Published online Oct 6, 2011. doi: 10.4292/wjgpt.v2.i5.42
Revised: September 20, 2011
Accepted: September 28, 2011
Published online: October 6, 2011
Gilbert’s syndrome is characterized by a benign indirect hyperbilirubinemia. It has often been underestimated and undiagnosed because of its mild symptoms; although it is not as rare as was once believed when its frequency was estimated using data originating from biochemical tests. Based on molecular techniques, the occurrence of Gilbert’s syndrome has changed, increasing to 10% in the Caucasian population. This molecular defect was described, by Bosma et al, in 1995, and affects the promoter region of the UGT 1A1 gene. In this case report, our aim is to present a new combination of two molecular defects in a Greek patient with Gilbert’s syndrome. A 13-year-old Greek girl was examined for Gilbert’s syndrome using molecular techniques, and an uncommon genotype was revealed comprising the rare mutation G71R in trans with A(TA)7TAA motif. The G71R mutation according to the literature, as well as our epidemiological data, is rare in Caucasians, while it is common in Asian populations. This is the first case study in the Greek population to report a new genotype for Gilbert’s syndrome manifestation in the Caucasian population.
- Citation: Kalotychou V, Karakosta M, Tzanetea R, Stamoulakatou A, Konstantopoulos K, Rombos Y. Contribution of G71R mutation to Gilbert’s syndrome phenotype in a Greek patient: A case report. World J Gastrointest Pharmacol Ther 2011; 2(5): 42-45
- URL: https://www.wjgnet.com/2150-5349/full/v2/i5/42.htm
- DOI: https://dx.doi.org/10.4292/wjgpt.v2.i5.42
Gilbert’s syndrome is characterized usually as a mild unconjugated hyperbilirubinemia in the absence of liver disease and/or overt hemolysis. The molecular basis of this syndrome was described in 1995 by Bosma et al[1], they found a defect on the UGT 1A1 gene resulting in a reduced synthesis of the UDP-glucoronosyltransferase 1 enzyme, critical for bilirubin metabolism. The first description of the molecular basis of this syndrome, concerned a dinucleotide insertion in the promoter region of the UGT 1 A1 gene. A TA was inserted into the TATA box extending the length of the sequence by two bases, creating the A(TA)7TAA motif, instead of the A(TA)6TAA, which is concerned with the reference sequence. Using the Hu h7 cell line in expression experiments, Bosma et al[1] showed that the mutated sequence A(TA)7TAA reduced the expression levels of the luciferase reporter gene, which was driven by the mutated UGT1A1 promoter compared to the wild type promoter. The TA insertion affects the binding site in the beginning and then the binding affinity of the TFIID transcription factor, resulting in a reduced expression of the underlying gene. Four different size motifs have been found and reported in this region namely: A(TA)8TAA, A(TA)7TAA, A(TA)6TAA and A(TA)5TAA; with A(TA)8TAA and A(TA)5TAA motifs being mainly common to African people. Transient expression experiments have shown that there is an inverse relationship between the numbers of TA repeats and the activity of the promoter of the UGT 1 A1 gene throughout the range of (TA)5-(TA)8 repeats[2]. Numerous mutations have been detected for Gilbert’s syndrome, mutations affecting the promoter region, as well as the coding region.
Epidemiological studies have shown a geographic distribution of specific molecular defects. In Caucasians, the promoter mutation A(TA)7TAA is the prevalent defect leading to Gilbert’s syndrome when it appears in homozygosity. In Asians, mutations in the coding region are the most common causes of Gilbert’s syndrome, characterized by nucleotide substitutions G71R and Y486D. Expression experiments, using a Cos-7 cell line that was transiently transfected with normal and mutant cDNA of the UGT1A1 gene, showed that the expression levels of cDNA carrying the G71R and Y486D mutations, were lower compared to normal cDNA levels. In addition, Western blotting showed that the UGT1A1 G71R and Y486D protein levels were also reduced[3,4].
A 13-year-old girl, presenting high levels of unconjugated bilirubin (3.74 mg/dL), was referred to an outpatient’s clinic of a children’s hospital. Her clinical examination was normal, as well as her biochemical tests for liver function. The suspicion was raised of Gilbert’s syndrome, and the girl was consequently examined for it.
