Published online Sep 5, 2022. doi: 10.4292/wjgpt.v13.i5.77
Peer-review started: October 23, 2021
First decision: December 16, 2021
Revised: January 22, 2022
Accepted: August 14, 2022
Article in press: August 14, 2022
Published online: September 5, 2022
Processing time: 311 Days and 8.5 Hours
Percutaneous Endoscopic Gastrostomy (PEG) tubes are often placed for dysp
To assess the safety of early (≤ 7 d post stroke) vs late (> 7 d post stroke) PEG tube placement and evaluate whether pre-procedural risk factors could predict mortality or complications.
We performed a retrospective study of patients undergoing PEG tube placement for dysphagia following a stroke at two hospitals in Saint Louis, MO between January 2011 and December 2017. Patients were identified by keyword search of endoscopy reports. Mortality, peri-procedural complication rates, and post-procedural complication rates were compared in both groups. Predictors of morbidity and mortality such as protein-calorie malnutrition, presence of an independent cardiovascular risk equivalent, and presence of Systemic inflammatory response syndrome (SIRS) criteria or documented infection were evaluated by multivariate logistic regression.
154 patients had a PEG tube placed for dysphagia following a stroke, 92 in the late group and 62 in the early group. There were 32 observed deaths, with 8 occurring within 30 d of the procedure. There was an increase in peri-procedural and post-procedural complications with delayed PEG placement which was not statistically significant. Hospital length of stay was significantly less in patients with early PEG tube placement (12.9 vs 22.34 d, P < 0.001). Protein calorie malnutrition, presence of SIRS criteria and/or documented infection prior to procedure or having a cardiovascular disease risk equivalent did not significantly predict mortality or complications.
Early PEG tube placement following a stroke did not result in a higher rate of mortality or complications and significantly decreased hospital length of stay. Given similar safety outcomes in both groups, early PEG tube placement should be considered in the appropriate patient to potentially reduce length of hospital stay and incurred costs.
Core Tip: Percutaneous Endoscopic Gastrotomy (PEG) tubes are often placed for nutrition for dysphagia following a stroke. The 2011 ASGE guidelines recommend delaying PEG tube placement for two weeks, although this guideline is based on weak evidence. There is increasing demand for early PEG tube placement to meet requirements for timely discharge to rehab facilities. This is the first study to compare outcomes such as mortality or complications of PEG tubes based on timing of placement in stroke patients. Early PEG tube placement did not result in a higher rate of mortality or complications and significantly decreased hospital length of stay.
- Citation: Reddy KM, Lee P, Gor PJ, Cheesman A, Al-Hammadi N, Westrich DJ, Taylor J. Timing of percutaneous endoscopic gastrostomy tube placement in post-stroke patients does not impact mortality, complications, or outcomes. World J Gastrointest Pharmacol Ther 2022; 13(5): 77-87
- URL: https://www.wjgnet.com/2150-5349/full/v13/i5/77.htm
- DOI: https://dx.doi.org/10.4292/wjgpt.v13.i5.77
Enteral nutrition is the recommended method for providing sufficient nutrition and calories to patients who are unable to tolerate oral feeding[1]. Stroke patients commonly require enteral nutrition. Post stroke oropharyngeal dysphagia occurs in over 65% of cases[2]. While most post-stroke dysphagia will improve within the first four weeks, 20% of patients may require short-term enteral tube feeding, and 8% require tube feeding for more than six months[3,4]. Malnutrition will occur after an acute stroke in 8% to 34% of cases[5].
Methods to provide enteral feeding include nasogastric (NG) tubes, percutaneous endoscopic gastrostomy (PEG) tubes, and percutaneous gastrostomy tubes placed by interventional radiology or surgery. Nasoenteral tubes are recommended for short-term use in patients who are expected to resume oral nutrition within 30 d [1]. According to the 2011 ASGE guidelines, if a patient is unable to resume oral feeding after 2-3 wk with a nasoenteric tube, then PEG tube placement is recommended[1]. This recommendation is graded as low quality and the authors suggest that further research is needed to strengthen the evidence supporting a 2-3 wk waiting period[1]. This study aims to evaluate the safety of early vs delayed placement of PEG tubes in patients after an acute stroke.
The original recommendation for delayed PEG tube placement for two weeks seems to be derived from the observation that about half of patients with dysphagia improve within 2 wk, whereas only 15% of patients have persistent dysphagia after 1 mo[2]. Two of the earliest studies assessing safety of PEG tube placement after a stroke were published in the 1990s. Both studies found PEG feeding to be safe and effective when placed at least 2 wk after a stroke, however neither of these studies addressed early (< 2 wk post stroke) PEG tube placement[6,7]. In 2005, Dennis and colleagues were the first to evaluate outcomes of early PEG tube placement in 321 patients who were randomized to early PEG vs NG tube placement after an acute stroke, and found no major differences in survival, but non-statistically significant improvement in functional outcomes if NG feeding was used in the first 2-3 wk, followed by PEG if needed[8,9]. Since the publication of the 2011 ASGE guidelines, there has been one additional retrospective chart review study looking at timing of PEG tubes in 34623 stroke patients, in which early PEG placement (< 7 d post stroke) was associated with a significant decrease in length of hospital stay (10.4 vs 20.5 d)[10]. Furthermore, there was no significant difference in inpatient mortality rates and other complications, however overall mortality was not evaluated.
The primary objective of our study was to assess the safety of early PEG tube placement in stroke patients by evaluating mortality and complications in patients who underwent PEG tube placement within 7 d vs after 7 d of an acute stroke. Our secondary objectives included the identification of predictors of morbidity and mortality such as malnutrition, pre-procedural infections, and independent cardiovascular risk equivalents. We hypothesized that there would be no difference in outcomes, mortality, or complications with early PEG tube placement unless independent risk factors for complications were present. We hope our study will contribute to evidence-based decision making regarding the timing of PEG tube placement following a stroke.
This is a bi-center retrospective study of all adult patients who underwent PEG tube placement for dysphagia following an acute stroke between January 2011 and December 2017 at Saint Louis University Hospital and Saint Mary’s Hospital in Saint Louis, Missouri. This study was approved by the institutional review board at Saint Louis University School of Medicine. Patients were identified by performing a keyword search of upper endoscopy reports on the ProVation (ProVation Medical, Minneapolis, MN) database using the keywords “gastrostomy” and “PEG.” Individual patient records and endoscopy reports were then reviewed to confirm that the indication for placement was dysphagia following an acute stroke, and that endoscopic tube placement was performed via the standard “pull through” technique as described by Ponsky and Gauderer[11]. Exclusion criteria included PEG tubes placed for indications other than dysphagia from acute stroke, using a nonstandard endoscopic tube placement method, or tubes placed by surgery or interventional radiology.
At both hospitals, standard dysphagia management following a stroke required assessment of dysphagia by both the neurology service and a speech therapist. Enteral nutrition with a nasogastric tube was initiated immediately after determination of dysphagia was made and if PEG tube placement would be delayed. If it was determined that prolonged PEG tube placement would be required, the gastroenterology service was consulted. Active infection delayed placement of PEG tube but pre-procedure malnutrition, defined as albumin < 3.2, did not delay the procedure. All anti-platelet and anti-coagulant agents were held prior to PEG placement, according to ASGE guidelines[12]. Patients with early PEG tube placement, defined as within 7 d from the stroke event, were compared to those with later interventions. A dose of antibiotics was given prior to all procedures. The PEG site was examined for infection and bleeding 24 h after tube placement, and tube feeds were initiated once the tube was deemed safe to use by the inpatient gastroenterology team.
Retrospective data was collected from review of the patients’ electronic medical records available at the two study centers, including inpatient and outpatient follow up data. Baseline characteristics including age, gender, and comorbidities were compared for both groups. Outcomes evaluated included the rate of peri-procedural complications, the rate of post-procedural complications, and 90-d all-cause mortality after PEG placement. Peri-procedural complications were defined as those occurring during or within 24 h of PEG placement and included cardiovascular and cerebrovascular events, aspiration, bleeding, organ injury, perforation, or infection. Post-procedural complications were defined as those occurring after 24 hours and within 3 mo from PEG placement and included cardiovascular and cerebrovascular events, aspiration, bleeding, infection, and PEG tube site complications. Complication and mortality rates were calculated for both groups. Patients were censored at death or 90-d follow-up from date of PEG placement. The Kaplan-Meier Method was used to perform a time to event analysis. Predictors of morbidity and mortality were evaluated by multivariate logistic regression and included documentation of pre-procedure protein-calorie malnutrition (defined as albumin ≤ 3.2), having an independent cardiovascular risk equivalent outside of the current stroke (type 2 diabetes, prior stroke, abdominal aortic aneurysm, peripheral vascular disease, or Framingham risk score > 20%), and presence of Systemic inflammatory response syndrome (SIRS) criteria or documented infection (positive urine or blood culture). All statistical analyses were performed using SAS 9.4 (SAS, Cary, NC) and results were considered significant with a P value ≤ 0.05. Statistical Review of the study was performed by a biomedical statistician.
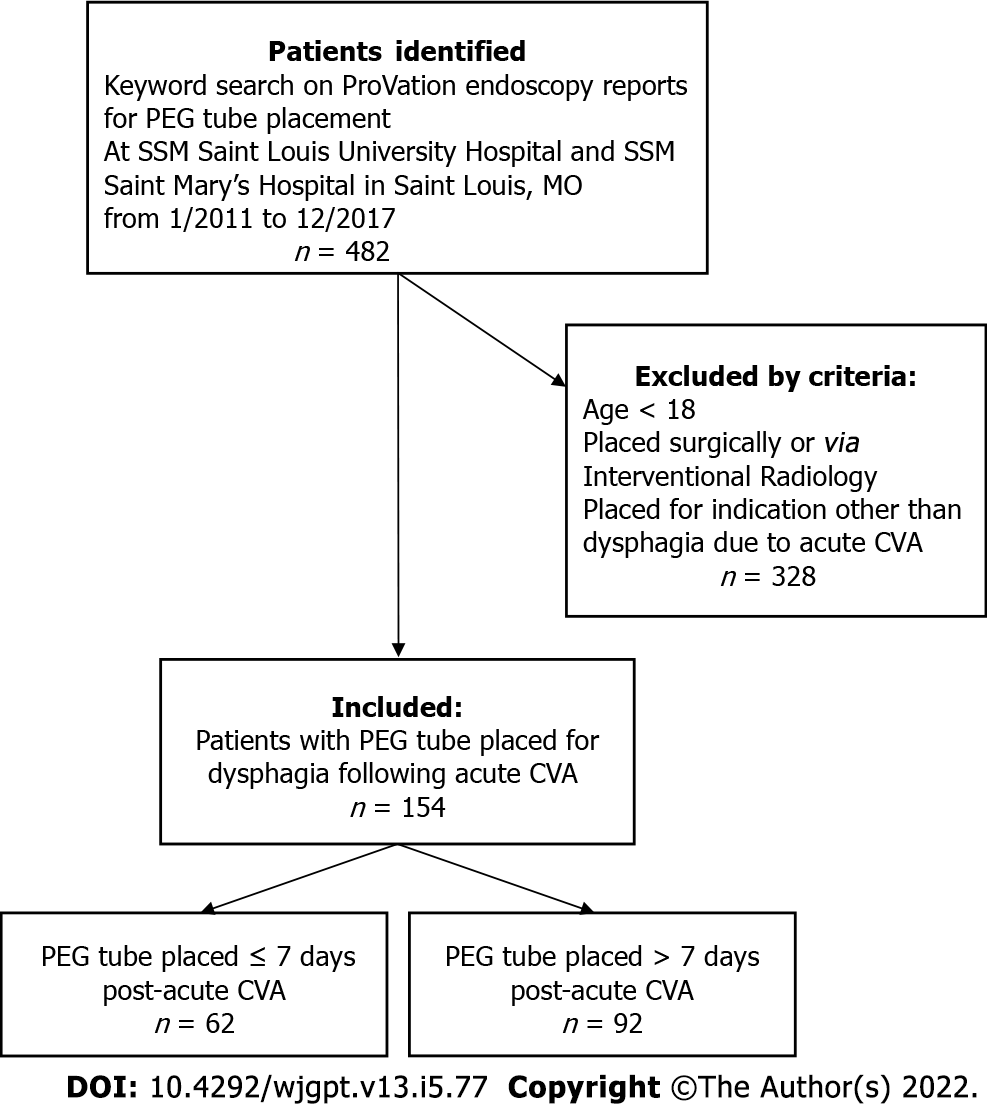
Initial keyword search identified 482 cases on the ProVation endoscopy report database. 154 patients were included in the study after manually reviewing charts to exclude patients that did not meet the inclusion criteria. Among these, 62 patients underwent early PEG placement, while the remaining 92 patients had PEG tube placement after 7 d (See Figure 1). Baseline patient demographics (Table 1) and clinical comorbidities are listed (Table 2). There was a statistically significant difference in age between patient’s undergoing early vs late PEG placement (74.7 vs 66.2 years, P = 0.0005). There was otherwise no significant difference in sex or the total number of comorbidities as described below. Among specific comorbidities however, there was a statistically significant difference in chronic kidney disease (3.2% vs 13.0%) and peripheral arterial disease (8.1% vs 1.1%) between patients undergoing early and late interventions.
| All (N = 154) | PEG ≤ 7 d (N = 62, 40.26%) | PEG > 7 d (N = 92, 59.74%) | P value | ||||
| N | SD | N | SD | N | SD | ||
| Age | 69.62 | 15.02 | 74.65 | 13.89 | 66.24 | 14.87 | 0.00051 |
| Sex | 0.0658 | ||||||
| Female | 78 | 50.65 | 37 | 59.68 | 41 | 44.57 | |
| Male | 76 | 49.35 | 25 | 40.32 | 51 | 55.43 | |
| Any comorbidity | 147 | 95.45 | 59 | 95.16 | 88 | 95.65 | 1.00002 |
| Number of comorbidities | 0.43393 | ||||||
| 0 | 7 | 4.55 | 3 | 4.84 | 4 | 4.35 | |
| 1 | 26 | 16.88 | 12 | 19.35 | 14 | 15.22 | |
| 2 | 37 | 24.03 | 13 | 20.97 | 24 | 26.09 | |
| 3 | 41 | 26.62 | 17 | 27.42 | 24 | 26.09 | |
| 4 | 26 | 16.88 | 12 | 19.35 | 14 | 15.22 | |
| 5 | 8 | 5.19 | 4 | 6.45 | 4 | 4.35 | |
| 6 | 5 | 3.25 | 0 | 0.00 | 5 | 5.43 | |
| 7 | 3 | 1.95 | 1 | 1.61 | 2 | 2.17 | |
| 8 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | |
| 9 | 1 | 0.65 | 0 | 0.00 | 1 | 1.09 | |
| Types of comorbidities | All (N = 154) | PEG ≤ 7 d (N = 62, 40.26%) | PEG > 7 d (N = 92, 59.74%) | P value | |||
| N | % | N | % | N | % | ||
| Acute respiratory distress syndrome | 1 | 0.65 | 0 | 0.00 | 1 | 1.09 | 1.00001 |
| Acute kidney injury | 2 | 1.30 | 0 | 0.00 | 2 | 2.17 | 0.5158 |
| Alcohol abuse | 5 | 3.25 | 2 | 3.23 | 3 | 3.26 | 1.00001 |
| Amyotrophic lateral sclerosis | 1 | 0.65 | 0 | 0.00 | 1 | 1.09 | 1.00001 |
| Anemia | 4 | 2.60 | 2 | 3.23 | 2 | 2.17 | 1.00001 |
| Aortic dissection | 1 | 0.65 | 0 | 0.00 | 1 | 1.09 | 1.00001 |
| Aortic stenosis | 1 | 0.65 | 0 | 0.00 | 1 | 1.09 | 1.00001 |
| Arrhythmias | 7 | 4.55 | 4 | 6.45 | 3 | 3.26 | 0.44031 |
| Atrial fibrillation | 32 | 20.78 | 17 | 27.42 | 15 | 16.30 | 0.0955 |
| Breast cancer | 3 | 1.95 | 2 | 3.23 | 1 | 1.09 | 0.56521 |
| Coronary artery disease | 29 | 18.83 | 9 | 14.52 | 20 | 21.74 | 0.2609 |
| Cirrhosis | 1 | 0.65 | 1 | 1.61 | 0 | 0.00 | 0.40261 |
| Chronic kidney disease | 14 | 9.09 | 2 | 3.23 | 12 | 13.04 | 0.0377 |
| Colon cancer | 2 | 1.30 | 2 | 3.23 | 0 | 0.00 | 0.16051 |
| Chronic obstructive pulmonary disease | 9 | 5.84 | 1 | 1.61 | 8 | 8.70 | 0.08531 |
| Dementia | 15 | 9.74 | 5 | 8.06 | 10 | 10.87 | 0.5648 |
| Diabetes mellitus | 52 | 33.77 | 19 | 30.65 | 33 | 35.87 | 0.5014 |
| Deep vein thrombosis | 1 | 0.65 | 1 | 1.61 | 0 | 0.00 | 0.40261 |
| Heart failure | 24 | 15.58 | 11 | 17.74 | 13 | 14.13 | 0.5445 |
| Hyperlipidemia | 45 | 29.22 | 20 | 32.26 | 25 | 27.17 | 0.4963 |
| Hypertension | 121 | 78.57 | 45 | 72.58 | 76 | 82.61 | 0.1369 |
| Lung cancer | 1 | 0.65 | 0 | 0.00 | 1 | 1.09 | 1.00001 |
| Nonalcoholic steatohepatitis | 1 | 0.65 | 0 | 0.00 | 1 | 1.09 | 1.00001 |
| Obstructive sleep apnea | 3 | 1.95 | 1 | 1.61 | 2 | 2.17 | 1.00001 |
| Peripheral artery disease | 6 | 3.90 | 5 | 8.06 | 1 | 1.09 | 0.03911 |
| Parkinson's disease | 2 | 1.30 | 0 | 0.00 | 2 | 2.17 | 0.51581 |
| Patent Foramen Ovale | 1 | 0.65 | 1 | 1.61 | 0 | 0.00 | 0.40261 |
| Prostate cancer | 2 | 1.30 | 0 | 0.00 | 2 | 2.17 | 0.51581 |
| Pulmonary hypertension | 1 | 0.65 | 1 | 1.61 | 0 | 0.00 | 0.40261 |
| Seizure disorder | 4 | 2.60 | 2 | 3.23 | 2 | 2.17 | 1.00001 |
| Stroke | 38 | 24.68 | 14 | 22.58 | 24 | 26.09 | 0.62061 |
| Substance abuse | 1 | 0.65 | 0 | 0.00 | 1 | 1.09 | 1.00001 |
| Thrombocytopenia | 2 | 1.30 | 1 | 1.61 | 1 | 1.09 | 1.00001 |
| Thyroid cancer | 1 | 0.65 | 1 | 1.61 | 0 | 0.00 | 0.40261 |
Peri-procedural and post-procedural complications were compared between patients undergoing early vs late PEG placement (See Table 3). There were 3 peri-procedural complication events in the early group, compared to 8 in the late PEG placement group. There were 19 post-procedural complications in the early group, compared to 35 in the late PEG placement group. These differences, however, were not statistically significant. Similarly, there were no statistical differences in the type of peri-procedural or post-procedural complications based on timing of PEG tube placement (See Table 4).
| Early PEG group | Late PEG group | Analysis value (P) Fisher’s exact | |
| Peri procedural complication | |||
| Pulled PEG tube | 0 | 1 | 1 |
| Pulmonary edema | 0 | 1 | 1 |
| NSTEMI | 0 | 1 | 1 |
| Bleeding | 0 | 4 | 0.15 |
| Pneumonia | 1 | 1 | 1 |
| Evolving stroke/herniation | 1 | 0 | 0.4 |
| Post procedural complications | |||
| Aspiration | 8 | 14 | 0.69 |
| Pneumonia | 10 | 16 | 0.84 |
| Bleeding | 2 | 2 | 1 |
| Buried bumper | 1 | 0 | 0.4 |
| Cardiac arrest | 2 | 1 | 0.57 |
| Death | 7 | 8 | 0.59 |
| Ileus | 0 | 1 | 1 |
| Infection | 5 | 4 | 0.49 |
| Necrotizing pancreatitis | 0 | 1 | 1 |
| Pulled PEG tube | 3 | 9 | 0.36 |
| Pulmonary embolism | 0 | 3 | 0.27 |
| Respiratory failure | 1 | 3 | 0.65 |
| Seroma | 1 | 0 | 0.4 |
| Stroke | 1 | 2 | 1 |
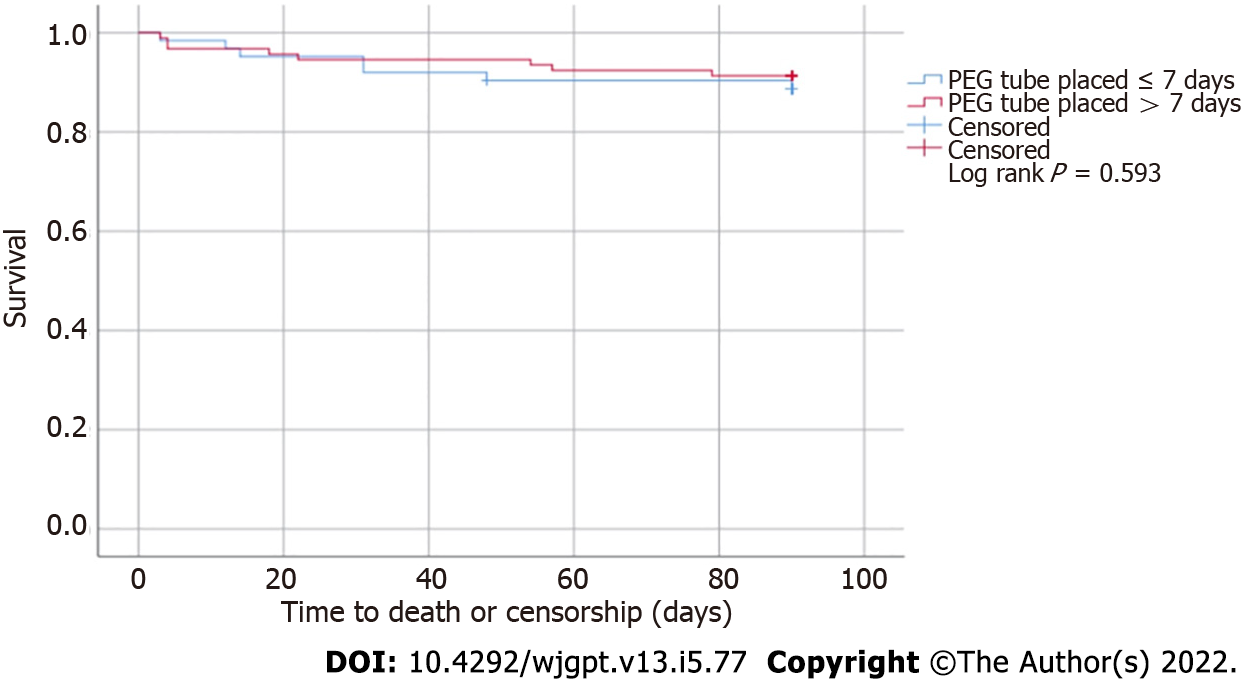
There were 32 total observed deaths, however only 8 occurred within 30 d from PEG placement, including 3 deaths in the early group vs 5 deaths in the late PEG placement group. The Kaplan-Meier Method was used to perform a time to event (death) analysis. As seen in Figure 2, there was no statistical difference in survival between both groups (log rank P = 0.593).
Pre-procedural risk factors for complications and mortality, such as protein calorie malnutrition, presence of SIRS or documented infection, and cardiovascular disease risk equivalents independent of current stroke, were evaluated by multivariate regression analysis. None of the risk factors studied significantly impacted 30- or 90-d mortality. Protein-calorie malnutrition [OR 1.31, (0.37-4.7)] and presence of infection or SIRS criteria [OR 1.55, (0.38-6.28)] were associated with an increase in peri-procedural and post-procedural complications, however these did not reach statistical significance (See Table 5).
| Death in 30 d, OR [95%CI] | Death in 90 d, OR [95%CI] | Peri-procedural complication, OR [95%CI] | Post-procedural complications, OR [95%CI] | |
| Protein calorie malnutrition (albumin ≤ 3.2) | 0.757 [0.179-3.21] | 0.854 [0.289-2.527] | 1.31 [0.37-4.7] | 1.07 [0.54-2.11] |
| Documented SIRS criteria or infection pre-procedure | 0.524 [0.06-4.52] | 0.56 [0.12-2.67] | 1.55 [0.38-6.28] | 1.92 [0.85-4.35] |
| Presence of cardiovascular risk equivalent independent of this stroke | 0.24 [0.05-1.24] | 0.35 [0.11-1.09] | 0.65 [0.19-2.23] | 1.63 [0.82-3.24] |
Total hospital length of stay was significantly lower in patients undergoing early PEG tube placement (12.9 vs 22.3 d, P < 0.001).
This study compared mortality, complications, and risk factors for morbidity in 154 patients with dysphagia following an acute stroke undergoing PEG tube placement within 7 d vs after 7 d of acute stroke. We had hypothesized that there would be no difference in outcomes, mortality, or complications with early PEG tube placement unless independent risk factors for complications were present. Our results support our hypothesis. There were no differences in mortality or rates of peri-procedural and post-procedural complication. Patients undergoing early PEG placement had no worse outcomes and had a shorter length of hospital stay.
The only other study to directly compare early PEG placement to later PEG placement was an observational study of the US Nationwide Inpatient Sample by George et al[10], which identified 34623 stroke patients undergoing PEG placement and found no difference in inpatient mortality. Our study results match its findings. Our study, which directly reviewed patients’ charts rather than nationwide hospital data, extends its conclusions by additionally finding no difference in mortality at 30 and 90 d after hospitalization. Finally, both studies found a significant decrease in length of hospital stay associated with early PEG placement.
Our study defined “early” vs “late” PEG placement using a cutoff of 7 d after the acute stroke. This cutoff was defined prior to our data collection and was not the result of a “post-hoc” analysis. We chose this cutoff for several reasons. First, our clinical experience suggested that we were consulted by neurology for PEG placement earlier than 2 wk in the patients’ hospital course, usually around 7 d. Second, using the same cutoff as the study by George et al[10] would allow for a direct comparison with their results. Finally, using an earlier cutoff than the guideline-based 2 wk, would allow us to better answer our hypothesis, which was that PEG placement earlier than 2 wk does not lead to worsened outcomes.
As previously discussed in our introduction, this 7 d interval is earlier than the 2 wk interval recommended in the 2011 ASGE guidelines[1]. Most recently, the 2019 guidelines for early management of acute ischemic stroke by the American Heart Association and the American Stroke Association also recommend the use of nasogastric tubes for feeding “in the early phase of stroke” and to place PEG tubes “in patients with longer anticipated persistent inability to swallow safely (> 2 to 3 wk)”[13]. The strength of this recommendation is graded as moderate, and the level of evidence is based on expert opinion and clinical experience[13].
Despite these recommendations to delay PEG placement for 2 wk, there is a high demand to initiate early placement. Stroke patients often require intensive inpatient or outpatient rehab and skilled nursing facility care; both of which require patients to resume oral nutrition or have a PEG tube given the high risk of nasoenteric tube displacement or blockage[6,7]. Delayed PEG placement could limit how quickly stroke patients can begin intensive rehabilitation and jeopardize their neurologic recovery. For this reason, determining the optimal timing of PEG placement is clinically important, but few studies have examined this directly. The 2005 “FOOD” randomized controlled trial comparing PEG tube vs nasoenteric tube feeding appeared to show the former was associated with a small increase in absolute risk of death or poor outcomes[8,9]. A later 2012 Cochrane review found that although there was insufficient data to offer definitive answers, PEG and NG tube feeding did not differ in terms of death or functional outcomes in patients with dysphagia following an acute or subacute stroke. Instead, PEG tubes were associated with improved food delivery, less treatment failures, and less GI bleeding[14]. However, neither of these studies specifically looked at the timing of placement. Our study, and the study by George et al[10], introduce new evidence for this discussion.
Our study had several limitations, most notably the relatively small population size, which likely contributed to an underpowered study and inability to identify significant differences in mortality, complication rates, or risk factors for these adverse outcomes. We attempted to account for any comorbidities contributing to the patients’ clinical status and outcomes, and our demographic and comorbidity data were broadly similar for both patient groups. Nevertheless, there remains the possibility of confounding bias when assessing the mortality and hospital length of stay. It is possible that sicker patients required longer recovery before they could undergo PEG placement, affecting length of stay.
Another limitation of our study was its retrospective design, as all health care events for a given patient may not have been captured (i.e., patients presenting to an outside hospital for PEG tube related complications). Additionally, pre-procedural platelet count and antiplatelet, anticoagulant, or immunosuppressant use were not examined.
Our study did assess age, which is a risk factor for prolonged dysphagia and poorer outcomes. There was a significant age difference between the early and late PEG placement groups, with the early group trending older (74.65 vs 66.24). If this were to affect results, it should have favored the late placement group. However, this study did not evaluate other identified risk factors for prolonged dysphagia and therefore need for enteral feeding, including National Institutes of Health Stroke Scale (NIHSS), presence of a bilateral infarction, pre-procedural clinical signs of aspiration, or stroke location in the frontal operculum or insular cortex[15]. These risk factors would have been assessed by the neurology service who evaluated the stroke patients prior to consulting the gastroenterology service for gastrostomy tube placement. Unassessed differences in these risk factors for persistent dysphagia could contribute to differences in our study populations’ outcomes.
A study published in Annals of Gastroenterology identified low albumin and positive urine cultures as predictors of mortality in patients who underwent PEG placement[16]. Other studies have found that patients with abnormal leukocyte counts were four times more likely to experience early and late complications[17] and that 30-d mortality was significantly higher in patients with a platelet count < 100000/μL[18]. Although our study did see an increase in peri-procedural complications in patients with protein-calorie malnutrition and pre-procedural SIRS or infection; as well as an increase in post-procedural complications in patients with protein-calorie malnutrition, pre-procedural SIRS or infection, or pre-existing cardiovascular risk equivalents, the findings were not statistically significant. The observed differences may have reached statistical significance with a larger population size.
Overall, our outcomes are comparable to those seen in the FOOD trial, which showed around a 40% incidence of any adverse outcome (e.g., pulmonary embolism, infection, etc.) over an 8 month follow up period, a 10% mortality rate for non-malnourished patients at 3 mo, and nearly a 30% mortality rate for malnourished patients.
In conclusion, our study shows that early PEG tube placement, less than 7 d following an acute stroke, was not associated with increased mortality or complications when compared to delayed PEG placement. Although not statistically significant, there were fewer deaths and complications in patients undergoing early PEG tube placement. While the risk factors studied showed no statistically significant difference in complication rates or mortality, a large-scale study may favor delayed PEG tube placement in patients with significant protein-calorie malnutrition, concurrent SIRS, or infection. As expected, hospital length of stay was significantly reduced in patients with early PEG tube placement. Given similar safety outcomes in both groups, early PEG tube placement should be considered in appropriate candidates. Prospective studies and cost analyses are warranted.
In conclusion, our study shows that early PEG tube placement, within 7 d of an acute stroke, was not associated with increased mortality or complications when compared to delayed PEG placement. Although not statistically significant, there were fewer deaths and complications in patients undergoing early PEG tube placement. While the risk factors studied showed no statistically significant difference in complication rates or mortality, a large-scale study may favor delayed PEG tube placement in patients with significant protein-calorie malnutrition, concurrent SIRS, or infection. As expected, hospital length of stay was significantly reduced in patients with early PEG tube placement. Given similar safety outcomes in both groups, early PEG tube placement should be considered in appropriate candidates. Prospective studies and cost analyses are warranted.
Stroke patients commonly require enteral nutrition for dysphagia. Percutaneous endoscopic gastrostomy (PEG) tubes and nasogastric tubes are options for enteral feeding, but the optimal timing determining when PEG tubes should be placed is uncertain. The 2011 ASGE guidelines recommend waiting 2 wk for assessment of resolution of dysphagia prior to placing a PEG tube, but the recommen
There is a demand for earlier placement of PEG tubes to facilitate earlier patient discharge to intensive rehab for neurologic recovery. An observational study using the Nationwide Patient Survey data found no difference in inpatient mortality or complication rates following early (within 7 d) PEG placement compared to delayed PEG placement after 7 d. This study was based on hospital data and could not provide longer term post-hospitalization outcomes or mortality. Further studies looking at the safety of early PEG placement are warranted.
This study aims to evaluate the safety of early (within 7 d) vs delayed (after 7 d) placement of PEG tubes in patients for dysphagia after acute stroke. Primary objectives were evaluation of 30- and 90-d mortality and rates of peri- and post-procedural complication. Secondary objectives included identification of predictors of morbidity and mortality in multivariate analysis.
This bi-center, retrospective chart review identified 482 patients undergoing PEG placement based on endoscopy reports. After excluding patients with age < 18, PEG placed by surgery or interventional radiology, and indications other than dysphagia from acute stroke, 154 patients were identified for review, including 62 PEGs placed within 7 d of stroke and 92 placed after 7 d. Retrospective data was collected, and outcomes evaluated included rate of peri-procedural complications, rate of post-procedural complications, and 90-d all-cause mortality. Demographics and predictors of morbidity and mortality were also collected and evaluated in multivariate logistic regression.
Demographics and comorbidities were similar between groups, except for age (early 74.7 vs delayed 66.2 years, P = 0.0005). There was no statistically significant difference in peri- or post-procedural complication rate or mortality between groups. None of the proposed risk factors studies significantly impacted 30- or 90-d mortality, although protein-calorie malnutrition and presence of infection or SIRS criteria were non-significantly associated with an increase in complication rate. Finally, hospital length of stay was significantly lower in patients undergoing PEG tube placement (12.9 vs 22.3 d, P < 0.001).
Early PEG placement was not associated with an increase in mortality or complications compared to delayed PEG placement in this retrospective chart review. This suggests early PEG placement is safe.
Further prospective study to evaluate the safety of early PEG placement and reconsideration of the 2-wk delay in PEG placement is warranted.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Byeon H, Fagundes RB A-Editor: Yao QG, United States S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | ASGE Standards of Practice Committee; Jain R, Maple JT, Anderson MA, Appalaneni V, Ben-Menachem T, Decker GA, Fanelli RD, Fisher L, Fukami N, Ikenberry SO, Jue T, Khan K, Krinsky ML, Malpas P, Sharaf RN, Dominitz JA. The role of endoscopy in enteral feeding. Gastrointest Endosc. 2011;74:7-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (1)] |
| 2. | Martino R, Foley N, Bhogal S, Diamant N, Speechley M, Teasell R. Dysphagia after stroke: incidence, diagnosis, and pulmonary complications. Stroke. 2005;36:2756-2763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1142] [Cited by in RCA: 1281] [Article Influence: 64.1] [Reference Citation Analysis (0)] |
| 3. | Rowat A. Enteral tube feeding for dysphagic stroke patients. Br J Nurs. 2015;24:138, 140, 142-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Turner M, Barber M, Dodds H, Dennis M, Langhorne P, Macleod MJ; Scottish Stroke Care Audit. Agreement between routine electronic hospital discharge and Scottish Stroke Care Audit (SSCA) data in identifying stroke in the Scottish population. BMC Health Serv Res. 2015;15:583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Ojo O, Brooke J. The Use of Enteral Nutrition in the Management of Stroke. Nutrients. 2016;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 6. | Park RH, Allison MC, Lang J, Spence E, Morris AJ, Danesh BJ, Russell RI, Mills PR. Randomised comparison of percutaneous endoscopic gastrostomy and nasogastric tube feeding in patients with persisting neurological dysphagia. BMJ. 1992;304:1406-1409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 258] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 7. | Norton B, Homer-Ward M, Donnelly MT, Long RG, Holmes GK. A randomised prospective comparison of percutaneous endoscopic gastrostomy and nasogastric tube feeding after acute dysphagic stroke. BMJ. 1996;312:13-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 259] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 8. | Dennis MS, Lewis SC, Warlow C; FOOD Trial Collaboration. Effect of timing and method of enteral tube feeding for dysphagic stroke patients (FOOD): a multicentre randomised controlled trial. Lancet. 2005;365:764-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 228] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 9. | Dennis M, Lewis S, Cranswick G, Forbes J; FOOD Trial Collaboration. FOOD: a multicentre randomised trial evaluating feeding policies in patients admitted to hospital with a recent stroke. Health Technol Assess. 2006;10:iii-iiv, ix. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 97] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 10. | George BP, Kelly AG, Albert GP, Hwang DY, Holloway RG. Timing of Percutaneous Endoscopic Gastrostomy for Acute Ischemic Stroke: An Observational Study From the US Nationwide Inpatient Sample. Stroke. 2017;48:420-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 11. | Ponsky JL, Gauderer MW. Percutaneous endoscopic gastrostomy: a nonoperative technique for feeding gastrostomy. Gastrointest Endosc. 1981;27:9-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 287] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 12. | ASGE Standards of Practice Committee; Acosta RD, Abraham NS, Chandrasekhara V, Chathadi KV, Early DS, Eloubeidi MA, Evans JA, Faulx AL, Fisher DA, Fonkalsrud L, Hwang JH, Khashab MA, Lightdale JR, Muthusamy VR, Pasha SF, Saltzman JR, Shaukat A, Shergill AK, Wang A, Cash BD, DeWitt JM. The management of antithrombotic agents for patients undergoing GI endoscopy. Gastrointest Endosc. 2016;83:3-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 455] [Article Influence: 50.6] [Reference Citation Analysis (1)] |
| 13. | Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, Jauch EC, Kidwell CS, Leslie-Mazwi TM, Ovbiagele B, Scott PA, Sheth KN, Southerland AM, Summers DV, Tirschwell DL. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2019;50:e344-e418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1907] [Cited by in RCA: 4102] [Article Influence: 683.7] [Reference Citation Analysis (0)] |
| 14. | Geeganage C, Beavan J, Ellender S, Bath PM. Interventions for dysphagia and nutritional support in acute and subacute stroke. Cochrane Database Syst Rev. 2012;10:CD000323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 113] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 15. | Galovic M, Stauber AJ, Leisi N, Krammer W, Brugger F, Vehoff J, Balcerak P, Müller A, Müller M, Rosenfeld J, Polymeris A, Thilemann S, De Marchis GM, Niemann T, Leifke M, Lyrer P, Saladin P, Kahles T, Nedeltchev K, Sarikaya H, Jung S, Fischer U, Manno C, Cereda CW, Sander JW, Tettenborn B, Weder BJ, Stoeckli SJ, Arnold M, Kägi G. Development and Validation of a Prognostic Model of Swallowing Recovery and Enteral Tube Feeding After Ischemic Stroke. JAMA Neurol. 2019;76:561-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 82] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 16. | Gumaste VV, Bhamidimarri KR, Bansal R, Sidhu L, Baum J, Walfish A. Factors predicting early discharge and mortality in post-percutaneous endoscopic gastrostomy patients. Ann Gastroenterol. 2014;27:42-47. [PubMed] |
| 17. | Lee SP, Lee KN, Lee OY, Lee HL, Jun DW, Yoon BC, Choi HS, Kim SH. Risk factors for complications of percutaneous endoscopic gastrostomy. Dig Dis Sci. 2014;59:117-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Pih GY, Na HK, Ahn JY, Jung KW, Kim DH, Lee JH, Choi KD, Song HJ, Lee GH, Jung HY. Risk factors for complications and mortality of percutaneous endoscopic gastrostomy insertion. BMC Gastroenterol. 2018;18:101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 74] [Article Influence: 10.6] [Reference Citation Analysis (1)] |