Published online Jul 5, 2022. doi: 10.4292/wjgpt.v13.i4.47
Peer-review started: January 3, 2022
First decision: March 10, 2022
Revised: March 24, 2022
Accepted: May 16, 2022
Article in press: May 16, 2022
Published online: July 5, 2022
Processing time: 178 Days and 12.7 Hours
Acute pancreatitis (AP) presenting as an initial manifestation of primary hyperparathyroidism (PHPT) is uncommon, and its timely diagnosis is crucial in preventing recurrent attacks of pancreatitis.
To determine the clinical, biochemical, and radiological profile of PHPT patients presenting as AP.
This is a retrospective observational study, 51 consecutive patients admitted with the diagnosis of PHPT during January 2010 and October 2021 at a tertiary care hospital in Puducherry, India was included. The diagnosis of AP was established in the presence of at least two of the three following features: abdominal pain, levels of serum amylase or lipase greater than three times the normal, and characteristic features at abdominal imaging.
Out of the 51 consecutive patients with PHPT, twelve (23.52%) had pancreatitis [5 (9.80%) AP, seven (13.72%) chronic pancreatitis (CP)]. PHPT with AP (PHPT-AP) was more common among males with the presentation at a younger age (35.20 ± 16.11 vs 49.23 ± 14.80 years, P = 0.05) and lower plasma intact parathyroid hormone (iPTH) levels [125 (80.55-178.65) vs 519.80 (149-1649.55, P = 0.01)] compared to PHPT without pancreatitis (PHPT-NP). The mean serum calcium levels were similar in both PHPT-AP and PHPT-NP groups [(11.66 ± 1.15 mg/dL) vs (12.46 ± 1.71 mg/dL), P = 0.32]. PHPT-AP also presented with more gastrointestinal symptoms like abdominal pain, nausea, and vomiting with lesser skeletal and renal manifestations as compared to patients with PHPT-NP.
AP can be the only presenting feature of PHPT. Normal or higher serum calcium levels during AP should always draw attention towards endocrine causes like PHPT.
Core Tip: Acute pancreatitis (AP) is a rare complication of primary hyperparathyroidism (PHPT) and can be its only presenting symptom. In this study, we retrospectively analyzed our single-center data of 51 PHPT patients between 2010 and 2021. The study showed that 9.8% of PHPT patients presented with AP. Patients with PHPT with AP patients presented at a younger age with a male preponderance and a lower frequency of skeletal and renal involvement as compared to patients with PHPT without pancreatitis. Early diagnosis and surgery for PHPT in AP will prevent recurrent attacks of AP and other PHPT-related complications.
- Citation: Rashmi KG, Kamalanathan S, Sahoo J, Naik D, Mohan P, Pottakkat B, Kar SS, Palui R, Roy A. Primary hyperparathyroidism presenting as acute pancreatitis: An institutional experience with review of the literature. World J Gastrointest Pharmacol Ther 2022; 13(4): 47-56
- URL: https://www.wjgnet.com/2150-5349/full/v13/i4/47.htm
- DOI: https://dx.doi.org/10.4292/wjgpt.v13.i4.47
Primary hyperparathyroidism (PHPT) is an endocrine disease characterized by excessive secretion of parathyroid hormone (PTH) from one or more parathyroid glands[1]. PHPT has become an asym
Traditionally, 80% of acute pancreatitis (AP) cases are related to alcohol abuse and biliary stone disease, and < 10% have metabolic causes such as diabetic ketoacidosis, hypertriglyceridemia, and hypercalcemia with or without PHPT as an etiology[5]. PHPT has been linked with the development of both AP and chronic pancreatitis (CP). The first case report of AP in PHPT was published by Smith and Cook in 1940[6]. Later, in 1980, a study by Bess et al[7] from the Mayo Clinic involving 1153 patients with histopathologically confirmed PHPT showed that only 17 (1.5%) had coexisting or prior pancreatitis. This frequency was comparable to the reported incidence of pancreatitis among patients admitted to a hospital without PHPT. However, the link between the two diseases cannot be excluded based on data from hospitals with a large number of symptomatic PHPT patients[8-11]. Shepherd reported this association in Australia, where seven (5.1%) of 137 PHPT patients had pancreatic disease[12]. Western studies have shown a pancreatitis prevalence in PHPT patients that ranges from 5.1 to 8.1 percent, with predominantly AP cases[8,9,12]. However, studies from India have reported a higher prevalence of AP in PHPT patients, ranging from 12.9 to 16 percent, with a roughly equal number of CP and AP patients[11,13]. Despite its rarity, the fact that parathyroidectomy has been shown to prevent the recurrence of pancreatitis attacks suggests a causal link between the two diseases[14-16].
