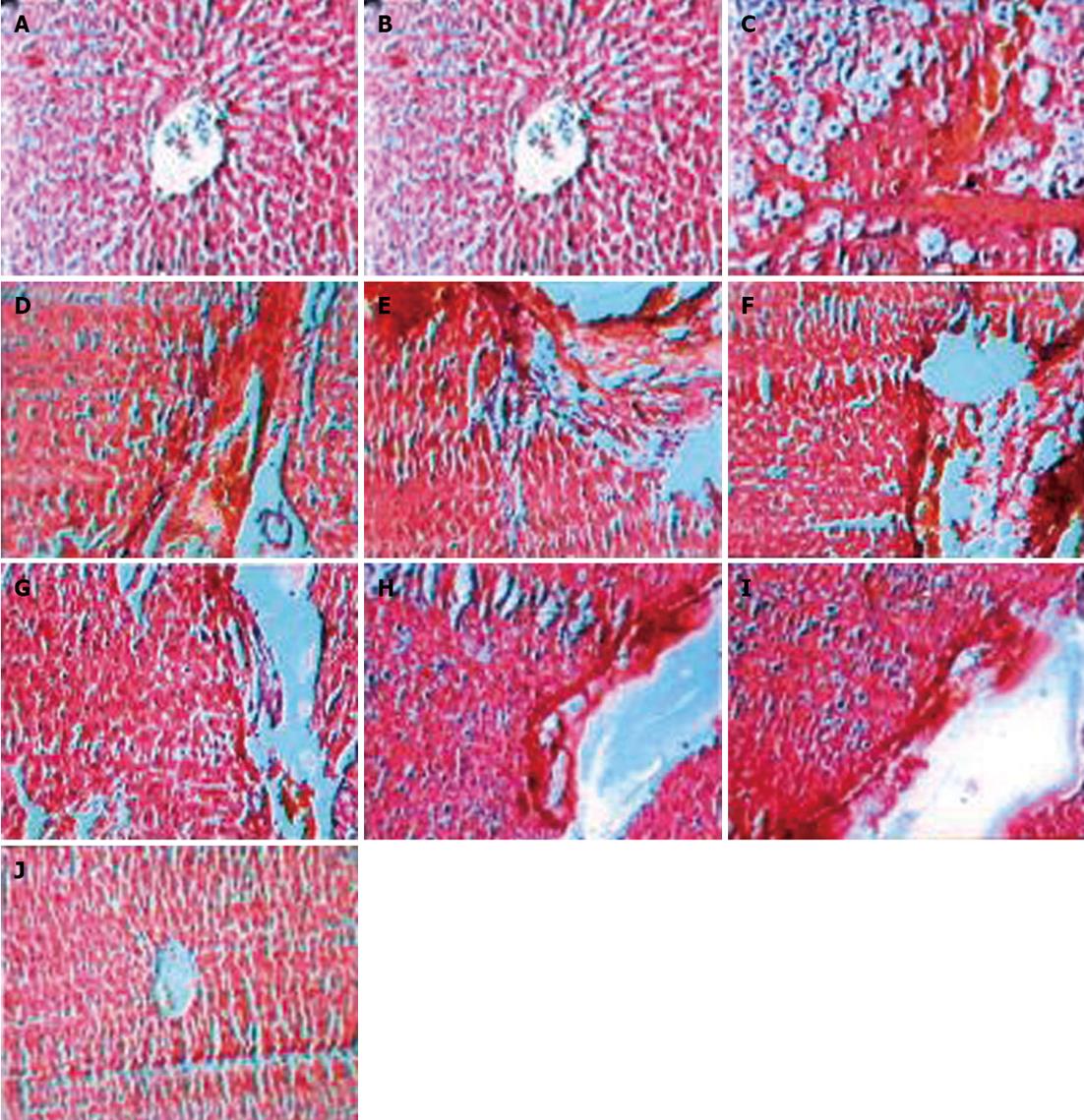
Liver histopathology in the control group showed development of large septa of connective tissue penetrating into the parenchyma with resultant initiation of fibrous septa formation. Progressive septa formation which leads to nodule formation was observed in more severe cases. Treatment with silymarin, heparin and a silymarin and heparin combination reduced the severity of histopathological lesions compared to the control group. Silymarin, heparin and a silymarin and heparin combination treatment resulted in progressive breakage association with dissolution of fibrous septa and reduction in the area of fibrosis (Figure 4 and Table 2).
| Parameters | Normal control | CCl4 control | Silymarin (100 mg/kg) | Heparin (2000 IU/kg) | Silymarin 100 mg/kg and heparin 2000 IU/kg treated (combination) group | Control treated |
| Degree (area) of fibrosis | 0 | 4 | 3 | 2 | 1 | 0 |
| Percentage area of fibrosis | Fibrosis not observed | 58 | 24.7dA | 15.7dA,aB | 12.9dA,bC | Fibrosis not observed |
Liver fibrosis is a disease of chronic liver injury, which is characterized by deposition of ECM[2]. It is one of the major causes of death worldwide. All cells in the liver can synthesize and secrete ECM, which regulates the proliferation, differentiation and metabolism of liver cells. The abnormal metabolism and deposition of ECM leads to liver fibrosis. The goal of liver fibrosis therapy is to prevent dysfunction of liver and consequent liver failure. It has been suggested that thrombin plays a detrimental role in chronic liver injury[10]. Fibrin deposition in liver leads to fibrosis[2]. It is well known that thrombin plays an important role in formation of fibrin. Fibrin deposition in vascular beds leads to occlusive thrombi contributing to downstream liver injury.
Thrombin is a multifunctional serine protease that plays an important role in thrombosis and hemostasis. Formed by proteolytic cleavage of prothrombin by the prothrombinase complex, thrombin initiates the final step of the blood coagulation cascade by cleaving soluble fibrinogen to fibrin[14]. There are at least two mechanisms through which thrombin generation could be involved in fibrogenesis. Firstly, clot formation by itself leads to flow disturbance and local hypoxia which is a cofactor for fibrosis[14,15]. Secondly, thrombin signals via specific cellular receptors that belong to the family of PAR[15]. The thrombin receptor PAR-1 is up regulated in human liver during acute and chronic injury[6]. It has been reported that thrombin stimulates the proliferation of rat hepatic stellate cells[15]. Chronic thrombin inhibition by synthetic thrombin antagonist SSR182289 reduced the extent of fibrosis deposition in the CCl4 model of liver fibrosis in the rat[9]. So, it is understood that thrombin plays an important role in liver fibrosis. PAR-1 antagonism protects against experimental liver fibrosis[15]. However, altogether, the case is strong for a role of coagulation proteases in liver fibrogenesis. Heparin is cheap and safe. It has a stronger and direct effect on thrombin. Heparin has been shown to inactivate the coagulation system and prevent acute liver injury[10]. Heparin is reported to produce beneficial effects in patients suffering from liver fibrosis due to chronic hepatitis[12].
We have studied the antifibrotic action of heparin in experimentally induced chronic model of liver fibrosis in rats. Oral administration of CCl4 is one of the easiest, fastest and reliable techniques to develop fibrosis and can be used to screen antifibrotic agents[9,16-18]. CCl4 accumulates in the hepatocytes, where its activation by oxidases that are involved in the hemolytic breakage of the C-Cl bond occurs. This activation occurs in the hepatic endoplasmic reticulum via an enzyme system of electron transport from reduced nicotinamide adenine dinucleotide phosphate to oxygen, and this leads to formation of a reactive metabolite, the trichloromethyl radical (CCl3). The CCl3 radical then binds covalently to a series of molecular structures and particularly to the lipid of endoplasmic reticulum membranes, resulting in the formation of lipid radicals. The lipid radicals react with molecular oxygen to produce peroxy radicals which is involved in initiation of lipid peroxidation[15]. The lipid peroxides produce breakdown of the biomembrane at cellular and subcellular levels. This leads to hepatocyte destruction and release of intracellular enzymes like GOT, GPT and ALP in blood[19,20]. The increased activities of liver marker enzymes such as GOT, GPT and ALP in the serum of CCl4 induced rats indicate damage to hepatic cells. Both heparin and silymarin treated animals possessed significantly lower SGOT, SGPT and ALP levels compared to untreated animals, suggesting the protective effect of heparin against CCl4 induced hepatocytes lyses. The much lower levels of SGOT, SGPT and ALP were observed in animals treated with both heparin-silymarin when compared with an individual drug. The combination is more effective in preventing CCl4 induced hepatotoxicity (Figure 1).
Bilirubin is the excretory end product of heme degradation. It is conjugated in the liver to form bilirubin diglucuronide and excreted in bile. Increase in serum bilirubin levels may be found in hepatocellular damage, hemolytic jaundice and hepatitis[21]. CCl4 injury causes significant degeneration of hepatocytes and blockage of bile ducts resulted in a significant increase in serum total, direct and indirect bilirubin levels. Heparin and silymarin treated animals had significantly lower total, direct and indirect bilirubin levels compared to untreated animals. The combination of heparin-silymarin shows a much lower level in total, direct and indirect bilirubin levels compared to an individual drug (Figure 2).
The liver participates in synthesis of plasma proteins. Injury to a liver cell causes alteration in liver function by cell necrosis. CCl4 injury shows decreased total protein levels which was not statistically significant. Heparin, silymarin and heparin-silymarin treated animals had increased total protein levels which is not statistically significant (Figure 2).
