Published online Oct 6, 2012. doi: 10.4292/wjgpt.v3.i5.74
Revised: August 24, 2012
Accepted: August 25, 2012
Published online: October 6, 2012
AIM: To study the safety and effectiveness associated with accelerated infliximab infusion protocols in patients with inflammatory bowel disease (IBD).
METHODS: Original protocols and infusion rates were developed for the administration of infliximab over 90-min and 60-min. Then the IBD patients on stable maintenance infliximab therapy were offered accelerated infusions. To be eligible for the study, patients needed a minimum of four prior infusions. An initial infusion of 90-min was given to each patient; those tolerating the accelerated infusion were transitioned to a 60-min infusion protocol at their next and all subsequent visits. Any patient having significant infusion reactions would be reverted to the standard 120-min protocol. A change in a patient’s dose mandated a single 120-min infusion before accelerated infusions could be administered again.
RESULTS: The University of Virginia Medical Center's Institutional Review Board approved this study. Fifty IBD patients treated with infliximab 5 mg/kg, 7.5 mg/kg and 10 mg/kg were offered accelerated infusions. Forty-six patients consented to participate in the study. Nineteen (41.3%) were female, five (10.9%) were African American and nine (19.6%) had ulcerative colitis. The mean age was 42.6 years old. Patients under age 18 were excluded. Ten patients used immunosuppressive drugs concurrently out of which six were taking azathioprine, three were taking 6-mercaptopurine and one was taking methotrexate. One of the 46 study patients used corticosteroid therapy for his IBD. Seventeen of the patients used prophylactic medications prior to receiving infusions; six patients received corticosteroids as pre-medication. Four patients had a history of distant transfusion reactions to infliximab. These reactions included shortness of breath, chest tightness, flushing, pruritus and urticaria. These patients all took prophylactic medications before receiving infusions. 46 patients (27 males and 19 females) received a total of fifty 90-min infusions and ninety-three 60-min infusions. No infusion reactions were reported. There were no adverse events, including drug-related infections. None of the patients developed cancer of any type during the study timeframe. Total cost savings for administration of the both 90-min and 60-min accelerated infusions compared to standard 120-min infusions was estimated to be $53 632 ($116 965 vs $63 333, P = 0.001). One hundred and eighteen hours were saved in the administration of the accelerated infusions (17 160 min vs 10 080 min, P = 0.001). In the study population, overweight females [body mass index (BMI) > 25.00 kg/m2] were found to have statistically higher BMIs than overweight males (mean BMI 35.07 ± 2.66 kg/m2vs 30.08 ± 0.99 kg/m2, P = 0.05), finding which is of significance since obesity was described as being one of the risk factors for Crohn’s disease.
CONCLUSION: We are the first US group to report substantial cost savings, increased safety and patient satisfaction associated with accelerated infliximab infusion.
- Citation: McConnell J, Parvulescu-Codrea S, Behm B, Hill B, Dunkle E, Finke K, Snyder K, Tuskey A, Cox D, Woodward B. Accelerated infliximab infusions for inflammatory bowel disease improve effectiveness. World J Gastrointest Pharmacol Ther 2012; 3(5): 74-82
- URL: https://www.wjgnet.com/2150-5349/full/v3/i5/74.htm
- DOI: https://dx.doi.org/10.4292/wjgpt.v3.i5.74
Crohn’s disease (CD) and ulcerative colitis (UC) represent two classes of progressive inflammatory diseases of the gastrointestinal tract that can present with life-threatening episodes and complications over the course of a patient’s life. The use of infliximab, a chimeric monoclonal antibody against tumor necrosis factor (TNF), has changed the management of inflammatory bowel disease (IBD) through its ability to improve both short- and long-term outcomes in patients.
The Food and Drug Administration approved infusion time for infliximab in the United States is 120-min[1]. Given the need for repeated infusions of the drug, it would be preferable for patients and health care providers alike to be able to improve the speed of this administration process. There are several European studies showing that the incidence of adverse reactions to infliximab are comparable whether it is given over the standard 120 min or over a shorter time period[2-5]. A Canadian study has demonstrated that faster infusions of the drug result in fewer infusion reactions than the standard 120-min administration[2]. However, no United States studies have been conducted showing the safety of a rapid infusion protocol and the vast majority of publications on this topic used a standard dose between 3 and 5 mg/kg[6].
With the passing of the Affordable Care Act, new measures for health care quality improvement have been developed. These measures include incentives for utilizing hospital resources more efficiently and improving patient satisfaction scores[7]. Given the time savings to the patients, the resultant cost savings to the hospital, and the reduced rate of infusion reactions, accelerated infusions of infliximab in United States patients may help improve these metrics. This study aims to be the first United States study investigating the safety and cost-effectiveness associated with the use of an accelerated infusion protocol for infliximab in patients receiving variable doses of the drug.
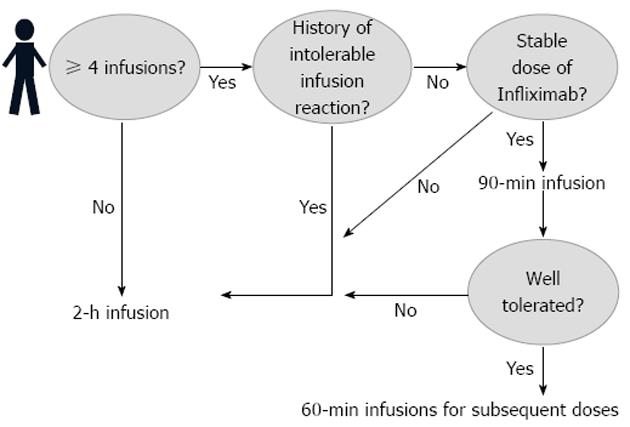
In 2011, the Digestive Health Center at the Medical Center of the University of Virginia introduced a new clinical protocolized management of infliximab administration. The new practice introduced an optional choice for the patients interested in receiving accelerated infliximab infusions. An infusion was considered to be accelerated if it was administered over 90 min and/or over 60 min. Concomitantly, original infusion rates were developed for the accelerated infusions (Table 1).
| Standard infliximab infusions | Accelerated infliximab infusions | ||||||||
| 120 min, 250 mL | 90 min, 250 mL | 60 min, 250 mL | 90 min, 500 mL | 60 min, 500 mL | |||||
| Rates (mL/h) | Dose (mL) | Rates (mL/h) | Dose (mL) | Rates (mL/h) | Dose (mL) | Rates (mL/h) | Dose (mL) | Rates (mL/h) | Dose (mL) |
| 10 | 2.5 | 10 | 2.5 | 20 | 2.5 | 20 | 5 | 50 | 5 |
| 20 | 5 | 20 | 5 | 40 | 5 | 40 | 10 | 100 | 10 |
| 40 | 10 | 40 | 10 | 80 | 10 | 80 | 20 | 350 | 40 |
| 80 | 20 | 80 | 20 | 160 | 20 | 300 | 40 | 500 | 80 |
| 150 | 75 | 500 | 190 | 300 | 75 | 700 | 420 | 750 | 350 |
| 250 | 135 | 550 | 135 | ||||||
A prospective cohort of consecutive patients were then assessed and recruited for this protocol. From August 2011 to March 2012, 50 IBD patients on stable dosing of infliximab, with at least four prior infusions, were offered the accelerated protocol. Initially, patients were only included if they did not use pre-medications and if they had no history of any infusion reactions. Later, patients who used pre-medications became eligible for the accelerated protocol. Eventually, patients with a remote history of infusion reactions were also made eligible. Patients under age 18 were excluded.
Doses of infliximab included 5 mg/kg, 7.5 mg/kg, and 10 mg/kg. Patients included in the study followed the protocol seen in Figure 1. Patients were initially transitioned to a 90-min infusion protocol; those that tolerated this infusion were subsequently given 60-min infusions at all future visits.
The University of Virginia Medical Center’s Institutional Review Board (IRB) approved this study (IRB No. = 15928). Fifty IBD patients were offered accelerated infusions and 46 patients consented to participate in the study. Nineteen (41.3%) were female, five (10.9%) were African American and nine (19.6%) had UC. The mean age was 42.6 years old. Complete demographics and other characteristics of the study population are shown in Tables 2 and 3.
| Characteristics | Data (%) |
| Gender | Female: 19/46 (41.3) |
| Male: 27/46 (58.7) | |
| Race | African American: 5/46 (10.9) |
| Caucasian: 41/46 (89.1) | |
| Age, yr | Mean: 42.6 |
| Median: 41.5 | |
| Range: 21-89 | |
| Disease type | Ulcerative colitis: 9/46 (19.6) |
| Crohn's: 37/46 (80.4) | |
| Use of premedications | 17/46 (37.0) |
| On corticosteroid therapy | 1/46 (2.2) |
| On immunosuppressive therapy | 10/46 (21.7) |
| Dose | 5 mg/kg: 25/46 (54.3) |
| 7.5 mg/kg: 5/46 (10.9) | |
| 10 mg/kg: 16/46 (34.8) | |
| Number of infusions prior to accelerated protocol | Mean: 27.4 |
| Median: 22 | |
| Range: 4-65 | |
| Body mass index | Normal (18.5-25.0): 19/46 (41.3) |
| Low (< 18.5): 1/46 (2.2) | |
| High (> 25.0): 26/46 (56.5) |
| No | Patient | Age (yr) | BMI | Dose1 | Date | Diagnose | Diagnosis | |
| 90 min infusion | 60 min infusion3 | |||||||
| 1 | Male: Caucasian | 37 | 170lbs (77 kg)BMI = 21.83 kg/m2 | 22 infusions5 mg/kg per 8 wk | 12/16/2011 | 02/20/2012 | Ulcerative colitis | Tylenol 650 mgClaritin 10 mgSolucortef 100 mg |
| 2 | Male: Caucasian | 56 | 202lbs (91 kg)BMI = 28.98 kg/m2 | 13 infusions7.5 mg/kg per 8 wk | 04/02/2012 | Crohn's disease | ||
| 3 | Male: Caucasian | 39 | 146lbs (66 kg)BMI = 19.80 kg/m2 | 50 infusions10 mg/kg per 7 wk | 12/19/2011 | 02/09/201203/29/2012 | Crohn's disease | Hydrocortisone 100 mg |