Serum calcium plays a crucial role in the pathogenesis of pancreatitis. Three mechanisms have been suggested for the development of AP in patients with PHPT. The earliest abnormalities of AP arise within the acinar cells. Calcium is a vital intracellular second messenger in acinar cells that initiates enzyme release through phosphorylation cascades. Elevated extracellular calcium levels due to PHPT may augment intracellular calcium signaling[17], and activate calcium-dependent proteins, such as calcineurin, as well as pancreatic proteases (especially trypsin)[18,19], or activate NF-κB,[20]leading to initiation of the pancreatic inflammatory cascade. In addition, hypercalcemia can lead to the formation of pancreatic calculi, ductal obstruction, and subsequent attacks of AP or CP[21]. Felderbauer et al[22] showed that PHPT patients with AP had a higher prevalence of mutations in serine protease inhibitor kazal type 1 (SPINK-1), cystic fibrosis transmembrane conductance regulator (CFTR), and chymotrypsin C genes. Hence, hypercalcemia per se, as well as genetic factors, may be implicated in the pathogenesis of pancreatitis in PHPT. Hence, we conducted this retrospective study to determine the prevalence of AP in PHPT patients and to distinguish PHPT-AP patients from PHPT-NP patients based on their clinical, biochemical, and radiological profiles.
This retrospective study included 51 patients admitted with a diagnosis of PHPT between January 2010 and October 2021. Data from these patients were obtained from the PHPT registry of the Department of Endocrinology, Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry, India. The study was approved by the Institutional Ethics Committee (JIP/IEC/2021/329). PHPT was diagnosed based on elevated levels of plasma intact parathyroid hormone (iPTH) in the presence of hypercalcemia or normocalcemia. All patients had sporadic PHPT. Patients with a diagnosis of multiple endocrine neoplasia, a history of alcoholism and smoking, evidence of hypertriglyceridemia, and the presence of gallstone disease on abdominal imaging were excluded. The medical records of all the patients were studied for clinical, biochemical, and radiological parameters. The clinical symptoms were categorized as skeletal, renal, gastrointestinal, and neuropsychiatric symptoms. Data on biochemical parameters [serum calcium, serum phosphorous, serum magnesium, serum albumin, serum alkaline phosphatase (ALP), serum 25-OH vitamin D, and plasma iPTH] and radiological investigations were obtained from case records as well as from the Hospital Information System (HIS) and the Picture Archiving and Communication System (PACS).
AP was diagnosed by the presence of two of the following three features: (1) Abdominal pain consistent with AP; (2) Serum lipase (or amylase) elevated more than 3-fold the upper normal range; and (3) Characteristic features of AP, such as edema or pancreatic necrosis and/or collection[23]. The diagnosis of CP was made based on clinical and radiological investigations. A thorough diagnostic evaluation was performed in patients with chronic abdominal pain (> 6 mo duration), and the diagnosis of CP was established if there was evidence of pancreatic calcification on abdominal X-ray and/or ultrasonography and/or abdominal computed tomography[24]. Parathyroid tumor localization was performed using ultrasound of the neck or technetium-99m sestamibi parathyroid single-photon emission computed tomography (SPECT)/contrast-enhanced computerized tomography.
Serum calcium [reference range (RR), 8.8-10.6 mg/dL], inorganic phosphorous (RR, 2.5-5 mg/dL), magnesium (RR, 1.9-2.5 mg/dL), albumin (RR, 3.5-5.2 g/dL), serum alkaline phosphatase (ALP) (RR, 30-120 IU/L), serum creatinine (RR, 0.5-0.9 mg/dL), serum lipase (RR, 0-67 IU/L), and serum amylase (RR, 22-80 IU/L) were measured using an autoanalyzer (Beckman-Coulter AU5800 clinical chemistry analyzer). Serum 25-OH vitamin D (RR, 20-100 ng/mL) and plasma iPTH (RR, 18.4-80.1 pg/mL) were quantified using the ADVIA Centaur XP VitD assay (Rev. C, 2012-08, Siemens) kit and ADVIA Centaur XP PTH assay (Rev. B, 2017-07, Siemens) kits, respectively. Vitamin D deficiency was defined as a serum 25-OH vitamin D level of < 12 ng/mL.