Fibrin deposits were in pericentral necrosis areas and within fibrotic septa during liver fibrogenesis, after CCl4 induced liver damage[2]. During activation of the coagulation system, plasma fibrinogen is converted to fibrin, resulting in a decrease in plasma fibrinogen concentration[10,13]. The decrease in fibrinogen levels is used as a biomarker for activation of the coagulation system and as a monitor of anticoagulant effectiveness. CCl4 treated animals have shown a significant decrease in fibrinogen levels. Heparin and silymarin treated animals significantly increased fibrinogen levels. However, the heparin- silymarin combination group has shown significant increase in fibrinogen levels (Figure 3).
Histological studies showed that CCl4 intoxication resulted in development of large septa of connective tissue flowing together, penetrating into parenchyma and progressive fibrous septa formation which resulted into nodule formation. Silymarin treated animals showed progressive breakage of fibrous septa with a mild degree of fibrolysis. Heparin treatment resulted in progressive breakage of fibrous septa with a moderate degree of fibrolysis. The heparin-silymarin combination group resulted in progressive breakage and almost dissolution of fibrous septa with a severe degree of fibrolysis.
In conclusion, it can be suggested that heparin produces significant antifibrotic effects in CCl4 intoxicated rats. This protective effect could be due to the anticoagulant property of heparin. Heparin binds with antithrombin III and forms the complex which binds with thrombin and blocks its action. Thus, the fibrotic effect of thrombin in liver fibrosis is blocked by heparin, by both blocking the coagulant action of thrombin and also indirectly preventing thrombin to bind to PAR-1 receptors. The heparin-silymarin combination group showed more of a synergistic effect than either drug alone. However, further studies may be required to support the above conclusion.
Liver fibrosis is the main complication of chronic liver disease that eventually leads to cirrhosis. Liver fibrosis is characterized by an excessive deposition of extracellular matrix (ECM) components in the liver parenchyma. The treatment of liver fibrosis or cirrhosis is always a problem in clinical practice. To control and stop liver fibrosis towards cirrhosis is of utmost importance. Evidence incriminates the serine proteinase, thrombin, in liver fibrogenesis, either through its procoagulant function or its signaling via cell-surface receptors. Recently, thrombin inhibitors have attracted attention as a new class of drugs for the treatment of liver fibrosis.
Activation of the coagulation cascade classically proceeds through activation of thrombin. There are a few reports suggesting the beneficial effects of thrombin antagonism in liver fibrosis. Many studies reported that direct inhibition of thrombin by a synthetic antagonist decreased lung collagen accumulation in experimental pulmonary fibrosis. Moreover, heparin could inhibit the growth of the Ito cells effect in vitro, which suggests that heparin does display some thrombin inhibitory activity.
Heparin has been reported to inactivate the coagulation system and prevent acute liver injury from a hepatotoxic dose of lipopolysaccharide, also effective in cholestatic liver injury in rat. Moreover, the effects of heparin on liver fibrosis in patients with chronic hepatitis B have been reported. There are no clear data for liver fibrosis. The aim of the present study was to evaluate the effect of chronic thrombin inhibition by heparin on experimentally induced chronic liver injury (liver fibrosis) in rats.
The study results suggest that heparin is a potential therapeutic agent that could be used in preventing liver fibrosis. Additionally, treatment with a combination drug (heparin and silymarin) produced a beneficial protective effect on liver fibrosis.
Liver fibrosis: liver fibrosis is characterized by an excessive deposition of ECM components in the liver parenchyma. Thrombin is a multifunctional serine protease that plays an important role in thrombosis and hemostasis. Formed by proteolytic cleavage of prothrombin by the prothrombinase complex, thrombin initiates the final step of the blood coagulation cascade by cleaving soluble fibrinogen to fibrin.
This is a good descriptive study in which the authors evaluate the preventive effect of heparin, a thrombin antagonist, on liver fibrosis induced by carbon tetrachloride in rats. The results are interesting and suggest that heparin is a potential therapeutic agent that could be used in preventing liver fibrosis.
Peer reviewer: Frank Tacke, MD, PhD, Professor, Department of Medicine III, University Hospital Aachen, Pauwelsstr. 30, 52074 Aachen, Germany
S- Editor Zhai HH L- Editor Roemmele A E- Editor Zheng XM
| 1. | Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;275:2247-2250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1567] [Cited by in RCA: 1597] [Article Influence: 63.9] [Reference Citation Analysis (0)] |
| 2. | Neubauer K, Knittel T, Armbrust T, Ramadori G. Accumulation and cellular localization of fibrinogen/fibrin during short-term and long-term rat liver injury. Gastroenterology. 1995;108:1124-1135. [PubMed] |
| 3. | Hattori N, Degen JL, Sisson TH, Liu H, Moore BB, Pandrangi RG, Simon RH, Drew AF. Bleomycin-induced pulmonary fibrosis in fibrinogen-null mice. J Clin Invest. 2000;106:1341-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 210] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 4. | Corpechot C, Barbu V, Wendum D, Kinnman N, Rey C, Poupon R, Housset C, Rosmorduc O. Hypoxia-induced VEGF and collagen I expressions are associated with angiogenesis and fibrogenesis in experimental cirrhosis. Hepatology. 2002;35:1010-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 369] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 5. | Chambers RC, Leoni P, Blanc-Brude OP, Wembridge DE, Laurent GJ. Thrombin is a potent inducer of connective tissue growth factor production via proteolytic activation of protease-activated receptor-1. J Biol Chem. 2000;275:35584-35591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 172] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 6. | Marra F, DeFranco R, Grappone C, Milani S, Pinzani M, Pellegrini G, Laffi G, Gentilini P. Expression of the thrombin receptor in human liver: up-regulation during acute and chronic injury. Hepatology. 1998;27:462-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 76] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Howell DC, Goldsack NR, Marshall RP, McAnulty RJ, Starke R, Purdy G, Laurent GJ, Chambers RC. Direct thrombin inhibition reduces lung collagen, accumulation, and connective tissue growth factor mRNA levels in bleomycin-induced pulmonary fibrosis. Am J Pathol. 2001;159:1383-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 128] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 8. | Yuan TX, Zhang JS, Zhang YE, Chen Q. [Culture of rat liver Ito cells and the observation of inhibitory effect of heparin on Ito cells]. Shanghai Yike Daxue Xuebao. 1996;23:90-93. |
| 9. | Duplantier JG, Dubuisson L, Senant N, Freyburger G, Laurendeau I, Herbert JM, Desmoulière A, Rosenbaum J. A role for thrombin in liver fibrosis. Gut. 2004;53:1682-1687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 63] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Yee SB, Harkema JR, Ganey PE, Roth RA. The coagulation system contributes to synergistic liver injury from exposure to monocrotaline and bacterial lipopolysaccharide. Toxicol Sci. 2003;74:457-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Abdel-Salam OM, Baiuomy AR, Ameen A, Hassan NS. A study of unfractionated and low molecular weight heparins in a model of cholestatic liver injury in the rat. Pharmacol Res. 2005;51:59-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Shi J, Hao JH, Ren WH, Zhu JR. Effects of heparin on liver fibrosis in patients with chronic hepatitis B. World J Gastroenterol. 2003;9:1611-1614. [PubMed] |
| 13. | Shivaraj G, Prakash BD, Sonal V, Shruthi K, Vinayak H, Avinash M. Thyroid function tests: a review. Eur Rev Med Pharmacol Sci. 2009;13:341-349. [PubMed] |
| 14. | Berry CN, Lassalle G, Lunven C, Altenburger JM, Guilbert F, Lalé A, Hérault JP, Lecoffre C, Pfersdorff C, Herbert JM. SSR182289A, a novel, orally active thrombin inhibitor: in vitro profile and ex vivo anticoagulant activity. J Pharmacol Exp Ther. 2002;303:1189-1198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Fiorucci S, Antonelli E, Distrutti E, Severino B, Fiorentina R, Baldoni M, Caliendo G, Santagada V, Morelli A, Cirino G. PAR1 antagonism protects against experimental liver fibrosis. Role of proteinase receptors in stellate cell activation. Hepatology. 2004;39:365-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 137] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 16. | Copple BL, Banes A, Ganey PE, Roth RA. Endothelial cell injury and fibrin deposition in rat liver after monocrotaline exposure. Toxicol Sci. 2002;65:309-318. [PubMed] |
| 17. | Vogel HG. Carbontetrachloride induced liver fibrosis in rats. Drug discovery and evaluation: pharmacological assays. New York: Springer-verlag 2002; 942-943. |
| 18. | Poon CY. Clinical analysis. Remington: The science and practice of pharmacy. Philadelphia: Lippincott Williams and Wilkins 2006; 565-598. |
| 19. | Shahjahan M, Sabitha KE, Jainu M, Shyamala Devi CS. Effect of Solanum trilobatum against carbon tetrachloride induced hepatic damage in albino rats. Indian J Med Res. 2004;120:194-198. [PubMed] |
| 20. | Ryeom HK, Kim SH, Kim JY, Kim HJ, Lee JM, Chang YM, Kim YS, Kang DS. Quantitative evaluation of liver function with MRI Using Gd-EOB-DTPA. Korean J Radiol. 2004;5:231-239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 117] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 21. | Murray RK. Porphyrins and bile pigments. Harper’s illustrated biochemistry. 26th ed. New York: McGraw-Hill Inc 2003; 270-285. |