Peripheral blood (2 mL sample) was collected from both her and her parents. DNA was isolated using the Qiagen midi blood kit, and amplified by polymerase chain reaction (PCR) using the appropriate pair of primers that bordered a 71-bp promoter region of the UGT1A1 gene containing the TATA-box. PCR products of different sizes were separated by electrophoresis on a 12% polyacrylamide gel.
Molecular analysis of the promoter region of the UGT1A1 gene revealed that the proposita was a heterozygote with the A(TA)7TAA/A(TA)6TAA genotype, while her mother was carrying the A(TA)7TAA/A(TA)7TAA genotype compatible with Gilbert’s syndrome (Figure 1). Despite this genotype, the proposita’s mother presented normal unconjugated bilirubin levels (0.65 mg/dL). The father was found to be a homozygote for the wild type genotype A(TA)6TAA/A(TA)6TAA, and also presented normal unconjugated bilirubin levels (0.46 mg/dL) as did his wife. However, the proposita’s heterozygosity for Gilbert’s syndrome could not explain the persistent high level (3.74 mg/dL) of unconjugate bilirubinemia, and raised the question of other possible mutations affecting the UGT1A1 gene.
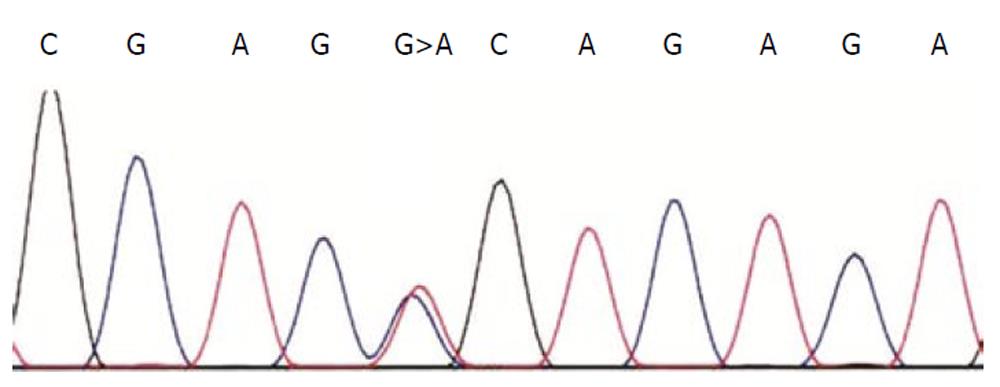
We continued the study, amplifying the coding regions of the UGT1A1 gene with the appropriate primers and proceeded with sequencing. We amplified the regions covering exon I, exon II-IV and exon V. PCR primers and sequencing primers were the same as those described by Takeuchi et al[5]. Nucleotide analysis of the above regions using PCR primers, revealed that the proposita was a carrier for the G71R mutation (G>A at nt211, Figure 2).
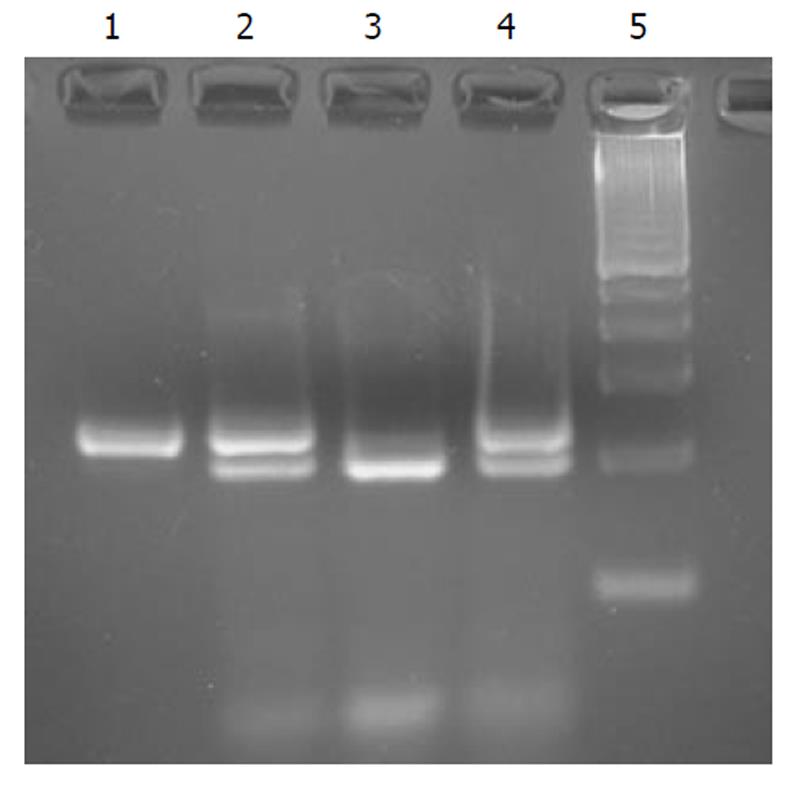
Her parents were examined for the above mutation using PCR-RFLP analysis. The primers suggested by Ando et al[6] were used, and the PCR product after a nested PCR (235 bp) was digested with the appropriate restriction enzyme that recognized the mutated sequence. The enzyme was Msp I (New England BioLabs®Inc.) and the recognized sequence was CCGG. Ten microliters of PCR product was digested under the following conditions: 2 h at 37°C using 10 U of Msp I enzyme. After digestion of the mutated allele, this revealed a 235 bp fragment, while the wild type digested allele revealed two 203 bp and 32 bp fragments. Her father was also found to be a carrier of this rare mutation in the Caucasian population, while her mother, was found to have a normal sequence for this mutation (Figure 3). The proposita’s father was carrying the wild genotype for Gilbert’s syndrome, but he was heterozygote for the newfound mutation. He was clinically normal, without any symptoms of hyperbilirubinemia.
We continued to look for this rare mutation in the Greek population, but out of a total of 146 individuals tested, (47 presenting unconjugated hyperbilirubinemia, and 99 with normal levels of unconjugated bilirubin) found only one other carrier of the G71R mutation. He was a carrier of the genotype A(TA)6TAA/A(TA)6TAA, with normal levels of unconjugated bilirubin.