Statistical analysis was carried out using MedCal Statistical software version 20.015. Categorical variables are described in terms of frequency and percentage. Continuous data are expressed as the mean ± SD or median with interquartile range (IQR). The student’s t test was used for normally distributed data, while the Mann-Whitney U test was used for comparing nonparametric variables. P < 0.05 was considered statistically significant.
A total of 51 PHPT patients were included in the study. The patients’ ages ranged from 38.25 to 60 years, and the mean age of presentation was 47.80 ± 14.51 years, with a female-to-male ratio of 1.55:1, as shown in (Table 1). The major clinical presentation of PHPT was bone pain (54.90%), followed by fatigue (49.01%), abdominal pain (43.13%), nephrolithiasis (33.33%), and nephrocalcinosis (21.56%). The mean serum calcium level was 12.41 ± 1.58 mg/dL, and the median plasma iPTH level was 328.10 (143-1111) pg/mL.
| Parameters | n = 51 |
| Age (yr) | 47.80 ± 14.51 |
| Female gender | 31 (60.78%) |
| Sex (female: male) | 1.55:1 (female 31, male 20) |
| Bone pain | 28 (54.90%) |
| Fracture | 04 (7.84%) |
| Pain abdomen | 22 (43.13%) |
| Nausea and vomiting | 16 (31.37%) |
| Weight loss | 12 (23.52%) |
| Fatigue and weakness | 25 (49.01%) |
| Anorexia | 16 (31.37%) |
| Psychiatric features | 06 (11.76%) |
| Nephrolithiasis | 17 (33.33%) |
| Nephrocalcinosis | 11 (21.56%) |
| Cholelithiasis | 04 (7.84%) |
| Serum creatinine (mg/dL) | 0.80 (0.63-1.14) |
| Serum corrected calcium(mg/dL) | 12.41 ± 1.58 |
| Serum phosphorous (mg/dL) | 2.7 (2.2-3.17) |
| Serum magnesium (mg/dL) | 1.80 (1.7-2) |
| Serum albumin (g/dL) | 3.90 (3.5-4.2) |
| Serum alkaline phosphatase (IU/L) | 196 (127.5-502) |
| Serum 25-OH vitamin D (ng/mL) | 18.09 ± 9.93 |
| Plasma iPTH (pg/mL) | 328.10 (143-1111) |
Twelve out of 51 (23.52%) PHPT patients had pancreatitis. Five (9.8%) patients had AP, and seven (13.72%) had CP. All of them had abdominal pain (100%) as the major presentation, followed by nausea with vomiting (91.67%) and anorexia (83.33%). The mean serum calcium level of PHPT-AP/CP patients was 12.26 ± 1.05 mg/dL, and the mean plasma iPTH was 283.48 pg/mL.
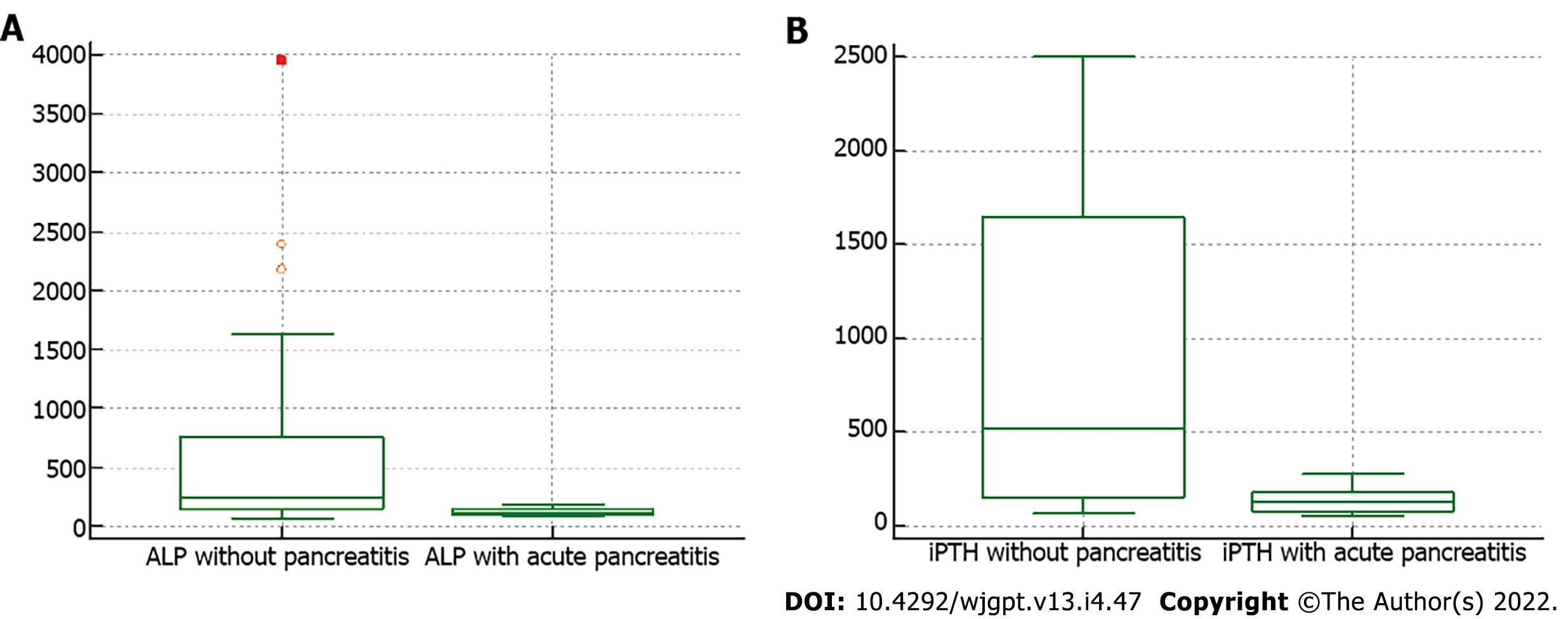
