ANAL FISSURE
Definition
An anal fissure (AF) is a longitudinal tear or crack in the skin of the anal canal. Superficial fissures look much like a paper cut and they usually self-heal within a few weeks but some anal fissures become deep and do not heal. If an AF does not heal in at least six weeks, it may be recognized as CAF but the chronological definition of AF is rather loose[1,10,13].
A morphological description offers a more precise definition. The CAF presents thickened edges with usually visible, internal anal sphincter fibers at the fissure base. It may also be associated with an external skin tag (the sentinel pile) at the lower end of the fissure and/or a present papilla at the upper end of a fissure (hypertrophied anal papilla). These features of fissure chronicity are attributed to chronic infection and are caused by development of fibrotic connective tissue[1,10,13].
Etiology and epidemiology
There is a deficit in epidemiological studies examining this often encountered disease. 235 000 new cases of anal fissure are reported every year in the US and about 40% of them persist for months and even years [14]. The exact etiology of AF is unknown but trauma caused by (especially hard) faecal mass and hypertonicity of the internal sphincter are thought to be the initiating factors. Despite these findings, only 25% patients with CAF have constipation[15]. Furthermore, diarrhea is a predisposing factor in about 6% patients[13,15]. For this reason, AF may be a consequence of bariatric procedure in obese people[16].
Microtrauma of the anus by constant saddle vibration in professional mountain bikers can lead to chronic inflammation and a resultant AF[17]. There is also a suspicion that the water stream from bidet-toilets may be a cause of anterior fissure-in-ano[18]. 3%-11% of anal fissures are associated with childbirth and typically this type of etiology predisposes to fissure localization in the anterior anal commissure[19]. Links between sexual abuse and AF have been considered[20].
It’s possible that Nicorandil may increase a risk of anal fissure. Painful ulceration(s) in the anus and mouth have been observed after therapy with this potassium-channel activator. The size of Nicorandil anal ulcers varies and their edges are undermined as with CAF. This medication may also cause other cutaneous ulcerations outwith the anus[21,22].
Diet is not without significance. Consumption of spicy food like hot chili peppers aggravates symptoms in patients with an acute AF[23]. The literature identifies that lifestyle-related factors such as diet, bowel habit and employment play an important role in the etiology of anal fissure. The literature only touches on the interrelationship between these factors, however, with no strong, evidence-based research to provide constructive lifestyle recommendations.
Location of primary and secondary fissure
The most common location for primary AF (where there is no obvious trigger) is the posterior anal midline. Only 10% of females and 1% of males have a fissure located in the anterior midline[13,15,24]. Secondary fissures as a result of inflammatory bowel disease, previous anal surgery and venereal, dermatological, infectious or neoplastic disease also occur in the lateral position of the anus. These causes should be considered when the localization of AF is atypical, especially Crohn’s disease and infectious agents including tuberculosis, herpes or cytomegalovirus, Chlamydia, Haemophilus ducreyi and human immunodeficiency virus. Despite this, secondary AF can demonstrate the typical localization of primary fissures[1,13,15].
Pathophysiology
Anal fissure has been associated with increased anal tone for many years. This has been substantiated by a highly successful surgical treatment for anal fissure - internal sphincterotomy which reduces resting anal pressure[25]. In 1994, this opinion was further reinforced by Shouten et al[26,27] who identified a relationship between anal pressure and anodermal blood flow, revealing that the internal anal sphincter (IAS) resting pressure was inversely related to the blood flow at the posterior midline. They also confirmed, using laser Doppler flowmetry, earlier findings from Klosterhalfen et al[28] that blood supply was significantly lower at the posterior midline than anywhere else in the anal canal in healthy individuals. Klosterhalfen et al[28] discovered a scarcity of small arteriolar collaterals between the end branches of the left and right inferior rectal artery dorsally during post-mortem angiographic studies. The work of Shouten et al[27] also revealed that there is a significantly lower anodermal blood flow at the fissure site than at the posterior anal midline of control groups.
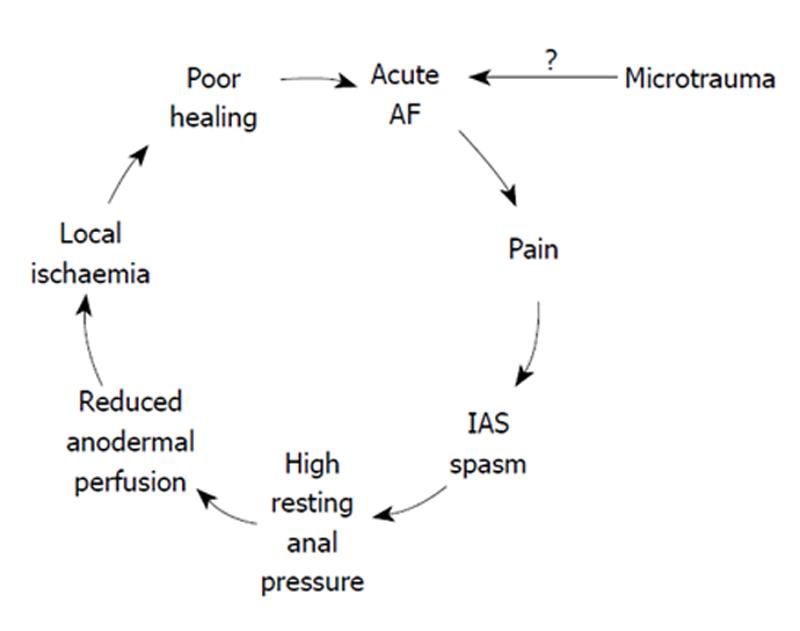
These facts constitute the logical basis for the explanation that microtrauma of the anoderma and AF cause anal pain which provokes a spasm of the IAS and a high anal pressure. This, in turn, leads to a reduction in anodermal perfusion. Ischemia in the fissure region ensues (Figure 1)[29]. A study by Farrouk et al[30] supported this belief, reporting a reduction in the normal spontaneous cyclic anal sphincter relaxation in patients with AF[30].
Figure 1 Pathophysiology of anal fissure.
Pain-spasm-ischaemia- non healing vicious cycle.
It is widely accepted that the reduction of anal pressure by LIS is associated with an improvement in anodermal perfusion; therefore, we conclude that this surgical procedure promotes anal fissure healing. This conception has been unshakable despite the fact that other surgical procedures such as anal advancement flap or tailored anal sphicterectomy can heal anal fissures without reducing maximum anal resting pressure (MARP). Moreover, they also have very high healing rate[31,32].
Despite this fact, there was common anticipation that oral or topical CS for therapy of AF would mimic surgical sphincterotomy, again by reducing anal resting pressure and causing its healing. CS performed with botulinum toxin (BTX) injections or the topical application of ointments such as calcium blockers, nitric oxide donors, a potassium channel agonist (minoxidil), inhibitors of angiotensin-converting enzyme, phosphodiesterase inhibitors, cholinomimetic (bethanechol) and an alfa-adrenoreceptor antagonist (indoramin) usually reduce anal resting pressures but with a diminished healing rate compared to LIS[33]. Indoramine, for example, had no satisfactory effect on fissure healing in double-blind randomized trials despite significant reductions in anal resting pressure. It was found to reduce anal resting pressure in patients with AF by an average of 35.8%[34].
Moreover, Ho et al[35] studies showed that LIS was associated with significantly improved fissure healing rates than CS with oral nifedipine, revealing simultaneously a similar or even lower value of mean resting anal pressure and maximum squeeze pressure in patients treated with this calcium blocker. Also Thornton et al[36] discovered that anal fissure healing is not related to anal pressures following CS but dependent upon the pre-treatment anal resting pressure and fissure grade. Furthermore, Pascual et al[37] confirmed no statistically significant differences between healing and not healing CAF when manometric and endosonographic findings were compared.
Before these studies cast some doubt upon the commonly accepted anal fissure pathophysiology, the first alternative hypothesis was suggested[38]. According this theory, a reduction of anal pressure is a consequence of pharmacological or surgical therapy for AF and is not a prerequisite for AF healing. It was theorised that a good response to surgical or pharmacological sphincterotomy is related to the presence of conserved vascular endothelium which conditions the ‘stretchability’ of the anal sphincters by the increased preservation of muscular blood flow. LIS reduces a risk of eruption of tissue in the fissure region during defecation and causes AF healing. This is thought to be attributed to the fact that when the anal sphincters stretchability is insufficient, erupted tissues release contraction vessel mediators which have a tendency to arrest fissure healing[33,38]. This perspective offers a simple explanation for differences between surgical and pharmacological sphincterotomy outcomes in that the various methods applied allow anal sphincter distention which will prevent tissue eruption in varying degrees.
THERAPEUTIC ASPECTS
Effectiveness of therapies for anal fissure
BTX was applied therapeutically for CAF for the first time in 1993[39,40]. Around this period, nitric oxide donors were also being considered as a remedy for CAF. Since this introduction of CS, many studies have supported its application and consider it a promising therapeutic approach for CAF. Some research has provided evidence to the contrary[41]. Siproudhis et al[42] in a multi-center, placebo-controlled, randomized, double-blind study showed no significant difference between BTX and placebo. Also, a meta-analysis by Nelson[41] revealed that CS for CAF in adults may be applied with a chance of cure that is marginally better than placebo and far less effective than surgery. Therefore, exclusively, prospective randomized controlled trials on the effectiveness of LIS vs BTX have been taken into consideration in this article.
In PubMed and Embase (January 1993 - January 2011), only 7 studies (encompassing 539 patients) were identified which compare the application BTX vs LIS (setting aside repetitive reports)[43-49]. All studies demonstrated that, although LIS associates with a high rate of minor anal incontinence (incontinence to flatus) as compared to BTX, it is statistically more effective as a therapy for CAF than BTX. The rate of CAF recurrence was also significantly less in group of patients treated with LIS[43-49].
In 2006, Nelson’s meta-analysis[41] (where crude criteria were applied) revealed that GTN, BTX and surgery had overall response rates of about 55%, 65% and 85% respectively. The third revision of the American Society of Colon and Rectal Surgeons’ (ASCRS) guidelines (published in 2010) offer that BTX injection allows healing in 60%-80% of fissures and higher rates than placebo with recurrence in up to 42% of cases[24]. According to ASCRS, topical nitrates are marginally superior to placebo with regards to healing. Topical calcium channel blockers have a lower incidence of adverse effects than topical nitrates but insufficient data exists to conclude whether they are superior to placebo in CAF healing[24].
ASCRS provide a strong recommendation for LIS as the therapy of choice (based on high-quality grade 1a evidence). However, ASCRS maintains that non-operative treatment continues to be safe, has few side effects and should usually be considered as a first step in CAF therapy (based on moderate-quality evidence grade 1b recommendation)[24].
Adverse effects of therapies for anal fissure
The identified complications following BTX are relatively benign. They may be sub-divided into obligatory and facultative side effects[50]. The first type of complication relates to excessive weakness of anal sphincters and/or injury of anal wall tissues. In this category, transitory incontinence for flatus (18%) or faeces (5%) and perianal thrombosis or hematoma belong[24]. Jost et al[51] reported a high rate of thrombosis (19.2%) but others observed a much lower frequency (only 1.1% in one study)[52]. It was not found to be related to the number of injection sites nor to the size of the needles (25 and 27 gauge)[50,52].
Facultative side effects are related to BTX spreading from the target tissue to distant muscles by hematogenic diffusion. One study found flu-like syndrome as a rare consequence of the therapy (3%). Epididymitis was only incidental[50,52].
Safety of BTX injection was also reinforced by autonomic system examination (using Ewing’s protocol) whilst using the English BTX preparation (Dysport) which is thought to have a greater tendency to leave the site of injection and enter systemic circulation[50,53]. All the identified side effects are transient and completely reversible[50,52].
Chemical sphincterectomy with nitrates may generate headaches in 20%-30%[24] of cases or even a higher rate[54]. The same symptom was identified in 27% of individuals within the placebo group[54]. The dose of GTN (0.2% or 0.4%) was not found to influence the efficacy but did increase the incidence of side effects, particularly headache, which occurred in about a quarter of patients[24,41]. Perianal itch was observed less frequently, in 10% cases, and allergic dermatitis is only accidental[41].
Calcium blockers treat AF with the benefit of a lower incidence of side effects than topical nitrates (topical therapy with diltiazem is rarely associated with headache and only incidentally perianal itch was observed)[24]. Nitrate-induced hypotension has not been well established and applying this to topical vasodilatators for AF is questionable.
Alongside the associated risks of anesthesia, LIS also carries a risk of perianal infection, hemorrhage, fecal incontinence, urinary retention and keyhole defects[5,41,55,56]. It should be emphasized that these complications are rare, however, gas and fecal incontinence are a significant concern which has been observed in as many as 36% of cases[41].
A novel combination of known therapies
Perhaps as a consequence of the previously established risks following LIS surgery, surgeons continue to search for alternative solutions in CAF therapy. In 2004, Lindsey et al[57] published a prospective pilot study of CAF patients in whom medical therapy was failing. They reported a CAF healing rate of 93% after fissurectomy combined with injection of BTX (25 UI of Botox). The further study showed that BTX injection into the IAS combined with fissurectomy was only slightly less successful (83.3%) than LIS (98.7%)[58].
Why may BTX be less successful than surgical sphincterectomy?
We know that smaller doses of BTX may be less successful than a higher dose[59,60] but the optimal dose of BTX has not been established[61-63]. We cannot ascertain all of the factors significant to formulating the most effective dose of BTX. This may explain some of the divergence amongst the study results for CS. The dose may be related to anal sphincter mass and also activity of surrounding inflammation. BTX also improves wound healing outside the anus where blood supply is not limited by sphincters[64,65]. This implies that distance from the anal fissure and the optimal angle of the needle during injection may cause variance in the injection method and, consequently, the effectiveness of the therapy[66].
Interestingly, even small doses of BTX when combined with fissurectomy have a high rate of CAF healing[57,58]. This begs the question, is the removal of fibrotic tissue during fissurectomy essential to the biochemistry of the anal fissure area? This question is particularly valid because there is a commonly accepted assumption that fibrosis in the internal anal sphincter, a consequence of chronic ischemia, could lie at the roots of CAF healing difficulties[29]. However, LIS performed even without fissurectomy brings a high rate of AF healing and, moreover, fibrous tissue inevitably develops following LIS at the site of muscle incision.
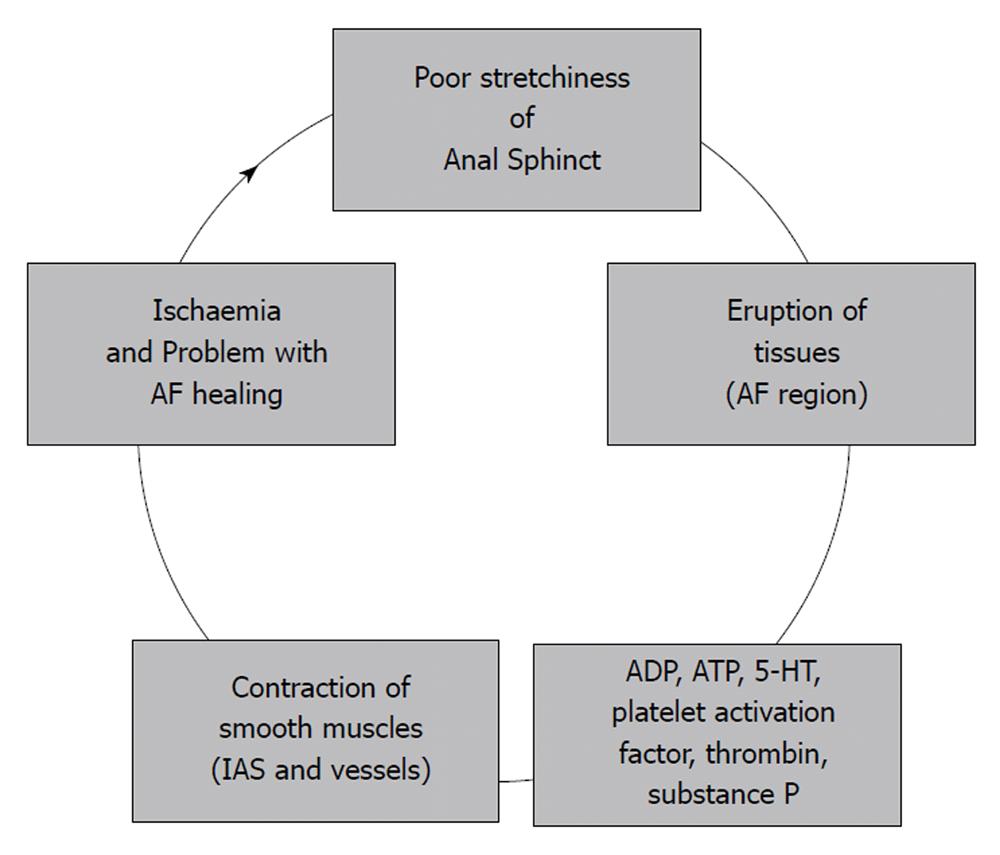
Yuksel et al[56] studied keyhole deformities following CAF therapy and concluded that nonhealing fissure and keyhole deformity are two ends of a spectrum of the same pathophysiological process. In an attempt at explanation, they referred to a new theory of AF healing[38,67]. They concur with the author that, if anal pressure drops and CAF is not healed, we should seek an explanation in certain vascular relaxing factors (adenosine diphosphate (ADP), adenosine triphosphate (ATP), 5-hydroxytryptamine (5-HT), platelet activation factor, as well as thrombin and substance P) as being responsible for it[38,56,67]. There is evidence that if endothelium is traumatized, platelet products contract smooth muscles (Figure 2)[68]. The same products relax the muscular coating of the arterioles when endothelium is intact. However, a trauma discontinues the feedback and platelet aggregation controlled by NO and prostacyclin. The area of AF is a place where the releasing of NO by regenerating cells is diminished[33,38]. A small GTPase encoded by the gene RhoA plays a key role in maintenance of the basal tone of the IAS[69]. It is also probably relevant to CAF healing. There is additional evidence that bacterial toxins are able to induce contraction of endothelial cells via the Rho/Rhokinase pathway[70]. These chemical mechanisms play a key role in altered and prolonged wound healing[36,56,71] as well as in CAF healing.
Figure 2 A new approach to physiology of anal fissure (AF).
In the past, the dorsal location of AF and elliptical arrangement of the IAS fibers was attributed to a lack of supportive tissue and fissures were considered to originate from phlebitis or cryptitis[29]. Today it should instead be accepted that CAF healing is resultant of several chemical processes. A tissue eruption during insufficient relaxation of the anal wall is one of the most relevant factors contributing to unfavorable CAF healing (Figure 2)[33,38]. Based on these arguments, we can conclude that the aim of any surgical technique for CAF should be the improvement of the anal sphincters ‘stretchability’ and the diminishment of chemical contractors of the arterioles. Alternately, infection can also generate problems with fissure healing[56,72,73].
Is it possible to predict anal fissure healing before we start therapy?
Thornton et al[36] applied a scoring system of IAS exposure (0 = healed; 1 = fissure with IAS muscle exposed; 2 = deeper fissure with IAS deeply exposed; 3 = deep undermined fissure; 4 = deep undermined fissure plus abscess or fistula) and observed that clinical healing of CAF positively correlated with a lower fissure score, a higher pre-treatment MARP in the mid anal canal and a greater percentage reduction of the MARP following topical application of Glyceryl Trinitrate (GTN).
Recently, Gil et al[74] evaluated ratio resting/voluntary contraction pressure. The study showed that the probability of CAF healing associates with an increase in the percentage change between resting and squeeze pressure (PI index). The patients with a PI of less than 150 have a low chance of successful CS and should be considered directly for LIS[74].
Ethical issues and algorithm treatment
As long as the patient is willing to accept the risk of fecal incontinence, we can justify the gold standard therapy (LIS) as the first-line treatment for CAF. It is prudent and in the patient’s interests, however, to consider the alternative CS when the initial diagnosis of AF is made. This may even be discussed prior to surgical referral with the general practitioner. It has been identified after all that diagnostic performance pertaining to benign anal diseases in the primary care setting may be insufficient[75]. It is reassuring that prospective studies emanating from the division of Colorectal Surgery in New York (US) revealed that there was no correlation between years of doctors’ experience and diagnostic accuracy of the benign disease[76]. Unfortunately, even there, the diagnostic accuracy for common benign anal pathologic conditions was suboptimal across all clinical specialties. Therefore, not only a good physical examination but also an improved training in the benign disease may improve the quality of patients` care.
At the point when the diagnosis of the AF is established, CS should be discussed. It may potentially improve comfort for several days before the patient is followed up by the proctologist. Although the diagnosis would be made in the proctology clinic, the surgery is not generally performed on the same day of the initial visit to the clinic. Therefore, CS can be justified as a straightforward, low risk therapy on the basis of patient comfort as an intermediate measure whilst awaiting surgical follow up.
GTN, botulinum toxin injection and surgery have overall response rates of about 55%, 65% and 85% respectively[41]. Therefore it should be recognised that surgery in the form of sphincterotomy is markedly superior. From an ethical perspective, however, we should at the very least, consider CS. The justification for this would be that we cannot predict the patient who receives a therapeutic benefit from the low risk process of CS would not have developed a severe complication following LIS. Moreover, the chance of successful CS is not low. The choice has to lie with the patient but the doctor`s responsibility is to inform him about the benefits and side effects of the available therapies for CAF. It may even be argued that a doctor should suggest CS if the patient meets the criteria described by Thornton et al[36] or Gil et al[74].
When the patient does not respond to nitrates (or calcium blockers), then BTX as a second line or both methods should be used. This algorithm of treatment for CAF was presented for the first time at the seventh United European Gastroenterology Week in 1999[72] and afterwards published in 2001[59] and 2002[52]. Many authors have followed this algorithm. A few days following its first publication (November 1999), this algorithm was also described in the New England Medical Journal where not only the possible increase of CS efficacy but also some economic aspects of this algorithm were suggested[77,78]. The cost of this algorithm (with stepwise escalation from topical nitroglycerin to BTX and LIS) was analyzed in 2005 by Essani et al[11] who considered it using a realistic economic model of the US healthcare system. This study demonstrated that 88% of patients could avoid surgery and assessed the algorithm as highly cost-efficient.
Due to a dispute in the literature regarding if BTX should be the first or second line treatment, in 2005, Madalinski[12] established how therapy with GTN (according the same algorithm) may diminish the cost of therapy with BTX.
In 2001, for the first time in the literature it was reported that administration of higher doses of botulinum toxin (50 or 100 units of Botox) could improve the results of CAF therapy with GTN[1,59]. BTX injection for a failure of CS with GTN was further supported by Lindsey et al[9] in prospective trials. Taking into account cost of anal fissure therapy, a method of BTX dispensing should be considered in which one vial should be effectively used for the maximum number of patients (depending on dose calculation)[79].
Since 1999, the author has expressed several times that a high dose of BTX should always be taken into account before surgery as the last line of CS[59,62,63,80]. An injection of higher doses of BTX may be a logical solution which outweighs doubts regarding the optimal dose for an individual. It may precede a potential fissurectomy. Taking into account the pharmacodynamics of BTX, fissurectomy could be performed in about 2-3 wk after applying a high dose of BTX if no symptomatic improvement follows an initial BTX injection. This combined approach (of fissurectomy with BTX) may be divided into two steps when applicable and could provide an additional rung of the therapeutic ladder worth consideration before LIS.