| 4NI | Female: African-American2 | 50 | 198lbs (89 kg)BMI = 31.01 kg/m2 | 22 infusions5 mg/kg per 7 wkHb = 12.3 g/dL | Crohn's disease | |||
| 5 | Male: Caucasian | 28 | 203lbs (92 kg)BMI = 29.17 kg/m2 | 38 infusions5 mg/kgper 8 wk | 09/27/2011 | 11/22/201101/23/201203/20/2012 | Crohn's disease | |
| 6 | Male: Caucasian | 30 | 115lbs (52 kg)BMI = 15.60 kg/m2 | 6 infusions5 mg/kg per 8 wk | Crohn's disease | |||
| 7 | Female: Caucasian | 53 | 300lbs (136.5 kg)BMI = 57.78 kg/m2 | 42 infusions5 mg/kg per 8wk | 08/08/2011 | 10/11/201112/21/201102/13/2012 | Crohn's disease (fistulizing) | |
| 8 | Female: CaucasianMoved to Germany Dec 2011 | 21 | 121lbs (55 kg)BMI = 22.18 kg/m2 | 12 infusions5 mg/kg per 8wk | 08/22/2011 | 10/17/201112/12/2011 | Crohn's disease | |
| 9 | Male: Caucasian | 50 | 179lbs (81 kg)BMI = 27.25 kg/m2 | 8 infusions5 mg/kg per 6 wk | 08/23/2011 | 10/04/201111/15/201112/27/201102/07/201203/21/2012 | Crohn's disease | |
| 10 | Male: Caucasian | 47 | 195.8lbs (89 kg)BMI = 28.96 kg/m2 | 15 infusions10 mg/kg per 6 wk | 11/08/2011 | 12/27/201102/06/201203/21/2012 | Crohn's disease | |
| 11 | Female: Caucasian | 58 | 157lbs (71 kg)BMI = 23.87 kg/m2 | 62 infusions7.5 mg/kg per 7 wk | 11/07/201112/29/201102/03/2012 | 04/02/2012 | Crohn's disease | |
| 12 | Female: CaucasianMoved to another hospital | 55 | 200lbs (90 kg)BMI = 34.16 kg/m2 | 50 infusions7.5 mg/kg per 8 wk | 09/26/2011 | 11/21/2011 | Crohn's disease | |
| 13 | Male: Caucasian | 29 | 154lbs (70 kg)BMI = 21.50 kg/m2 | 40 infusions5 mg/kg 400 mg per 6 wkHb = 15.6g/dL | 01/03/2012 | 02/14/201203/30/2012 | Crohn's disease | |
| 14 | Female: Caucasian | 36 | 248lbs (113 kg)BMI = 35.87 kg/m2 | 16 infusions5 mg/kg600-7.5 mg/kg shortened infusion D/C | 09/02/201110/28/2011Normal infusion time01/27/2012 (after dose increase) | 03/23/2012 | Ulcerative colitis | |
| 15 | Female: Caucasian | 34 | 167lbs (76 kg)BMI = 29.80 kg/m2 | 9 infusions5 mg/kg400 mg per 6-8wkHb = 12.1g/dL | 07/28/2011 | 09/16/201111/02/201112/20/201102/09/201203/22/2012 | Crohn's disease | |
| 16 | Female: Caucasian | 54 | 121lbs (54 kg)BMI = 20.14 kg/m2 | 48 infusions5 mg/kg per 8 wk | 03/05/2012 | Crohn's disease | ||
| 17 | Female:African-American | 68 | 191lbs (87 kg)BMI = 33.71 kg/m2 | 30 infusions 10 mg/kg per 4 wk | 11/23/2011 | 12/22/201101/16/201202/17/201203/16/2012 | Crohn's disease | |
| 18 | Female: Caucasian | 25 | 146lbs (66 kg)BMI = 22.87 kg/m2 | Since 20055 mg/kg per 8 wk | 01/05/201203/01/2012 | Crohn's disease | ||
| 19 | Female: Caucasian | 56 | 145lbs (66 kg)BMI = 22.87 kg/m2 | 51 infusions10 mg/kg per 5 wk | 10/21/2011 | 12/02/201201/13/201202/17/201203/30/2012 | Crohn's disease | |
| 20 | Female: Caucasian | 26 | 143lbs (65 kg)BMI = 23.85 kg/m2 | 10 infusions5 mg/kg per 7 wk | 07/25/2011 | 09/23/201111/10/201101/05/201202/23/2012 | Crohn's disease | |
| 21 | Male: Caucasian | 47 | 199lbs (90 kg)BMI = 22.75 kg/m2 | 48 infusions10 mg/kg per 6 wk | 12/20/2011 | 01/31/201202/12/2012 | Ulcerative colitis | |
| 22 | Female: Caucasian | 36 | 232lbs (105 kg)BMI = 35.28 kg/m2 | 5 infusions10 mg/kg,1100 mg in 500 mL per 6 wk | 01/13/201202/24/2012 | 04/06/2012 | Crohn’s disease | |
| 23 | Male: Caucasian | 21 | 202 lbs (91 kg)BMI = 27.40 kg/m2 | 4 infusions5 mg/kg per 8 wk | 01/23/2012 | 03/21/2012 | Crohn’s disease | |
| 24 | Female: Caucasian | 41 | 126lbs (57.5 kg)BMI = 19.86 kg/m2 | 14 infusions10 mg/kg per 8 wk | 02/22/2012 | 04/12/2004 | Crohn’s disease | |
| 25 | Male: CaucasianTransferred receives remicade locally (01/31/12) | 34 | 212lbs (96.8 kg)BMI = 32.46 kg/m2 | 24 infusions5 mg/kg per 8 wk | 08/11/2011 | 10/07/201112/02/2011 | Ulcerative colitis | |
| 26 | Male: Caucasian | 41 | 196lbs (88 kg)BMI = 32.62 kg/m2 | 21 infusions10 mg/kg per 6 wk | 04/04/2012 | Crohn’s disease | ||
| 27 | Male: Caucasian | 63 | 176lbs (80 kg)BMI = 26.76 kg/m2 | 61 infusions5 mg/kg 400 mg | 09/08/2011 | 11/29/201101/27/201203/14/2012 | Crohn’s disease | |
| 28 | Male: Caucasian | 38 | 149lbs (67.5 kg)BMI = 22.00 kg/m2 | 10 infusions10 mg/kg per 6 wk | 12/08/2011 | 01/20/201203/06/2012 | Crohn’s disease | |
| 29 | Female: Caucasian | 42 | 259lbs (117.5 kg)BMI = 41.80 kg/m2 | 46 infusions10 mg/kg1200 mg in 500 mL | 02/13/2012 | 04/02/2012 | Crohn’s disease | |
| 30 | Male: Caucasian | 40 | 192LBS (87 kg)BMI = 27.55 kg/m2 | 65 infusions7.5 mg/kg per 8 wk` | 12/13/2011 | 02/07/2012 | Crohn’s disease | |
| 32 | Male: Caucasian | 49 | 154LBS (70 kg)BMI = 23.42 kg/m2 | 49 infusions10 mg/kg700 mg per 8wk | 12/19/2011 | 02/10/2012 | Crohn’s disease | |
| 33 | Male: Caucasian | 23 | 131lbs (59.5 kg)BMI = 18.83 kg/m2 | 15 infusions10 mg/kg per 8 wk | 01/11/2012 | 03/09/2012 | Ulcerative colitis | |
| 34 | Male: Caucasian | 26 | 208lbs (94.5 kg)BMI = 26.71 | 7 infusions5 mg/kg per 8 wk | 09/12/2011 | 11/07/201101/03/201203/27/2012 | Crohn’s disease | |
| 35 | Female: African-American | 24 | 129lbs (58.5 kg)BMI = 22.14 kg/m2 | 11 infusions7.5 mg/kg per 8 wkHb = 13.1 g/dL | 01/23/2012 | 03/20/2012 | Crohn’s disease | |
| 36 | Male: Caucasian | 31 | 170lbs (77 kg)BMI = 23.11 kg/m2 | 13 infusions5 mg/kg per 8 wk Normal infusion on 02/21 because of dose increase: 600 mg | 11/14/2011 | 01/16/2012 | Crohn’s disease | |
| 37 | Male: African-American | 45 | 267lbs (121 kg)BMI = 40.60 kg/m2 | 27 infusions10 mg/kg1200 mg in 500 mL per 7 wk | 12/15/201102/15/201204/05/2012 | Crohn’s disease | ||
| 38 | Male: Caucasian | 89 | 184lbs (83.5 kg)BMI = 27.17 kg/m2 | 22 infusions5 mg/kg per 8 wk | 07/21/2011 | 09/15/201111/03/201112/29/201102/23/2012 | Crohn’s disease | |
| 39 | Male: Caucasian | 46 | 181lbs (82 kg)BMI = 22.62 kg/m2 | 24 infusions10 mg/kg per 5 wk | 11/29/2011 | 01/10/201202/14/201203/20/2012 | Crohn’s disease | |
| 40 | Male: Caucasian | 58 | 167lbs (76 kg)BMI = 27.79 kg/m2 | 19 infusions5 mg/kg per 8 wk | 09/12/2011 | 11/07/201101/09/201203/05/2012 | Ulcerative colitis | |
| 41 | Male: Caucasian | 72 | 169lbs (77 kg)BMI = 25.81 kg/m2 | 23 infusions 10 mg/kg per 7 wk | 11/19/2011 | 01/06/201202/24/2012 | Ulcerative colitis | |
| 42 | Female: Caucasian | 48 | 128lbs (58 kg)BMI = 22.32 kg/m2 | 54 infusions5 mg/kg per 6 wk | 09/30/2011 | 11/23/201101/17/201203/23/2012 | Crohn’s disease | |
| 43 | Caucasian30 yr old | 30 | 264lbs (120 kg)BMI = 35.88 kg/m2 | 44 infusions5 mg/kg per 8 wk | 08/04/2011 | 09/29/201111/28/201101/23/201203/19/2012 | Crohn’s disease | |
| 44 | Female:Caucasian | 44 | 138lbs (63 kg)BMI = 21.7 kg/m2 | 54 infusions10 mg/kg per 5 wk | 01/26/2012 | 03/01/201204/05/2012 | Crohn’s disease | |
| 45 | Male: Caucasian | 28 | 205lbs (93 kg)BMI = 27.80 kg/m2 | 10 infusions5 mg/kg per 8 wk | 01/26/2012 | 03/21/2012 | Ulcerative colitis | |
| 46 | Male: African-American | 43 | 277lbs (103 kg)BMI = 36.65 kg/m2 | 5 infusions5 mg/kg per 8 wk | 08/19/2011 | 10/10/201112/05/201101/18/201202/27/2012 | Crohn’s disease | |
| 47 | Female: Caucasian | 50 | 202lbs (92 kg)BMI = 34.81 kg/m2 | 7 infusions5 mg/kg per 6 wk | 07/21/2011 | 09/01/201110/10/201111/21/201112/29/201102/06/201203/19/2012 | Ulcerative colitis | |
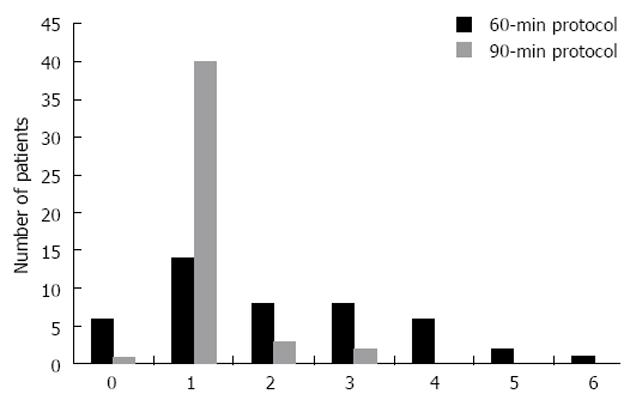
Of the 46 patients consenting to participate, one withdrew immediately prior to receiving the first accelerated infusion. The remaining 45 subjects were given at least one 90-min infusion and 39 subjects were given at least one 60 min infusion. In total, fifty 90-min infusions and ninety-three 60-min infusions were given. No infusion reactions of any type were noted. The distribution of the number of accelerated infusions of each type can be seen in Figure 2.
There were also no patient-reported infections or hospitalizations among the patients in the study. None of the patients developed cancer of any type during the study timeframe. Reasons for giving 90-min infusions multiple times to the same patient included change in infliximab dose, concerns about a specific patient’s blood pressure control, and physician preference. One patient experienced a local reaction at the infusion site during a 60-min infusion, which was due to catheter placement rather than a true infusion reaction; nevertheless this patient requested a 90-min infusion at the next visit. However, when the patient came for her next infusion, she opted to continue receiving it over 60 min. The prior local reaction was not replicated by the subsequent infusions. Thus, both the patient and the provider concluded that the previous reaction was induced by intravenous catheter malfunction.
Ten patients used immunosuppressive drugs concurrently out of which six were taking azathioprine, three were taking 6-mercaptopurine and one was taking methotrexate. One of the 46 study patients used corticosteroid therapy for his IBD. Six patients received corticosteroids as pre-medication.
Four patients had a remote history of infusion reactions to infliximab therapy. These reactions included shortness of breath, chest tightness, flushing, pruritus and urticaria. These patients all took prophylactic medications before receiving infusions. Other patients receiving pre-medications took them because of treatment re-initiation or because of physician preference. Prophylactic medications included acetaminophen, loratadine, famotidine, cetirizine, hydrocortisone and diphenhydramine.
Hospital cost savings were calculated by estimating the cost required to deliver infusions over 120-min vs using the accelerated infusion times. In this study, 118 h of infusion time and $53 632 were saved by using the accelerated protocols. Using a Mann-Whitney U test, these savings were found to be statistically significant: $116 965 vs $63 333 (P < 0.001).
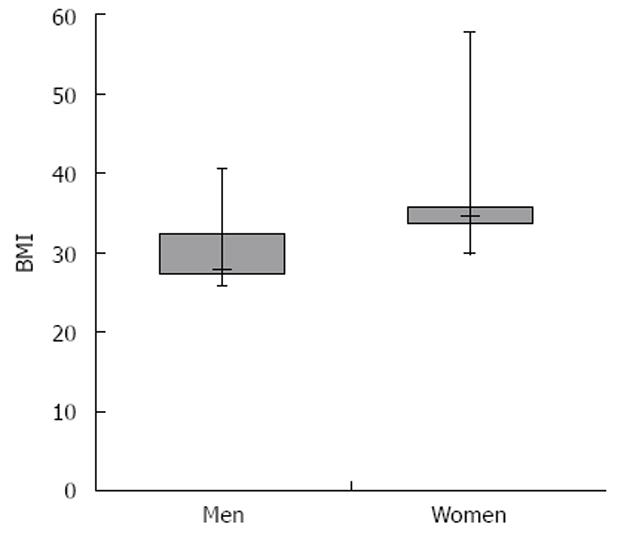
Body mass index (BMI) data for all patients were obtained. Thirteen of 46 patients (28.2%) fell under the definition of obese with a BMI > 30 kg/m2. Numerous BMI comparisons were made between subpopulations of patients in the study. In the subpopulation of overweight (BMI > 25.0 kg/m2) females vs males, females had statistically higher BMIs. Their mean BMI was 35.07 kg/m2, SD = 8.83, SE = 2.66 kg/m2vs 30.08 kg/m2, SD = 4.11, SE = 0.99, P < 0.05. The distribution of BMIs among these two populations is shown in Figure 3. Other subpopulation comparisons were not found to be significantly different, including comparisons between data of Caucasians and African Americans, patients receiving regular doses (5 mg/kg) and higher doses (7.5 mg/kg and 10 mg/kg) and males and females as a whole.
Though no systematic surveying of the study patients was performed, all study patients expressed increased satisfaction associated with the accelerated protocol vs the standard 120-min infusions. As a result, three patients, who had initially declined to participate, expressed the desire at a later date to receive accelerated infusions.
The current paradigm of infusing infliximab over 120 min was based on clinical trial data showing a tolerable incidence of side effects at this rate. A number of studies have shown that infusing at rates faster than the current practice is safe[2-5]. Accelerated infusions of infliximab were safe in the population of patients in this study. There were no infusion reactions observed after delivering a total of 143 accelerated infusions over the course of this study. Only one patient had to revert to a 120-min infusion after receiving accelerated infusions. This instance was due to an increase in the infliximab dose, which mandated a subsequent 120-min infusion per the study protocol. Several patients repeated the intermediate rate infusions for various reasons; however, these reasons did not include suspected infusion reactions. Similar to prior studies[2-5], this study provides further evidence that accelerated infusions of infliximab in IBD patients are well-tolerated regardless of dose, diagnosis, history of infusion reactions or treatment frequency.
Prior studies of faster infusion times have had different study populations. Our study is the first to use a dose of 7.5 mg/kg at faster rates and the largest number of patients receiving 10 mg/kg (n = 16, 34.78% of the study population). Noticeably, there is only one prior study that included patients who received a 10 mg/kg dose (n = 5, 2.8% of the study population)[5]. Our study population is also notable for containing a majority of patients not receiving prophylaxis; among those getting pre-medications, there were a number of reasons for the prophylaxis. Some of our patients had a remote history of infusion reactions, while others had had treatment re-initiations; these patients all received pre-medications and none had infusion reactions with the accelerated protocol. Compared to other studies, our patient population also had a larger percentage of UC (19.6%) and overweight patients (56.5%).
This diversity in our patient population helps to broaden the evidence for safe administration of infliximab at faster rates. Higher doses of infliximab may be used and patients with prior infusion reactions or treatment re-initiations may still safely receive their treatments with a faster protocol.
The need for anti-TNF therapy is increasing. Several studies have suggested that a more aggressive “top-down” approach to CD, such as involving early adoption of regular doses of infliximab, is more effective than the traditional “step up” method of treatment[8]. Using early aggressive therapy has been shown to decrease the need for surgery and reduce hospitalizations in patients with CD[8], and prompt induction therapy in UC has been shown to promote mucosal healing, an important prognostic factor[9]. The active ulcerative colitis trials 1 and 2, which investigated using infliximab as maintenance therapy for UC, demonstrated significant improvements in clinical response to treatment among patients with moderate-to-severe UC receiving infliximab, when compared to patients receiving conventional therapy[10]. Whether the use of infliximab can reduce the rate of colectomy for UC patients is not currently known, but current data is promising[11].
With the improvement in treatment options over the last decade comes the sobering fact that hospitalizations and inpatient charges for IBD, especially CD, have increased substantially: from 1998 to 2004, the hospitalization rate for CD-related health issues increased by 4.3% and total charges increased from $762 million to $1.33 billion[12]. Considering the proven effectiveness of anti-TNF therapy and the rising incidence of IBD, it is likely that the number of patients on maintenance infliximab regimens will increase in the future. With rising health care costs and a system-wide heightened interest in efficiency and patient satisfaction, finding ways to streamline the delivery of this drug is a crucial step toward improving therapy for IBD. The cost savings from this protocol were significant. A total of $ 53 632 was saved over the course of this nine month period of time. This number represents a significant underestimate. With only 46 patients enrolled and many starting the accelerated protocol in the latter months of the study, this figure represents only a fraction of the cost-savings that could be obtained if the protocol were implemented in a wider and more consistent fashion. Cost savings for minimizing adverse events were not included in the estimate, which would contribute significantly to enhance the effectiveness of the infliximab infusions and thereby being in concert with the requirements of the Affordable Care Act.
Infliximab is not a benign drug and has the potential for serious side effects; compared to other biologics, it is the only drug with a significantly higher rate of adverse events than controls[13]. Long-term effects include an increased risk of bacterial, viral, or fungal infections causing hospitalization[14] and a theoretical, though unproven, increased risk of Hodgkin and non-Hodgkin lymphoma in adolescent and young adult males[15]. Acute infusion reactions occur in 0.8% to 8.8% of infusions, affecting 10% to 23% of patients per year[16]. Delayed infusion reactions occurred in roughly 2% of patients receiving infliximab over the course of a year[17]. While this study did not examine the infectious or cancer risks, the complete lack of infusion reactions in our patients is a promising lead for making infliximab safer.
The incidence of infusion reactions to infliximab is highly related to the development of antibodies to the drug, known as human anti-chimeric antibodies (HACA)[18]. Loss of response to treatment has also been observed in patients who develop these antibodies. Thus, the prevention of antibody formation is paramount in keeping infliximab as a safe and effective treatment for IBD patients. The development of these antibodies can be reduced by maintaining higher trough levels, having consistent dosing schedules[18], and using pre-medications such as hydrocortisone[19]. Additionally, the use of immunomodulators such as methotrexate has been shown to reduce antibody formation against infliximab in patients with rheumatoid arthritis[20].
When reviewing the literature, Lee et al[2] concluded in their study that 90% of the adverse reactions associated with infliximab infusions occur during the first eight infusions. Since our study population had a mean of 27 infusions prior to the initiation of the accelerated protocol we cannot easily compare our lack of reactions with the traditional infusion rates. However, considering the role that anti-infliximab antibodies play in the incidence of infusion reactions and treatment failure[18], the infusion rate may be related to the development of these antibodies. Mori et al[21] demonstrated that higher trough infliximab levels are associated with better outcomes. The serum concentration of infliximab measured one hour after each infusion approximates the maximum serum concentration (Cmax). Thus, we hypothesize that a more rapid attainment of Cmax may decrease the immune response to the drug. Future work could be directed toward monitoring the development of HACA among patients receiving infusions at different rates. Due to restrictions imposed by cost and insurance policies, this study did not analyze the infliximab serum level after 1 h infusions and it did not investigate the presence or absence of the antibodies toward infliximab.
Obese patients with CD also tend to have a higher incidence of perianal disease and more post-surgical complications[22]. BMI has also been shown to be a significant factor in patients with CD since obesity itself may be a risk factor for CD[23]. Treatment failures with infliximab in conditions such as rheumatoid arthritis[24], ankylosing spondylitis[25], and psoriasis[26] are also more common in overweight and obese patients than in patients with normal BMIs. Over half of our patients were considered overweight by BMI and nearly a third were obese. With the need for effective treatment options being especially great for obese patients suffering from CD, it is reassuring to know that accelerated infliximab infusions were safe among this demographic, as demonstrated in this study.
There are a number of limitations to this study. There was no specifically defined control group to which the patients receiving faster infusions could be compared. The racial makeup of the study population was somewhat narrow as well. A few deviations from the study protocol occurred, with some patients receiving an intermediate-speed infusion multiple times due to physician preference rather than the clinical guidelines. However, despite these limitations, this study provides strong evidence for the benefits of implementing an accelerated infusion protocol for treating IBD patients on maintenance therapy. With such a protocol in place, costs to patients and hospitals in terms of both time and money would be decreased, patient safety and satisfaction may be increased, and a life-saving drug may be made more widely available. With additional data and further inquiry, faster infusions of infliximab may become the standard of care in the United States.
We would like to thank Mr. Rebecca Treakle, BSN-RN, for her help to clinically care and monitor some of the patients, who participated in this study.
Crohn’s disease and ulcerative colitis (UC) represent two classes of progressive inflammatory diseases of the gastrointestinal tract with a rising incidence that can present with life-threatening episodes and complications over the course of a patient’s life. The use of biologic agents, such as anti-tumor necrosis factor (anti-TNF) drugs, has changed the management of inflammatory bowel disease (IBD) through its ability to improve both short- and long-term outcomes in patients.
Considering the proven effectiveness of anti-TNF therapy it is likely that the number of patients on maintenance infliximab regimens will increase in the future. With rising health care costs and a system-wide heightened interest in efficiency and patient satisfaction, finding ways to streamline the delivery of this drug by improving the cost of the administration process is a crucial step toward improving therapy for IBD. The current study looked into the efficacy and safety of accelerated infliximab infusions in IBD patients attending the University of Virginia Medical Center’s Gastrointestinal Clinic from VA, United States.
No United States studies have been conducted showing the safety of a rapid infusion protocol and the vast majority of publications on this topic used a standard dose between 3 and 5 mg/kg. There is only one prior study that included patients who received a 10 mg/kg dose. The study is the first to use a dose of 7.5 mg/kg at faster rates and the largest number of patients receiving 10 mg/kg (n = 16; 34.78% of the study population), while employing innovative infusion rates for these doses. The serum concentration of infliximab measured one hour after each infusion approximates the maximum serum concentration (Cmax), which will be the case of the accelerated infliximab infusion. Thus, they hypothesize that a more rapid attainment of Cmax may decrease the immune response to the drug. Future work could be directed toward monitoring the development of Heads of Asbestos Coordination Authorities among patients receiving infusions at different rates. Their study population is also notable for its number of UC patients as well as for its majority of patients not receiving prophylaxis.
This study provides strong evidence for the benefits of implementing an accelerated infusion protocol for treating IBD patients on maintenance therapy. With such a protocol in place, costs to patients and hospitals in terms of both time and money would be decreased, patient safety and satisfaction may be increased, and a life-saving drug may be made more widely available. With additional data and further inquiry, faster infusions of infliximab may become the standard of care in the United States.
Infliximab is a biologic agent, a chimeric monoclonal antibody against TNF, which has changed the management of IBD through its ability to improve both short- and long-term outcomes in patients.
This is a very interesting short original paper. The attached short original report presents interesting data on accelerated infliximab infusions in IBD patients.
Peer reviewer: Wojciech Blonski, MD, PhD, University of Pennsylvania, Gastrointestinal Research-Ground Centrex, 3400 Spruce St, Philadelphia, PA 19104, United States
S- Editor Gou SX L- Editor A E- Editor Xiong L
| 1. | Infliximab-full prescribing information. PA: Centocor Biotech, Inc. 2012; . |
| 2. | Lee TW, Singh R, Fedorak RN. A one-hour infusion of infliximab during maintenance therapy is safe and well tolerated: a prospective cohort study. Aliment Pharmacol Ther. 2011;34:181-187. [PubMed] [DOI] [Full Text] |
| 3. | Bhat S, Sharma D, Doherty P, Tham TC, Caddy GR. Are accelerated infliximab infusions safe in patients with inflammatory bowel disease? Inflamm Bowel Dis. 2010;16:1922-1925. [PubMed] |
| 4. | Breynaert C, Ferrante M, Fidder H, Van Steen K, Noman M, Ballet V, Vermeire S, Rutgeerts P, Van Assche G. Tolerability of shortened infliximab infusion times in patients with inflammatory bowel diseases: a single-center cohort study. Am J Gastroenterol. 2011;106:778-785. [PubMed] |
| 5. | Van Assche G, Vermeire S, Noman M, Amant C, Weyts E, Vleminckx A, Vermeyen MJ, Rutgeerts P. Infliximab administered with shortened infusion times in a specialized IBD infusion unit: a prospective cohort study. J Crohns Colitis. 2010;4:329-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 6. | Rutgeerts P, Van Assche G, Vermeire S. Review article: Infliximab therapy for inflammatory bowel disease--seven years on. Aliment Pharmacol Ther. 2006;23:451-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 170] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 7. | Available from: http://www.communityhlth.org/communityhlth/files/files_resource/Community%20Benefit/PPACA-Sec9007-tax-exempt-hospitals.pdf. |
| 8. | D'Haens G, Baert F, van Assche G, Caenepeel P, Vergauwe P, Tuynman H, De Vos M, van Deventer S, Stitt L, Donner A. Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn's disease: an open randomised trial. Lancet. 2008;371:660-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 922] [Cited by in RCA: 938] [Article Influence: 55.2] [Reference Citation Analysis (0)] |
| 9. | Sohrabpour AA, Malekzadeh R, Keshavarzian A. Current therapeutic approaches in inflammatory bowel disease. Curr Pharm Des. 2010;16:3668-3683. [PubMed] |
| 10. | Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, Travers S, Rachmilewitz D, Hanauer SB, Lichtenstein GR. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462-2476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2744] [Cited by in RCA: 2872] [Article Influence: 143.6] [Reference Citation Analysis (2)] |
| 11. | Behm BW, Bickston SJ. Efficacy of infliximab for luminal and fistulizing Crohn's disease and in ulcerative colitis. Curr Treat Options Gastroenterol. 2007;10:171-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Nguyen GC, Tuskey A, Dassopoulos T, Harris ML, Brant SR. Rising hospitalization rates for inflammatory bowel disease in the United States between 1998 and 2004. Inflamm Bowel Dis. 2007;13:1529-1535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 122] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 13. | Singh JA, Wells GA, Christensen R, Tanjong Ghogomu E, Maxwell L, Macdonald JK, Filippini G, Skoetz N, Francis D, Lopes LC. Adverse effects of biologics: a network meta-analysis and Cochrane overview. Cochrane Database Syst Rev. 2011;CD008794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Curtis JR, Patkar N, Xie A, Martin C, Allison JJ, Saag M, Shatin D, Saag KG. Risk of serious bacterial infections among rheumatoid arthritis patients exposed to tumor necrosis factor alpha antagonists. Arthritis Rheum. 2007;56:1125-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 283] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 15. | Rosenblum H, Amital H. Anti-TNF therapy: safety aspects of taking the risk. Autoimmun Rev. 2011;10:563-568. [PubMed] |
| 16. | Cheifetz A, Smedley M, Martin S, Reiter M, Leone G, Mayer L, Plevy S. The incidence and management of infusion reactions to infliximab: a large center experience. Am J Gastroenterol. 2003;98:1315-1324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 356] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 17. | Cheifetz A, Mayer L. Monoclonal antibodies, immunogenicity, and associated infusion reactions. Mt Sinai J Med. 2005;72:250-256. [PubMed] |
| 18. | Ducourau E, Mulleman D, Paintaud G, Miow Lin DC, Lauféron F, Ternant D, Watier H, Goupille P. Antibodies toward infliximab are associated with low infliximab concentration at treatment initiation and poor infliximab maintenance in rheumatic diseases. Arthritis Res Ther. 2011;13:R105. [PubMed] |
| 19. | Farrell RJ, Alsahli M, Jeen YT, Falchuk KR, Peppercorn MA, Michetti P. Intravenous hydrocortisone premedication reduces antibodies to infliximab in Crohn's disease: a randomized controlled trial. Gastroenterology. 2003;124:917-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 390] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 20. | Maini RN, Breedveld FC, Kalden JR, Smolen JS, Davis D, Macfarlane JD, Antoni C, Leeb B, Elliott MJ, Woody JN. Therapeutic efficacy of multiple intravenous infusions of anti-tumor necrosis factor alpha monoclonal antibody combined with low-dose weekly methotrexate in rheumatoid arthritis. Arthritis Rheum. 1998;41:1552-1563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 21. | Mori S. A relationship between pharmacokinetics (PK) and the efficacy of infliximab for patients with rheumatoid arthritis: characterization of infliximab-resistant cases and PK-based modified therapy. Mod Rheumatol. 2007;17:83-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Blain A, Cattan S, Beaugerie L, Carbonnel F, Gendre JP, Cosnes J. Crohn's disease clinical course and severity in obese patients. Clin Nutr. 2002;21:51-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 175] [Article Influence: 7.6] [Reference Citation Analysis (1)] |
| 23. | Mendall MA, Gunasekera AV, John BJ, Kumar D. Is obesity a risk factor for Crohn's disease? Dig Dis Sci. 2011;56:837-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 24. | Gremese E, Carletto A, Padovan M, Atzeni F, Raffeiner B, Giardina AR, Favalli EG, Erre G, Gorla R, Galeazzi M. Obesity and reduction of the response rate to anti-tumor necrosis factor α in rheumatoid arthritis: An approach to a personalized medicine. Arthritis Care Res (Hoboken). 2013;65:94-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 148] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 25. | Ottaviani S, Allanore Y, Tubach F, Forien M, Gardette A, Pasquet B, Palazzo E, Meunier M, Hayem G, Job-Deslandre C. Body mass index influences the response to infliximab in ankylosing spondylitis. Arthritis Res Ther. 2012;14:R115. [PubMed] |
| 26. | Puig L. Obesity and psoriasis: body weight and body mass index influence the response to biological treatment. J Eur Acad Dermatol Venereol. 2011;25:1007-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 146] [Article Influence: 10.4] [Reference Citation Analysis (0)] |