PHPT-AP patients were younger than PHPT patients without pancreatitis (PHPT-NP) (35.20 ± 16.11 vs 49.23 ±14.80 years, P = 0.05), and all of them were male, as shown in (Table 2). The PHPT-AP patients also presented with more gastrointestinal symptoms, such as abdominal pain (100%), nausea, and vomiting (100%), whereas skeletal disease (bone pain 56.41% and fracture 10.26%) and renal manifestations (nephrocalcinosis 23.07% and nephrolithiasis 35.89%) were more frequently seen in PHPT-NP. The mean calcium levels in patients with PHPT-AP were above the normal range (8.8-10.6 mg/dL). Serum ALP and plasma iPTH were significantly higher in PHPT-NP patients than in PHPT-AP patients (242 vs 112 IU/L, P = 0.03 and 519.80 vs 125 IU/L, P = 0.01, respectively) (Figure 1). The biochemical and imaging characteristics of PHPT-AP are shown in (Table 3) wherever available.
| Parameters | PHPT-NP, n = 39 | PHPT-AP, n = 05 | P value |
| Age (yr) | 49.23 ± 14.80 | 35.20 ± 16.11 | 0.05 |
| Female gender | 27 (69.23%) | 0 | |
| Bone pain | 22 (56.41%) | 1 (20%) | 0.17 |
| Fracture | 04 (10.26%) | 0 | |
| Pain abdomen | 10 (25.64%) | 5 (100%) | < 0.01 |
| Nausea and vomiting | 05 (12.82%) | 5 (100%) | < 0.01 |
| Weight loss | 06 (15.38%) | 1 (20%) | 1 |
| Fatigue and weakness | 18 (46.15%) | 2 (40%) | 1 |
| Anorexia | 06(15.38%) | 4 (80%) | < 0.01 |
| Psychiatric features | 03 (7.69%) | 0 | |
| Nephrolithiasis | 14 (35.89%) | 0 | |
| Nephrocalcinosis | 09 (23.07%) | 0 | |
| Cholelithiasis | 02 (5.12%) | 1 (20%) | 0.31 |
| Serum creatinine(mg/dL) | 0.80 (0.60-1.19) | 0.74 (0.66-0.89) | 0.66 |
| Serum corrected calcium(mg/dL) | 12.46 ± 1.71 | 11.66 ± 1.15 | 0.32 |
| Serum phosphorous (mg/dL) | 2.7 (2.2-3.17) | 2.7 (2.02-2.90) | 0.62 |
| Serum magnesium (mg/dL) | 1.82 ± 0.33 | 1.76 ± 0.34 | 0.72 |
| Serum albumin (g/dL) | 3.90 (3.5-4.1) | 4.2 (3.7-4.75) | 0.18 |
| Serum ALP (IU/L) | 242 (148-764.5) | 112 (106.25-147) | 0.03 |
| Serum 25-OH vitamin D(ng/mL) | 15.48 (10.24-21.37) | 25.91 (13.88-31.72) | 0.33 |
| Plasma iPTH (pg/mL) | 519.80(149-1649.55) | 125 (80.55-178.65) | 0.01 |
| No | Age | Sex | S Amylase (IU/mL) | S lipase (IU/mL) | Imaging | Modified CT severity index |
| 1 | 46 | M | 160 | 770 | Acute pancreatitis on CECT | 4 |
| 2 | 27 | M | 1487 | NA | Acute interstitial pancreatitis on MRCP | NA |
| 3 | 20 | M | NA | NA | Acute pancreatitis on CECT | 6 |
| 4 | 25 | M | 514 | 260 | Acute necrotizing pancreatitis on CECT | 6 |
| 5 | 58 | M | 1276 | 1365 | Acute necrotizing pancreatitis on CECT | 8 |
In our study, ultrasonography of the neck was performed in 34 patients, technetium-99m sestamibi parathyroid SPECT was performed in 48 patients, and computed tomography of the neck was performed in 18 patients for localizing parathyroid lesions, with sensitivities of 79.06%, 95.83%, and 100%, respectively.
The causal relationship between PHPT and pancreatitis has been debated for decades. There have been at least 12 retrospective studies or case series[25,26] on pancreatitis associated with PHPT since 1980, originating from the United States, India, France, Australia, Spain, and Germany, as shown in (Table 4).
| Ref. | Country | Number of PHPT Patients | PHPT with pancreatitis n (%) | Type of pancreatitis |
| Bess et al[7] | USA | 1153 | 17 (1.5) | 10 AP (0.86%), 7 CP |
| Sitges-Serra et al[8] | Spain | 86 | 7 (8.1) | 3 AP (3.4%), 1 RP, 3 CP |
| Koppelberg et al[9] | Germany | 234 | 13 (5.6) | 9 AP (3.8%), 4 CP |
| Shepherd et al[12] | Australia | 137 | 7 (5.1) | All AP (5.1%) |
| Carnaille et al[10] | France | 1224 | 40 (3.3) | 18 AP (1.47), 8 RP, 14 CP |
| Agarwal et al[32] | India | 87 | 6 (6.9) | 5 RP, 1 CP |
| Jacob et al[13] | India | 101 | 13 (12.9) | 6 AP (5.94%), 6 RP, 1 CP |
| Bhadada et al[29] | India | 59 | 9 (15.3) | All CP |
| Khoo et al[33] | USA | 684 | 10 (1.5) | All AP (1.5%) |
| Felderbauer et al[22] | Germany | 1259 | 57 (4.52) | 16 AP (1.27%), 15 CP, 26 NA |
| Arya et al[11] | India | 218 | 35 (16) | 18 AP (8.25%), 17 CP |
| Misgar et al[5] | India | 242 | 15 (6.19) | 14 AP (5.78%), 1 CP |
| Total | 5484 | 229 (4.17) | 111 (2.02) AP | |
Our experience with five PHPT-AP patients and the findings of an additional 111 such patients in the literature establish an etiological relationship between PHPT and AP. In our study, 23.52% of PHPT patients had pancreatitis; of these, 9.80% presented with AP at the time of diagnosis. However, studies from the Western population have shown a prevalence of AP as 0.86%-5.1% in patients with PHPT (Table 4). Several factors, including severity, delays in diagnosis, and asymptomatic vs symptomatic presentation of PHPT, are possible explanations for the higher prevalence of pancreatitis in patients with PHPT in studies from India, including ours. Among the two Indian studies that have reported a higher prevalence of AP, Jacob et al[13] reported the occurrence of AP in 6 of 101 (5.94%) patients with PHPT, while Arya et al[11] reported it in 18 of 218 (8.25%) such patients. Nevertheless, pancreatitis seems to be at least ten times more common in PHPT patients than in the general population[27].
PHPT-AP presented at a younger age with a male preponderance, in contrast to the female preponderance (27 out of 39) seen in PHPT-NP. In both studies by Carnaille et al[10] from France and the study by Jacob et al[13] from India, a younger age of presentation with a male preponderance of AP was reported, which is in agreement with our observation. Abdominal pain was the most common (100%) clinical presentation of PHPT-AP in our study, and the same finding was reported by others[13,28,29]. Skeletal manifestations such as bone pain and fracture were respectively seen in only 20% and none of the PHPT-AP patients, which is lower than the corresponding prevalence among patients with PHPT-NP (56.4% and 10.6%, respectively). This observation is similar to those of Arya et al[11] and Yadav et al[30]. The exact pathogenesis of this younger age of presentation, male preponderance, and presence of fewer skeletal/renal manifestations has not been established. A possible explanation could be the earlier detection of disease due to AP at a relatively mild stage with a lesser degree of elevation in iPTH levels. Additionally, genetic risk factors for the development of AP could have contributed to the younger age of presentation. One patient in the PHPT-AP group had a history of gallstones and underwent treatment for the same condition elsewhere before coming to our institute with AP. In the background of hypercalcemia and other PHPT-related complications, such as renal stones, and the absence of evidence of cholelithiasis on subsequent imaging with CT of the abdomen, the possibility of AP due to hypercalcemia was considered in this patient.
Several studies[10,13] have reported elevated serum calcium levels among PHPT patients with pancreatitis compared to patients with PHPT-NP. The results suggest that the serum calcium level above a threshold predisposes PHPT patients to pancreatitis. However, in our study, PHPT patients with AP had serum calcium levels similar to those in PHPT patients without pancreatitis. At the time of presentation, normocalcemia was seen in 1 (20%) patient and could be attributed to saponification of calcium in the pancreatic tissue. The diagnosis of PHPT was suspected in this patient due to the absence of other risk factors for AP, and the patient was subsequently found to have increased plasma iPTH levels. Thus, a normal calcium level at the time of presentation in patients with an acute episode of pancreatitis does not exclude the possibility of PHPT. The current study highlights the importance of measuring intact PTH levels and rechecking calcium levels in patients with an unexplained etiology of AP. Because pancreatitis primarily occurs in severely hypercalcemic patients, it is rarely associated with PHPT in developed countries where PHPT is diagnosed at a much earlier and milder stage. Serum ALP was significantly higher in patients with PHPT-NP than in PHPT-AP patients, most likely due to more severe bone disease.
In patients with PHPT who undergo parathyroidectomy, the course of pancreatitis is unclear due to a lack of long-term studies. Most published reports had a follow-up of only approximately two years. Despite this short period, there was a 42%-100% reduction in pancreatitis recurrence rates[31]. In our study, all patients with PHPT and pancreatitis underwent parathyroidectomy. After successful parathyroidectomy, four out of five patients with PHPT-AP did not report a recurrence of pancreatitis over a median follow-up of 8 mo (range, 8-50 mo). Histopathology revealed parathyroid adenoma in 3 cases and parathyroid carcinoma in 1 case. However, the report was inconclusive in one patient. Additionally, this patient had recurrent episodes of pancreatitis, and further evaluation could not be performed, as the patient was lost to follow-up due to the COVID pandemic. Our findings, like others, emphasize the importance of parathyroid surgery in these patients. It has been suggested that parathyroid surgery should precede any pancreatic surgery because of its beneficial effect on the course of the latter.
The limitations of this study are that we were not able to perform a gene mutation analysis of SPINK-1 and CFTR; hence, the exact prevalence of genetic risk and idiopathic pancreatitis could not be established. Additionally, we could not determine the cause of the lower frequency of renal manifestations in patients with PHPT-AP compared to patients with PHPT-NP, as data on urinary calcium profiles were not available for all patients given the retrospective nature of this study.
The current study demonstrated a causal relationship between PHPT and AP. Compared to patients with PHPT-NP, patients with PHPT-AP were younger, had a male preponderance, and had a lower frequency of skeletal and renal involvement. Our findings emphasize the importance of thoroughly investigating for PHPT in any patient with pancreatitis and high-normal or elevated serum calcium levels, especially in the absence of other common causes of pancreatitis. Pancreatitis should be an anticipated complication of PHPT and may be the sole presenting complaint of PHPT. Early diagnosis and resection of parathyroid lesions will prevent recurrent attacks of AP and other PHPT-related complications.
Primary hyperparathyroidism (PHPT) is an endocrine disease characterized by excessive secretion of parathyroid hormone (PTH) from one or more parathyroid glands. PHPT has been linked with the development of both acute and chronic pancreatitis.
Early diagnosis and surgery for PHPT will prevent the recurrence of acute pancreatitis (AP).
To determine the prevalence of AP in PHPT patients and to distinguish PHPT with acute pancreatitis (PHPT-AP) from PHPT without pancreatitis (PHPT-NP) patients based on their clinical and biochemical and radiological profiles.
This is a retrospective observational study done on 51 consecutive patients admitted with the diagnosis of PHPT between January 2010 to October 2021 at a tertiary care hospital in Puducherry, India.
In our study, the prevalence of AP in PHPT was found to be 9.80%. PHPT with AP was more common among males with the presentation at a younger age with lower plasma intact parathyroid hormone levels compared to PHPT-NP.
The current study demonstrates a causal relationship between the PHPT and AP. Evaluation for PHPT should be considered in any patient with pancreatitis with high normal or elevated serum calcium levels, especially in the absence of other common causes of pancreatitis.
Pancreatitis should be an anticipated complication of PHPT and may be the sole presenting complaint of PHPT. Early diagnosis and surgery for PHPT in AP will prevent recurrent attacks of AP and other PHPT-related complications.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Ashkar M, United States; Cotoi L, Romania; Hakim GD, Turkey A-Editor: Gao W S-Editor: Wu YXJ L-Editor: A P-Editor: Wu YXJ
| 1. | Bilezikian JP, Brandi ML, Rubin M, Silverberg SJ. Primary hyperparathyroidism: new concepts in clinical, densitometric and biochemical features. J Intern Med. 2005;257:6-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 107] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 2. | Misgar RA, Dar PM, Masoodi SR, Ahmad M, Wani KA, Wani AI, Bashir MI. Clinical and laboratory profile of primary hyperparathyroidism in Kashmir Valley: A single-center experience. Indian J Endocrinol Metab. 2016;20:696-701. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Misgar RA, Sehgal A, Masoodi SR, Wani AI, Bashir MI, Malik AA, Wani MA, Wani MM, Wani IA. A Comparison between Silent and Symptomatic Renal Stones in Primary Hyperparathyroidism. Indian J Endocrinol Metab. 2019;23:46-49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Misgar RA, Mathew V, Pandit K, Chowdhury S. Primary hyperparathyroidism presenting as recurrent acute pancreatitis: A case report and review of literature. Indian J Endocrinol Metab. 2011;15:54-56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Misgar RA, Bhat MH, Rather TA, Masoodi SR, Wani AI, Bashir MI, Wani MA, Malik AA. Primary hyperparathyroidism and pancreatitis. J Endocrinol Invest. 2020;43:1493-1498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Bess MA, Edis AJ, van Heerden JA. Hyperparathyroidism and pancreatitis. Chance or a causal association? JAMA. 1980;243:246-247. [PubMed] [DOI] [Full Text] |
| 8. | Sitges-Serra A, Alonso M, de Lecea C, Gores PF, Sutherland DE. Pancreatitis and hyperparathyroidism. Br J Surg. 1988;75:158-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Koppelberg T, Bartsch D, Printz H, Hasse C, Rothmund M. [Pancreatitis in primary hyperparathyroidism (pHPT) is a complication of advanced pHPT]. Dtsch Med Wochenschr. 1994;119:719-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Carnaille B, Oudar C, Pattou F, Combemale F, Rocha J, Proye C. Pancreatitis and primary hyperparathyroidism: forty cases. Aust N Z J Surg. 1998;68:117-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 69] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Arya AK, Bhadada SK, Mukherjee S, Singh P, Rana SS, Dahiya D, Sood A, Saikia UN, Prakash M, Bhasin DK, Behera A, Walia R, Bhansali A. Frequency & predictors of pancreatitis in symptomatic primary hyperparathyroidism. Indian J Med Res. 2018;148:721-727. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Shepherd JJ. Hyperparathyroidism presenting as pancreatitis or complicated by postoperative pancreatitis. Aust N Z J Surg. 1996;66:85-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Jacob JJ, John M, Thomas N, Chacko A, Cherian R, Selvan B, Nair A, Seshadri M. Does hyperparathyroidism cause pancreatitis? ANZ J Surg. 2006;76:740-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Chowdhury SD, Kurien RT, Pal S, Jeyaraj V, Joseph AJ, Dutta AK, Chandramohan A, Abraham D, Augustine J, Hephzibah J, Simon EG. Acute pancreatitis and hyperparathyroidism: a case series. Indian J Gastroenterol. 2014;33:175-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Singh DN, Gupta SK, Kumari N, Krishnani N, Chand G, Mishra A, Agarwal G, Verma AK, Mishra SK, Agarwal A. Primary hyperparathyroidism presenting as hypercalcemic crisis: Twenty-year experience. Indian J Endocrinol Metab. 2015;19:100-105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Prinz RA, Aranha GV. The association of primary hyperparathyroidism and pancreatitis. Am Surg. 1985;51:325-329. [PubMed] |
| 17. | Sutton R, Criddle D, Raraty MG, Tepikin A, Neoptolemos JP, Petersen OH. Signal transduction, calcium and acute pancreatitis. Pancreatology. 2003;3:497-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 78] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 18. | Husain SZ, Grant WM, Gorelick FS, Nathanson MH, Shah AU. Caerulein-induced intracellular pancreatic zymogen activation is dependent on calcineurin. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1594-G1599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Shah AU, Sarwar A, Orabi AI, Gautam S, Grant WM, Park AJ, Shah AU, Liu J, Mistry PK, Jain D, Husain SZ. Protease activation during in vivo pancreatitis is dependent on calcineurin activation. Am J Physiol Gastrointest Liver Physiol. 2009;297:G967-G973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 20. | Hietaranta AJ, Saluja AK, Bhagat L, Singh VP, Song AM, Steer ML. Relationship between NF-kappaB and trypsinogen activation in rat pancreas after supramaximal caerulein stimulation. Biochem Biophys Res Commun. 2001;280:388-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 55] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Cope O, Culver PJ, Mixter CG Jr, Nardi GL. Pancreatitis, a diagnostic clue to hyperparathyroidism. Ann Surg. 1957;145:857-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 140] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 22. | Felderbauer P, Karakas E, Fendrich V, Lebert R, Bartsch DK, Bulut K. Multifactorial genesis of pancreatitis in primary hyperparathyroidism: evidence for "protective" (PRSS2) and "destructive" (CTRC) genetic factors. Exp Clin Endocrinol Diabetes. 2011;119:26-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Banks PA, Freeman ML; Practice Parameters Committee of the American College of Gastroenterology. Practice guidelines in acute pancreatitis. Am J Gastroenterol. 2006;101:2379-2400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1181] [Cited by in RCA: 1150] [Article Influence: 60.5] [Reference Citation Analysis (0)] |
| 24. | Braganza JM, Lee SH, McCloy RF, McMahon MJ. Chronic pancreatitis. Lancet. 2011;377:1184-1197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 343] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 25. | Gupta AK, Madnani M, Mistry J, Soni H, Shah A, Patel KS, Mehta N, Dileep P, Chandra S, Haribhakti S. Primary hyperparathyroidism with pancreatitis: experience of management in 5 patients with review of literature. Indian J Gastroenterol. 2014;33:484-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Lenz JI, Jacobs JM, Op de Beeck B, Huyghe IA, Pelckmans PA, Moreels TG. Acute necrotizing pancreatitis as first manifestation of primary hyperparathyroidism. World J Gastroenterol. 2010;16:2959-2962. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 27. | Goebell H. The role of calcium in pancreatic secretion and disease. Acta Hepatogastroenterol (Stuttg). 1976;23:151-161. [PubMed] |
| 28. | Bhadada SK, Udawat HP, Bhansali A, Rana SS, Sinha SK, Bhasin DK. Chronic pancreatitis in primary hyperparathyroidism: comparison with alcoholic and idiopathic chronic pancreatitis. J Gastroenterol Hepatol. 2008;23:959-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 29. | Russell CF, Edis AJ. Surgery for primary hyperparathyroidism: experience with 500 consecutive cases and evaluation of the role of surgery in the asymptomatic patient. Br J Surg. 1982;69:244-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 123] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 30. | Yadav SK, Mishra SK, Mishra A, Mayilvagnan S, Chand G, Agarwal G, Agarwal A, Verma AK. Changing Profile of Primary Hyperparathyroidism Over Two and Half Decades: A Study in Tertiary Referral Center of North India. World J Surg. 2018;42:2732-2737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 31. | Bai HX, Giefer M, Patel M, Orabi AI, Husain SZ. The association of primary hyperparathyroidism with pancreatitis. J Clin Gastroenterol. 2012;46:656-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 32. | Agarwal A, George RK, Gupta SK, Mishra SK. Pancreatitis in patients with primary hyperparathyroidism. Indian J Gastroenterol. 2003;22:224-225. [PubMed] |
| 33. | Khoo TK, Vege SS, Abu-Lebdeh HS, Ryu E, Nadeem S, Wermers RA. Acute pancreatitis in primary hyperparathyroidism: a population-based study. J Clin Endocrinol Metab. 2009;94:2115-2118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |