Published online Feb 6, 2011. doi: 10.4292/wjgpt.v2.i1.6
Revised: October 1, 2010
Accepted: October 8, 2010
Published online: February 6, 2011
Pegylated interferon plus ribavirin remains the first-line treatment for patients with hepatitis C virus (HCV). Interferon α has the most extensive clinical application and is used for the treatment of chronic hepatitis B virus and hepatitis D virus as well as acute and chronic HCV infections. The attachment of polyethylene glycol to interferon increases its half-life by reducing the rate of absorption after injection, reducing renal and cellular clearance and also decreasing immunogenicity. In this case report, we have described a patient with chronic hepatitis C who developed ischemic necrosis of her fingertips after completing her third course of pegylated interferon and ribavirin. The patient underwent a very extensive workup in order to determine the underlying cause of her digital ischemia which was finally determined to be secondary to the use of pegylated interferon.
- Citation: Hashash JG, Tackett SA, McAdams DJ. Digital ischemic necrosis caused by pegylated interferon in a patient with hepatitis C. World J Gastrointest Pharmacol Ther 2011; 2(1): 6-8
- URL: https://www.wjgnet.com/2150-5349/full/v2/i1/6.htm
- DOI: https://dx.doi.org/10.4292/wjgpt.v2.i1.6
Almost 170 million individuals are affected by chronic hepatitis C viral infection worldwide. The first-line treatment for such patients includes a combination of pegylated interferon and ribavirin for a total of 24 or 48 wk, depending on the viral genotype. Up to 70% of patients experience fatigue, myalgias and headache as a side effect of the above mentioned combination therapy[1]. Other more adverse side effects may also occur. These include depression, suicidal ideations, cardiovascular events and hemolytic anemia and are fortunately less frequently encountered[2]. There have been a few case reports of vascular events occurring in patients treated with interferon α. Regardless, prolonged digital cyanosis or necrosis associated with interferon is rare and none of the reported cases involved pegylated interferon.
A 53-year-old female with chronic hepatitis C genotype-1a presented to an outside hospital with a chief complaint of chest pain. The patient’s symptoms occurred one month after completing her third course of pegylated interferon α-2a (Pegasys injection 180 mcg) and ribavirin (600 mg). Of note, she had previously tolerated these treatment regimens without any acute complications. The chest pain was accompanied by shortness of breath and bluish discoloration of her right index fingertip. While her chest x-ray revealed a small pleural effusion, the remainder of her work-up including electrocardiography, computed tomography (CT) scan of the chest with contrast, transesophageal echocardiogram and cardiac catheterization was unremarkable. The cause of the finger discoloration was undetermined and the patient was discharged home.
A few days later, the discoloration of the patient’s right index finger worsened and the right middle and left index fingertips also became cyanotic and painful. On arrival at our institution, the patient was afebrile and chest pain free. The affected fingers were cool and cyanotic; however there were no deficits in sensation, strength or radial pulses or other notable skin findings. Initial work-up included basic labs, C3, C4, blood cultures, rheumatoid factor and cold agglutinins which were negative. Erythrocyte Sedimentation Rate was 20 and C-Reactive Protein was 5.3. Antinuclear antibody was measured at 1:40 with a speckled pattern. Autoantibody studies for anti-dsDNA, anti-smith, Anti-neutrophil cytoplasmic antibody, anti-topoisomerase, anti-centromere, anti-RNA PolIII, anti-Ro, anti-La, anti-RNP, anti-Jo1 and cryoglobulins were all negative. An upper extremity arterial Doppler ultrasound showed patent radial and ulnar arteries with vasospasm of the palmar arcades bilaterally. Vasospasm can be detected on ultrasound by spectral analysis with exposure to cold to provoke spasm and heat to relieve the spasm. In the presence of vasospasm, the flow pattern is extremely pulsatile. In retrospect, it was unclear whether or not her initial presentation with chest pain was a result of coronary vasospasm possibly related to the vasospastic process that was noted in her upper extremities.
The patient had multiple complications during her hospitalization and, interestingly, she developed hemolytic anemia and thrombocytopenia. prothrombin time, partial thromboplastin time, fibrinogen and fibrin degradation product levels were within normal limits. An ADAMTS13 level was borderline low which was attributed to inflammation rather than to thrombotic thrombocytopenic purpura (TTP). Hypercoagulable studies showed no clotting diathesis. Anti-hepatitis C virus (HCV) Ab was positive but HCV polymerase chain reaction was < 30 IU/mL. Human immunodeficiency virus, hepatitis A virus and hepatitis B virus (HBV) serologies were negative.
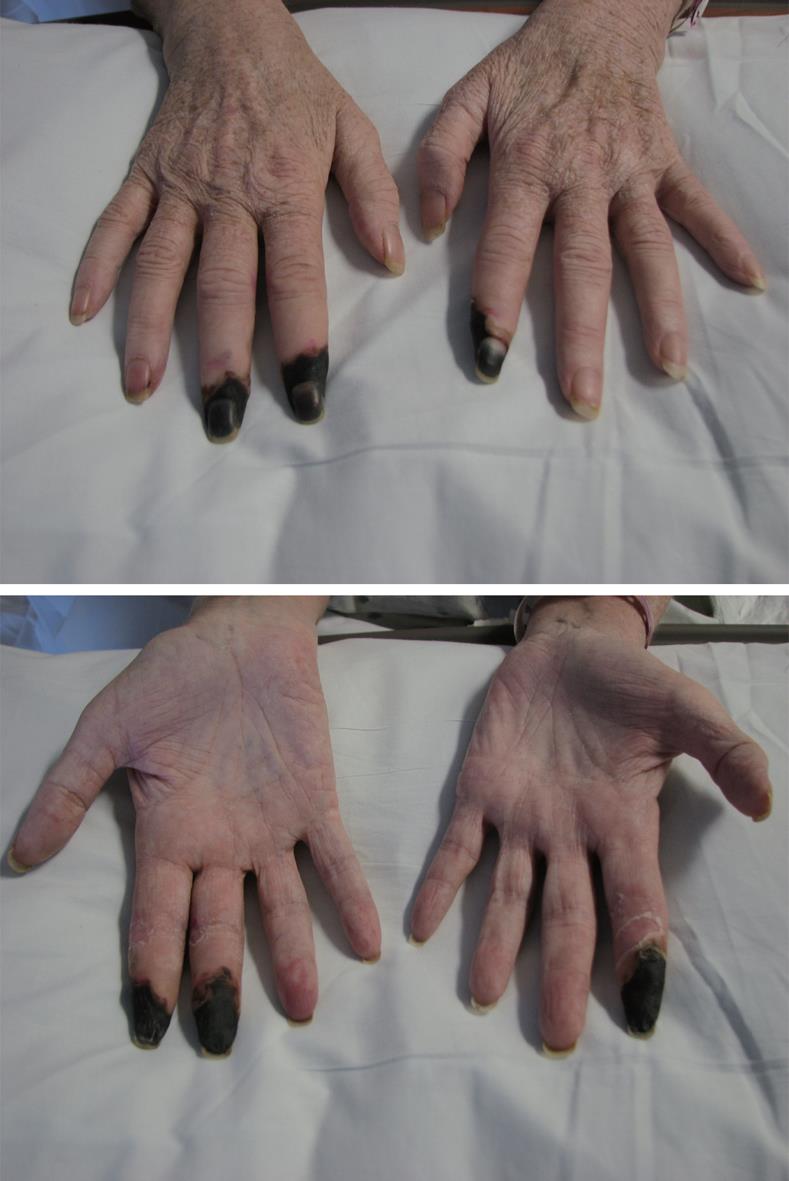
After the patient’s condition stabilized, attention returned to her ischemic fingers (Figure 1). Biopsy of her fingertips revealed a thrombotic coagulopathy. CT angiography of the upper extremities with pre and post dilatation images were obtained and showed no abnormalities. Invasive angiography, however, revealed occlusions of the left 2nd proper digital arteries and the right 2nd and 3rd proper digital arteries, without improvement after intra-arterial administration of papaverine, a vasodilator. Due to necrosis of her right index, right middle and left index fingers, the patient underwent amputation of those digits as well as radial and ulnar digital nerve surgery in attempt to prevent her pain from progressing. Surgical pathology reported ischemic necrosis of skin and adjacent soft tissues and prominent thickening of small and medium sized vessels with focal fibrin thromboemboli. There was no evidence of vasculitis. Grocott and periodic acid schiff with digestion (PASD) stains showed bacterial colonization as well as fungal hyphae in the necrotic tissue. No viral stains were performed.
DISCUSSION
Causes of digital ischemic necrosis are most commonly due to vasculitis, either autoimmune or drug-induced[3]. However, our patient had a negative rheumatological work-up and biopsy showed no inflammatory changes. The absence of cold agglutinins, although insensitive, did further assure us that this was less likely to be cryoglobulinemic digital ischemia. Digital ischemia can also be attributed to embolic phenomena such as endocarditis or cholesterol emboli; however these seem unlikely based on the work-up and the timing of the angiography. Our patient’s necrosis was due to microvascular occlusion. While the exact mechanism is not yet known, interferon treatment has led to microthrombi and endothelial cell injury without inflammatory cell infiltration in mouse models[4]. Based on this patient’s presentation and the extensive work-up, we concluded that the patient developed digital ischemic necrosis as a result of treatment with pegylated interferon α.
Pegylated interferon plus ribavirin remains the first-line treatment for patients with HCV[5]. Interferons are a family of cytokines that regulate resistance to viral infections, enhance innate and acquired immune responses and modulate normal and tumor cell survival and death. Interferon α has the most extensive clinical application and is currently primarily used for the treatments of chronic HBV and hepatitis D virus as well as acute and chronic HCV[6]. The attachment of polyethylene glycol to interferon increases its half-life by reducing the rate of absorption after injection. This in turn reduces renal and cellular clearance and also decreases immunogenicity, allowing pegylated interferon to achieve higher steady-state concentrations in the body. Randomized clinical trials have shown that pegylated interferon has a better sustained viral response rate when compared to standard interferon. Moreover, pegylated interferon tends to have less flu-like and depressive side effects.
Dermatological complications are known to occur with both HCV and interferon; however exactly which are due to HCV and which are due to interferon is not always clear. HCV has been isolated from biopsies of skin lesions from mixed cryoglobulinemia, lichen planus and psoriasis. Association of HCV with porphyria cutanea tarda and necrolytic acral erythema also occurs. We were unable to find any reports of HCV leading to digital necrosis. While more than 80% of patients receiving interferon will experience side effects (mostly flu-like), reports of digital ischemia are rare[7]. It is possible however, that interferon-related digital ischemia is under-reported. Al-Zahrani described a wide variety of vascular events associated with the use of interferon α, including Raynaud’s phenomenon, digital ulcerations and gangrene, pulmonary vasculitis and TTP/hemolytic uremic syndrome (HUS)[8]. In one series of 25 patients being treated with interferon α for chronic myeloid leukemia (CML), 13 (52%) reported that they had experienced symptoms consistent with Raynaud’s phenomenon[9]. Interestingly, there has also been a report of intestinal vasculitis with subsequent ischemia due to arteritis and arterial occlusion in a patient receiving pegylated interferon[10]. Regardless, prolonged digital cyanosis or necrosis associated with interferon is rare and none of these involved pegylated interferon or treatment for HCV.
In conclusion, given the high prevalence of chronic HCV infection, it is of utmost importance for physicians to be aware of the potential side effects of HCV therapy as many of those side effects lead to serious and detrimental consequences. In our patient, treatment with pegylated interferon α caused necrosis of the digits necessitating amputation.
Peer reviewers: Konstantinos Tziomalos, MD, PhD, Department of Clinical Biochemistry, Royal Free Campus, University College London Medical School, University College London, Pond Street, London NW3 2QG, United Kingdom; Osama Badary, PhD, Professor and Head, Department of Clinical Pharmacy, Faculty of Pharmacy, Ain Shams University, Abbassia, Cairo, Egypt; Francesca Cainelli, Department of Internal Medicine, University of Botswana, Private Bag 0022, Gaborone, Botswana
S- Editor Wang JL L- Editor Roemmele A E- Editor Lin YP
| 1. | McHutchison JG, Gordon SC, Schiff ER, Shiffman ML, Lee WM, Rustgi VK, Goodman ZD, Ling MH, Cort S, Albrecht JK. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. Hepatitis Interventional Therapy Group. N Engl J Med. 1998;339:1485-1492. |
| 2. | Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Gonçales FL Jr, Häussinger D, Diago M, Carosi G, Dhumeaux D. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975-982. |
| 3. | Chen KR, Carlson JA. Clinical approach to cutaneous vasculitis. Am J Clin Dermatol. 2008;9:71-92. |
| 4. | Dvorak HF, Gresser I. Microvascular injury in pathogenesis of interferon-induced necrosis of subcutaneous tumors in mice. J Natl Cancer Inst. 1989;81:497-502. |
| 5. | Strader DB, Wright T, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C. Hepatology. 2004;39:1147-1171. |
| 6. | Borden EC, Sen GC, Uze G, Silverman RH, Ransohoff RM, Foster GR, Stark GR. Interferons at age 50: past, current and future impact on biomedicine. Nat Rev Drug Discov. 2007;6:975-990. |
| 7. | Bachmeyer C, Farge D, Gluckman E, Miclea JM, Aractingi S. Raynaud's phenomenon and digital necrosis induced by interferon-alpha. Br J Dermatol. 1996;135:481-483. |
| 8. | Al-Zahrani H, Gupta V, Minden MD, Messner HA, Lipton JH. Vascular events associated with alpha interferon therapy. Leuk Lymphoma. 2003;44:471-475. |
| 9. | Creutzig A, Caspary L, Freund M. The Raynaud phenomenon and interferon therapy. Ann Intern Med. 1996;125:423. |
| 10. | Pompili M, Pizzolante F, Larocca LM, Covino M, Rapaccini GL, Gasbarrini G. Ischaemic jejunal vasculitis during treatment with pegylated interferon-alpha 2b and ribavirin for hepatitis C virus related cirrhosis. Dig Liver Dis. 2006;38:352-354. |