In this case report our aim has been to present a new combination of two molecular defects in a Greek patient with Gilbert’s syndrome. As has been mentioned, in the past, there has been a racial variability in Gilbert’s syndrome manifestation. A different genetic basis of hybilirubinemia has been found between Asian and Caucasian people. In Caucasians, Gilbert’s syndrome is due to an extra TA dinucleotide in the TATA box. It is inherited with the autosomal recessive character, and the clinical manifestation of this syndrome usually demands the homozygous status[2]. In Asian populations, other mutations have been referred to as causes for this syndrome. Mutations affecting the coding region of the UGT1A1 gene have interfered with unconjugated hyperbilirubinemia. Nucleotide substitutions like G>A at nt +211 (G71R), C>A at nt +686 (P229Q) in exon I and T>G at nt +1456 (Y486D) in exon V are common in Asian populations (Japanese, Korean and Chinese), with a high prevalence of G71R. The allele frequencies in Japanese, Korean and Chinese populations have been reported to be 13%, 23% and 23% respectively[7].
The rarity of the G71R mutation found in the Greek group of 146 individuals is inconsistent with previous publications regarding the Caucasian population. Reviewing the literature we found only a few publications concerning Caucasians carrying the G71R mutation. The first one concerned a case report published by Sava et al[8] in 2004, presenting a carrier for G71R mutation. He was a 64-year-old German male suffering from Gilbert’s syndrome. Gene analysis of 103 persons of German descent revealed that only 1 out of 103 was a carrier of this mutation[8]. Unfortunately, in this report there were no other data regarding the TATA-box configuration, and we were not able to make an estimation of its possible contribution to clinical phenotype. The second publication mentioned that only 2 persons out of a total of 136 Italian individuals (83 controls and 53 pediatric subjects) presented the G71R mutation[9].
In expression experiments by Yamamoto et al[3] in 1998, the enzymatic activity of the mutated enzyme carrying the G71R mutation was 60% of the normal gene. However, a lower enzymatic activity (30% of normal) is necessary in order for Gilbert syndrome to be clinically present.
In our proposita, there co-exists two molecular defects residing in trans on the UGT1A1 gene, the G71R mutation and the A(TA)7TAA motif. This new genotype for Greek and Caucasian population, consisting of a nucleotide substitution and a dinucleotide insertion, may affect the bilirubin levels of the proposita, leading to Gilbert’s syndrome manifestation. A single dose of G71R mutation seems to be insufficient for a clinical manifestation of Gilbert’s syndrome in the heterozygote father, who is the donor of this mutation.
Taking these findings into consideration, we propose that a synergistic reaction may occur between these two molecular defects, leading to reduced enzymatic activity of the underling gene product (UDP-glucoronosyltransferase 1 enzyme).
This is the first case report in the Greek population, describing a new genotype for Gilbert’s syndrome manifestation in the Caucasian population. These findings suggest that in some cases TATA box insertion, elongation is not sufficient to explain Gilbert’s phenotype, and further investigation is necessary.
We thank Mr. Kostas Kormas for the image processing and Mr. Peter Magee for his contribution to the English correction, http://www.anglais.webs.com.
Peer reviewer: Osama Badary, PhD, Professor, Head, Clinical Pharmacy Dept., Faculty Pharmacy, Ain Shams University, Abbassia, Cairo, Egypt
S- Editor Wang JL L- Editor Hughes D E- Editor Zheng XM
| 1. | Bosma PJ, Chowdhury JR, Bakker C, Gantla S, de Boer A, Oostra BA, Lindhout D, Tytgat GN, Jansen PL, Oude Elferink RP. The genetic basis of the reduced expression of bilirubin UDP-glucuronosyltransferase 1 in Gilbert’s syndrome. N Engl J Med. 1995;333:1171-1175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1077] [Cited by in RCA: 1003] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 2. | Beutler E, Gelbart T, Demina A. Racial variability in the UDP-glucuronosyltransferase 1 (UGT1A1) promoter: a balanced polymorphism for regulation of bilirubin metabolism? Proc Natl Acad Sci USA. 1998;95:8170-8174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 683] [Cited by in RCA: 634] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 3. | Yamamoto K, Sato H, Fujiyama Y, Doida Y, Bamba T. Contribution of two missense mutations (G71R and Y486D) of the bilirubin UDP glycosyltransferase (UGT1A1) gene to phenotypes of Gilbert’s syndrome and Crigler-Najjar syndrome type II. Biochim Biophys Acta. 1998;1406:267-273. [PubMed] |
| 4. | Jinno H, Tanaka-Kagawa T, Hanioka N, Saeki M, Ishida S, Nishimura T, Ando M, Saito Y, Ozawa S, Sawada J. Glucuronidation of 7-ethyl-10-hydroxycamptothecin (SN-38), an active metabolite of irinotecan (CPT-11), by human UGT1A1 variants, G71R, P229Q, and Y486D. Drug Metab Dispos. 2003;31:108-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 85] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 5. | Takeuchi K, Kobayashi Y, Tamaki S, Ishihara T, Maruo Y, Araki J, Mifuji R, Itani T, Kuroda M, Sato H. Genetic polymorphisms of bilirubin uridine diphosphate-glucuronosyltransferase gene in Japanese patients with Crigler-Najjar syndrome or Gilbert’s syndrome as well as in healthy Japanese subjects. J Gastroenterol Hepatol. 2004;19:1023-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 75] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 6. | Ando Y, Saka H, Ando M, Sawa T, Muro K, Ueoka H, Yokoyama A, Saitoh S, Shimokata K, Hasegawa Y. Polymorphisms of UDP-glucuronosyltransferase gene and irinotecan toxicity: a pharmacogenetic analysis. Cancer Res. 2000;60:6921-6926. [PubMed] |
| 7. | Akaba K, Kimura T, Sasaki A, Tanabe S, Ikegami T, Hashimoto M, Umeda H, Yoshida H, Umetsu K, Chiba H. Neonatal hyperbilirubinemia and mutation of the bilirubin uridine diphosphate-glucuronosyltransferase gene: a common missense mutation among Japanese, Koreans and Chinese. Biochem Mol Biol Int. 1998;46:21-26. [PubMed] |
| 8. | Sava M, Kraemer DM. Heterozygous G71R-mutation causing Gilbert’s syndrome in one of 103 random persons of German descent. J Hepatol. 2005;42:778-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Ferraris A, D’Amato G, Nobili V, Torres B, Marcellini M, Dallapiccola B. Combined test for UGT1A1 -3279T--& gt; G and A(TA)nTAA polymorphisms best predicts Gilbert’s syndrome in Italian pediatric patients. Genet Test. 2006;10:121-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |