Published online Mar 5, 2022. doi: 10.4292/wjgpt.v13.i2.11
Peer-review started: July 7, 2021
First decision: October 3, 2021
Revised: October 18, 2021
Accepted: January 19, 2022
Article in press: January 19, 2022
Published online: March 5, 2022
Processing time: 237 Days and 9.4 Hours
Melatonin (MLT) is a potent antioxidant molecule that is shown to have a beneficial effect in various pathological situations, due to its action against free radicals.
To evaluate the effect of MLT on carbon tetrachloride (CCl4) induced liver injury in rats in terms of oxidative stress, reticular stress, and cell damage.
Twenty male Wistar rats (230-250 g) were divided into four groups: Control rats, rats treated with MLT alone, rats treated with CCl4 alone, and rats treated with CCl4 plus MLT. CCl4 was administered as follows: Ten doses every 5 d, ten every 4 d, and seven every 3 d. MLT was administered intraperitoneally at a dose of 20 mg/kg from the 10th wk to the end of the experiment (16th wk).
MLT was able to reduce the release of liver enzymes in the bloodstream and to decrease oxidative stress in CCl4 treated rats by decreasing the level of thiobarbituric acid reactive substances and increasing superoxide dismutase activity, with a lower reduction in serum zinc levels, guaranteeing a reduction in liver damage; additionally, it increased the expression of nuclear factor (erythroid-derived 2)-like 2 and decreased the expression of Kelch-like ECH-associated protein 1. MLT also decreased the expression of the proteins associated with endoplasmic reticulum stress, i.e., glucose-regulated protein 78 and activating transcription factor 6, as well as of heat shock factor 1 and heat shock protein 70.
MLT has a hepatoprotective effect in an experimental model of CCl4-induced liver injury, since it reduces oxidative stress, restores zinc levels, and modulates endoplasmic reticulum stress.
Core Tip: Liver cirrhosis is a chronic condition of the liver that is characterized by inflammation, steatosis, and formation of fibrotic tissue that impair liver function. Several studies have demonstrated the relationship between oxidative stress and the development of different diseases. As oxidative stress can damage lipids, proteins, and DNA, causing changes in cell homeostasis, it is important to study therapeutic substances that can minimize or delay the effects of the disease. Hepatotoxic drugs are commonly used as an experimental model to assess different stages of liver disease. Melatonin was used in this work as a therapeutic strategy and showed hepatoprotective action in a chronic hepatotoxicity model.
- Citation: Bona S, Fernandes SA, Moreira ACJ, Rodrigues G, Schemitt EG, Di Naso FC, Marroni CA, Marroni NP. Melatonin restores zinc levels, activates the Keap1/Nrf2 pathway, and modulates endoplasmic reticular stress and HSP in rats with chronic hepatotoxicity. World J Gastrointest Pharmacol Ther 2022; 13(2): 11-22
- URL: https://www.wjgnet.com/2150-5349/full/v13/i2/11.htm
- DOI: https://dx.doi.org/10.4292/wjgpt.v13.i2.11
Chronic liver diseases are characterized by multistep processes that involve several molecules and cellular events to transform a normal parenchyma into a parenchyma with steatosis, increased collagen deposition, fibrosis, and cirrhosis[1]. Many studies have demonstrated the presence of overproduction of free radicals and reactive oxygen species (ROS) in inflammatory chronic diseases. ROS are able to oxidize macromolecules or activate transcription factors[2-4]. The relation between the development of chronic liver diseases and ROS has been widely discussed since oxidative stress may cause damage in lipid, protein, and DNA, producing alterations in cellular redox homeostasis[5].
Cellular homeostasis can be disrupted by a variety of stimuli, including metabolic imbalance, oxidative stress, and folding of malformed proteins. In response to these stressors, cells induce specific molecular pathways that usually involve the activation of signaling cascades or changes in gene expression. These responses allow cells to adapt to stress and to regain homeostasis. However, if stress is intense or prolonged, the cells are unable to reestablish homeostasis and, in turn, activate pathways that result in cell death[6].
Carbon tetrachloride (CCl4) is a hepatotoxic drug used in experimental models to evaluate different stages of liver disease and thus define therapeutic strategies. Exposure to CCl4 have a toxic effect on liver cells, promoting damage in tissue and causing changes in the antioxidant defense mechanism. This process results in an imbalance between ROS production and antioxidative enzymes release[5].
The antioxidant defense mechanism is dependent on some minerals, including zinc. This mineral is essential for a large number of structural proteins, enzymatic processes, and transcription factors. Its deficiency causes numerous clinical manifestations, such as appetite loss, smell and taste disturbances, cerebral and immune dysfunction, and reduced drug elimination capacity. These clinical characteristics have been observed in chronic liver diseases[7].
The alteration in cellular redox homeostasis either results in mitochondrial dysfunction or can affect other organelles, such as the endoplasmic reticulum (ER). The ER stress may impair protein synthesis, resulting in accumulation of misfolded proteins. ER stress induces the activation of the intracellular signaling pathway called unfolded protein response (UPR), which contributes to the pathogenesis of several chronic diseases[8,9].
Melatonin (N-acetyl-5-methoxytryptamine; MLT) is an indoleamine lipophilic derivative of tryptophan. It is produced primarily by the pineal gland of vertebrates and is also detected in other organs[10-13]. MLT has effects on sleep, mood, sexual maturation and reproduction, immune function, aging, and the antioxidative defense system[14,15], and exhibits numerous actions, including anti-inflammatory, antioxidant, and oncostatic properties. In hepatocytes, it was observed that MLT protects from oxidative damage. Its action is based on scavenging the free radicals and stimulating antioxidant enzymes[15-17]. Reducing the generation of ROS would be a way to slow the progression of cell damage observed in liver diseases.
Our aim was to study the effects of MLT on biochemical indexes, serum zinc levels, and oxidative stress in rats exposed to CCl4. Also, we evaluated the expression of proteins involved in cell damage, ER stress, and unfolded protein response in animals with liver injury induced by CCl4 and treated with MLT.
Twenty male Wistar rats with an average weight of 230-250 g were used. The animals were housed at 22 °C with 12 h light-dark cycles, had free access to water, and received a restricted diet (16 g of chow per day for each animal).
All experiments were performed in accordance with the Guiding Principles for Research Involving Animals (NAS) and the Committee of Ethics and Research in Health of the Graduate and Research Group of Hospital de Clinicas de Porto Alegre under protocol number 100316.
The animals were divided into four groups: Control (CO) rats, rats treated with MLT alone (MLT), rats treated with CCl4 alone (CCl4), and rats treated with CCl4 plus MLT (CCl4 + MLT). The CCl4 and CCl4 + MLT groups received 27 intraperitoneal doses of 0.5 mL of CCl4 dissolved in mineral oil (1:6). The first ten doses were given at an interval of 5 d, the following ten at an interval of 4 d, and the last seven at an interval of 3 d[18]. In order to promote cytochrome P450 enzyme induction, phenobarbital was added to the drinking water of each animal at a concentration of 0.3 g/L 7 d before the first application and throughout the experiment[19].
MLT (Sigma Aldrich, St. Louis, MO) was administered intraperitoneally to the MLT and CCl4 + MLT groups at a dose of 20 mg/kg/day from the 10th wk to the end of the experiment (16th wk)[20].
At 24 h after the last administration of CCl4, the animals were anesthetized with 1% xylazine and 10% ketamine, and then we collected blood samples from the retro-orbital plexus. Liver samples were obtained for the remaining analyses. At the end of the experiment, the animals were killed under deep anesthesia by exsanguination, as described in the guidelines of the American Veterinary Medical Association (AVMA) on Euthanasia (AVMA, 2007).
Serum levels of alanine aminotransferase (ALT) (U/L) and aspartate aminotransferase (AST) (U/L) were determined by the kinetic UV test. Alkaline phosphatase (AP) (U/L) was quantified by the colorimetric kinetic test. The levels of these enzymes were measured using routine laboratory methods of the Hospital de Clinicas de Porto Alegre by enzymatic methods (automated - Siemens Advia 1800 Chemistry system).
Frozen tissue from each rat was homogenized in ice-cold phosphate buffer (KCl 140 mmol/L, phosphate 20 mmol/L, pH 7.4) and centrifuged at 3000 rpm for 10 min. Protein concentration in liver homogenates was determined using a bovine albumin solution[21]. Lipid peroxidation (LPO) was determined by measuring the concentration of thiobarbituric acid reactive substances (TBARS) (nmol/mg protein)[22]. Spectrophotometric absorbance in the supernatant was measured at 535 nm. Cytosolic superoxide dismutase (SOD) (EC 1.15.1.1) was assayed as described previously[23]. The auto-oxidation rate of epinephrine, which is progressively inhibited by increasing amounts of SOD in the homogenate, was monitored spectrophotometrically at 560 nm. The amount of enzyme that inhibited 50% of epinephrine auto-oxidation was defined as 1 U of SOD activity.
Western blot analysis was performed using cytosolic and nuclear extracts prepared from liver homogenates as previously described[24]. The supernatant fraction was collected and stored at -80 °C in aliquots until use. Protein concentration was measured as described previously[21]. Lysate proteins were fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride membranes[25]. The membranes were then blocked with 5% nonfat dry milk in Tris-buffered saline containing 0.05% Tween 20 (TTBS) for 1 h at room temperature and probed overnight at 4 ºC with polyclonal antibodies against nuclear factor (erythroid-derived 2)-like 2 (Nrf2) (SC30915/57kDa), Kelch-like ECH-associated protein 1 (Keap1) (SC33569/69kDa), activating transcription factor 6 (ATF6) (SC166659/90kDa), and 78-kDa glucose-regulated protein (GRP78/BiP) (SC376768/78kDa) (Santa Cruz Biotechnology, Santa Cruz, CA, United States) at a dilution of 1:200-1000 with TTBS in 5% nonfat dry milk. Antibodies against heat shock factor 1 (HSF1) (H4163/75kDa) and heat shock protein 70 (HSP70) (H5147/73 and 72kDa) (Sigma Aldrich, St Louis, MO, United States) were used at a dilution of 1:5000 with TTBS in 5% nonfat dry milk, as well as antibodies against β-actin (A5060/42kDa) and anti-glyceraldehyde 3-phosphate dehydrogenase (G9545/37kDa) (Sigma Aldrich, St Louis, MO, United States) at a dilution of 1:2,000 with TTBS in 5% nonfat dry milk. After washing with TTBS, the membranes were incubated for 1 h at room temperature with secondary HRP conjugated antibody (Santa Cruz Biotechnology, Santa Cruz, CA, United States, 1:4000). Protein detection was performed via chemiluminescence using a commercial ECL kit (Amersham Pharmacia Biotech, Little Chalfont, UK)[24]. The density of the specific bands was quantified with imaging densitometer software (Scion Image, Maryland, MA).
The serum zinc concentration was measured using a Zinc Assay Kit (Abnova Corporation, Taipei City, Taiwan). The absorbance (425 nm) of the supernatant was measured by spectrophotometry, and the values are expressed in µg/dL.
The mean and standard deviation (SD) were calculated for all data. Significant differences between means were evaluated by one-way analysis of variance (ANOVA). Statistical significance was assessed using Tukey’s test. P values < 0.05 were deemed significant. All analyses were carried out using Statistical Package for Social Sciences (SPSS), version 18.0 (SPSS Inc., Chicago, IL).
We evaluated the hepatoprotective activity of MLT against CCl4-induced liver injury in rats (Table 1). Analyses of transaminase enzymes showed that in the CCl4 group, AST and ALT were increased compared to the CO, MLT, and CCl4 + MLT groups. Also, AP demonstrated a significant increase in the CCl4 group compared to the other groups, indicating the presence of hepatocellular injury. Animals treated with MLT showed reduced enzymatic levels when compared to the CCl4 group (P < 0.001).
| Parameter | CO | MLT | CCl4 | CCl4 + MLT |
| AST (U/L) | 175.4 ± 34.4 | 161.8 ± 20.3 | 1016.8 ± 340.8a | 519.6 ± 127.5 |
| ALT (U/L) | 50.2 ± 5.6 | 43.8 ± 6.6 | 270 ± 90.8a | 177 ± 42.7 |
| AP (U/L) | 80.2 ± 25.4 | 75 ± 14.3 | 395 ± 130.8a | 238 ± 24.5 |
| TBARS (nmol/mg protein) | 0.18 ± 0.01 | 0.15 ± 0.01 | 0.29 ± 0.03a | 0.18 ± 0.05 |
| SOD (U/SOD/mg protein) | 12.84 ± 1.08 | 11.43 ± 0.7 | 9.32 ± 0.3a | 13.18 ± 1.6 |
| Zinc (µg/dL) | 48.66 ± 4.9 | 0.62 ± 10.4 | 11.11 ± 4.31a | 28.96 ± 6.67 |
Determination of lipid peroxidation in liver tissue with liver injury induced by CCl4 and treated with MLT was performed by the TBARS method, which showed an increase of malondialdehyde (MDA) formation in tissues exposed to CCl4. Lipid peroxidation was significantly increased in the CCl4 group (+ 61%) vs control animals. The CCl4 + MLT group had LPO levels similar to the controls (Table 1). SOD activity was lower in the CCl4 group (- 27%) compared to animals of the control group. However, treatment with MLT reduced LPO and restored SOD activity in the CCl4 + MLT group in comparison with the CCl4 group (Table 1).
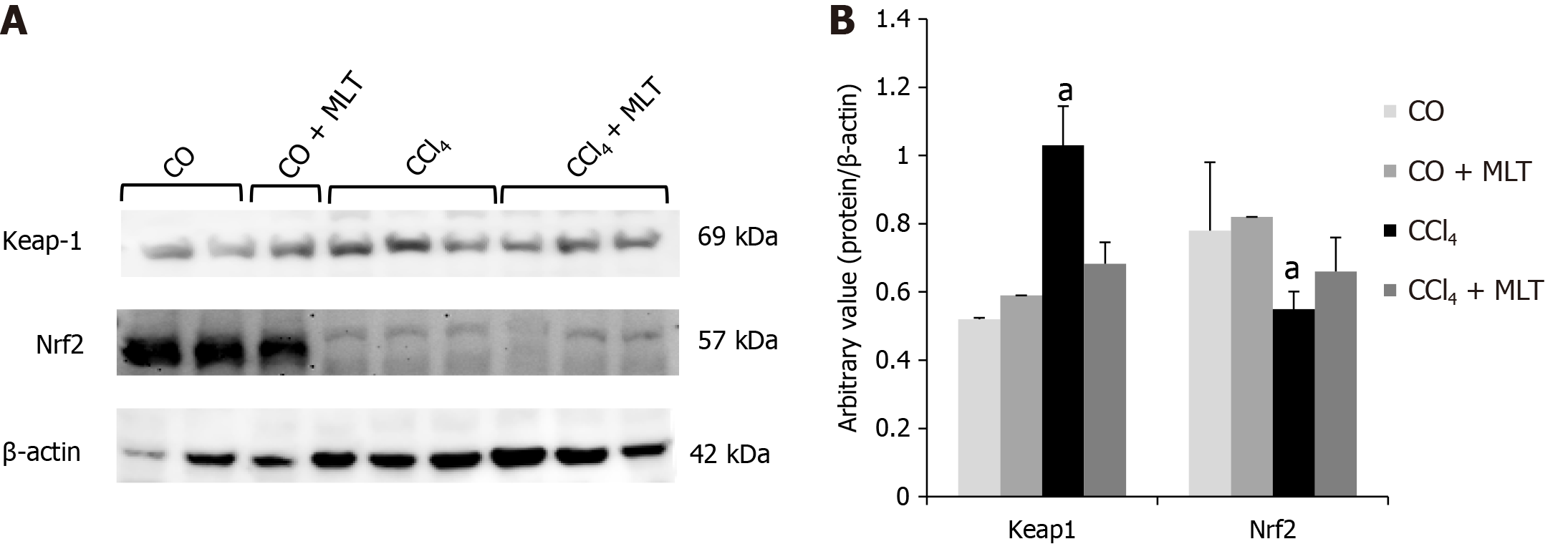
Protein markers related to oxidative stress were also evaluated. Animals in the CCl4 group overexpressed Keap1 and underexpressed nuclear factor Nrf2. Conversely, animals in the CCl4 + MLT group underexpressed Keap1 and overexpressed Nrf2 (Figure 1). The overexpression of Keap1 observed in the CCl4 group suggested an inhibitory action on nuclear factor Nrf2. However, the underexpression of Keap1 evidenced in the CCl4 + MLT group suggested that MLT shows a cytoprotective effect on hepatocytes by stimulating the action of Nrf2.
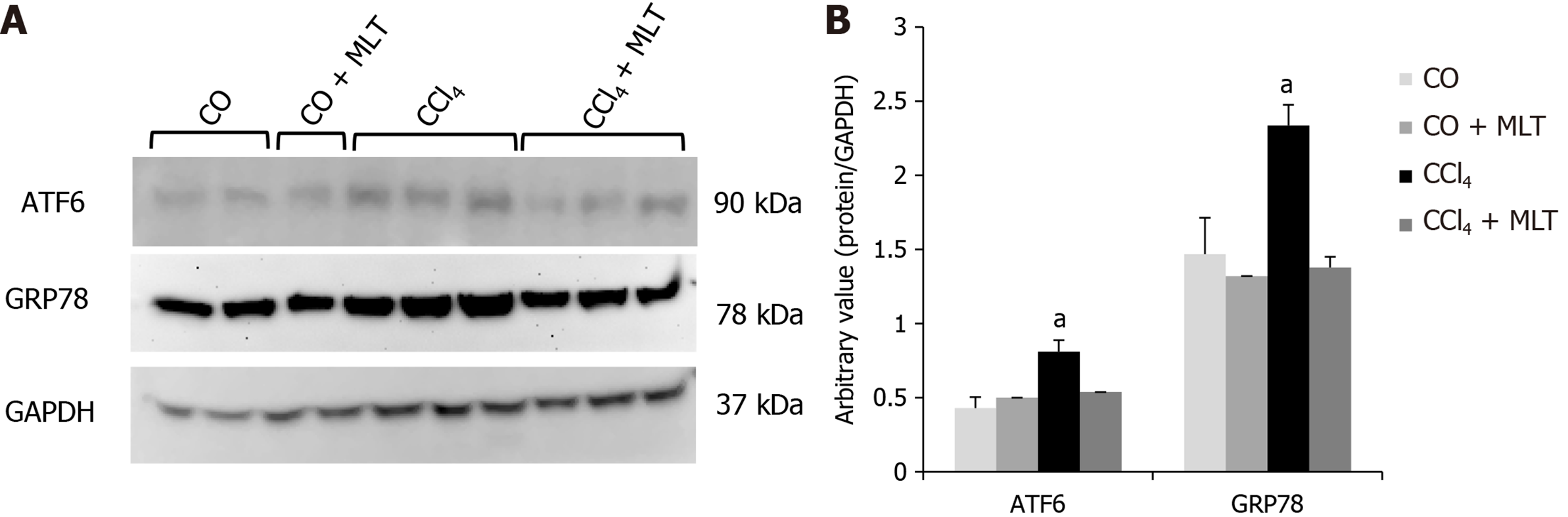
Concerning markers of ER stress, ATF6 and GRP78/BiP expression was strongly increased in animals treated with CCl4 (P < 0.05), while that in the CCl4 + MLT group had expression similar to the control group (Figure 2).
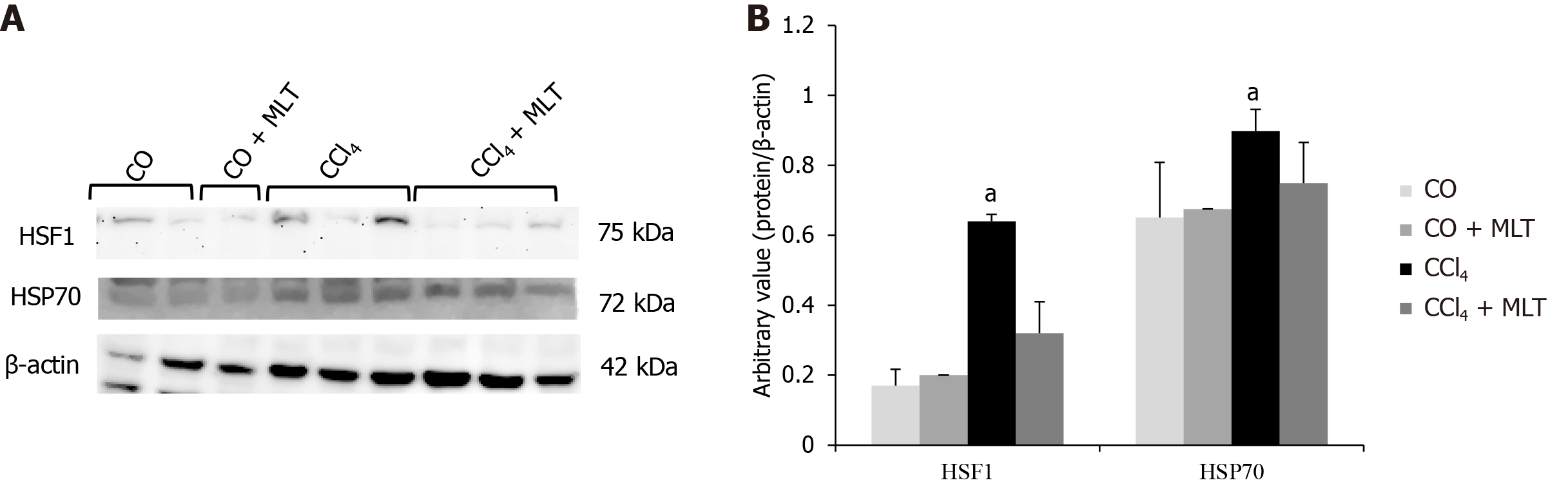
Stressful insults, such as exposure to toxic agents, stimulate HSF1 to act as a master activator of the response of HSPs, including HSP70[26]. HSF1 and HSP70 expression was significantly higher in animals from the CCl4 group compared with the control group and significantly reduced in animals from the CCl4 + MLT group compared with the CCl4 group (Figure 3).
When assessing serum zinc levels in different groups, we observed that the induction of liver injury by CCl4 caused damage to hepatocytes associated with a significant reduction in zinc levels. In contrast, animals treated with CCl4 + MLT showed zinc levels similar to those in the control group, indicating that MLT has a hepatoprotective effect (Table 1).
Many studies have demonstrated the presence of overproduction of free radicals and ROS in chronic liver diseases. ROS are able to oxidize macromolecules or activate transcription factors, resulting in oxidative stress and ER stress[27,28,29] Our study evidenced that MLT treatment in rats with liver injury chemically induced by CCl4 was promising. We observed that MLT was able to activate the Nrf2 pathway and modulate the unfolded protein response and ER stress in rats with liver injury. MLT plays a key role in hepatoprotection, which results in the reduction of hepatic enzymatic levels and oxidative stress.
We evaluated the efficacy of MLT regarding the protection of macromolecules against the oxidative damage during hepatotoxicity process in animals exposed to CCl4. In general, MLT consistently improved the pattern of the hepatic enzymes. Also, this study observed a reduction of oxidative stress in animals that were treated with MLT. With respect to oxidative damage, MLT stimulated the Keap1/Nrf2 pathway and restored the normal expression of the proteins involved in the ER stress.
In the present study, chronic exposure to CCl4 induced liver injury, as evidenced by the significant increase in the liver enzymes under study. MLT treatment protected the liver from toxicity promoted by the toxic agents (CCl4). AST and ALT in the CCl4 group increased significantly compared to the other groups. This analysis characterized a condition of necrosis or alteration of cell membrane permeability. This increase is indicative of acute liver injury and hepatocellular damage[30]. Animals treated with CCl4 + MLT had significantly reduced AST and ALT activities, indicating a significant cytoprotective effect for MLT on hepatocytes[31]. Other studies reported that the use of hepatotoxic agents such as CCl4 and thioacetamide to generate free radicals affects the permeability of hepatocyte membranes and increases serum levels of enzymatic biochemical parameters. The use of antioxidants such as quercetin was able to reduce the release of these enzymes, probably due to the restoration of liver parenchyma[32,33].
The involvement of ROS in liver injury and in the death of hepatocytes exposed to toxic agents has been extensively documented both in vitro and in vivo[8]. It is believed that the changes in redox homeostasis may play a significant role in the pathogenesis of many diseases characterized by chronic inflammation, activation of wound healing, and fibrogenesis[27,28,32,34]. The mechanism of CCl4 toxicity occurs through the generation of highly toxic free radicals. In the present study, this evidence is reinforced by the increase in TBARS levels in the CCl4 group. Among many effects attributed to MLT, we highlight its potent antioxidant effect[20,35-37]. Our results showed that MLT significantly reduced liver LPO, which is consistent with previous reports indicating that MLT is capable of removing free radicals and has a protective effect in experimentally induced hepatotoxicity[20,37,38]. There is evidence that MLT increases the efficiency of the electron transport chain and, as a consequence, reduces the generation of ROS[39,40]. This antioxidant property is due to the double bond existing in its chemical structure, which allows electrons to be transferred to unstable chemical species[40].
The antioxidant enzyme system, represented in this study by SOD, prevents the accumulation of oxygen and hydrogen peroxide, and thus is considered the main line of defense of the body[41]. CCl4 significantly decreased SOD activity, and MLT reversed this process. Reduced SOD activity, along with increased LPO in animals from the CCl4 group, establishes a situation of oxidative stress[41-43]. MLT protects hepatocytes against damage from free radicals by directly scavenging free radicals and stimulating antioxidant enzymes[44,45]. Similar to previous studies, our study found that MLT increased the activity of the antioxidant enzyme SOD, which occurred in parallel with Nrf2 activation[46,47].
Nrf2 is a major transcriptional regulator that secures a vast range of tissues and cells from ROS mediated induction because of its various antioxidant and phase II detoxification enzymes[4]. Furthermore, several chemopreventive compounds can abate tissue fibrosis by enhancing nuclear translocation of Nrf2 and induction of glutathione S-transferases (GSTs) and SOD expression[4]. One of these essential mechanisms responsible for the induction of enzymes in response to stress is the pathway of Nrf2 and its inhibitor Keap1. When translocated into the nucleus, Nrf2 binds to the antioxidant responsive element (ARE), regulating the expression of endogenous antioxidants and proteins involved in the regulation of cell cycle and death[46-50]. In an Nrf2-knockout mouse model after long term CCl4 treatment, the liver fibrosis was aggravated[4].
In this study, Nrf2 expression was significantly reduced, whereas Keap1 expression was greater in animals from the CCl4 group. Studies have shown that Nrf2 plays a protective role in liver disease, with Nrf2-deficient rats being more sensitive and susceptible to liver injury and fibrosis induced by hepatotoxins[51]. Our study showed that the improvement in liver damage was due to MLT treatment. This improvement was due to MLT that is able to activate the nuclear factor Nrf2, resulting in less oxidative stress[51-54]. It has also been documented that Nrf2 activators, a wide variety of compounds such as sulfur-containing compounds, polyphenols, terpenoids, carotenoids, and selenium, exert the cytoprotective effect and emerge to play a part in the antioxidative response, presumably providing a series of protections needed for normal cellular activities. Furthermore, it is widely recognized that reduced Nrf2-mediated antioxidant defense plays a key role in the process of CCl4-induced liver fibrosis. Nrf2 activators thus far exhibit hepatoprotective effects and significantly attenuate liver injury and fibrosis in clinical studies[4]. Kaufman et al in 2020 related that the modulation of the Nrf2 is dependent of zinc[55]. They evaluated cells incubated in zinc deficient medium and evidenced a reduction of the nuclear Nrf2 levels[55]. Zinc deficits aggravated the oxidative stress and reduced Nrf2 nuclear translocation[55-58].
As already observed in the study by Fernandes et al[59], cirrhotic animals showed significantly lower serum levels of zinc when compared to controls, showing an association of zinc deficiency with liver damage in sick animals.
Studies linking zinc and liver diseases often described that the replacement of the zinc element results in an improvement in the clinical and morphological condition[7,59]. We determined the zinc levels in the liver of rats exposed to CCl4. Our results demonstrated reduced levels of zinc in injured livers. MLT treatment was effective in restoring normal zinc levels. A study with old rats treated with MLT showed a link between zinc and MLT. It is believed that MLT is able to modulate zinc turnover, due to the synchronization of circadian patterns of zinc and MLT and the concomitant increase in the levels of both in animals treated with MLT[56-58].
Our results show that MLT treatment in animals treated with CCl4 resulted in an improvement in the liver enzyme pattern, lower oxidative stress, activation of Nrf2, and normalization of serum zinc levels. In addition to these findings, we measured the expression of ATF6 and GRP78/BiP, two important proteins involved in ER stress.
In the presence of stressors, GRP78/BiP is released, leading to the activation of the UPR signaling pathway, including ATF6[60]. Under the action of released GRP78/BiP, ATF6 decouples from the ER and undergoes cleavage in the Golgi system. At that moment, the activation of the nuclear factor (ATF6 50-kDa) that regulates the GRP78/ BiP and GRP94 proteins occurs, and the maintenance of this activation results in ER stress[61].
Physiological or pathological processes that disturb ER homeostasis led to a pathologic response called ER stress, causing the activation of the intracellular signaling pathway called UPR, thereby contributing to the pathogenesis of several conditions, including liver diseases[8,9,29,62]. A growing amount of evidence reinforces that increased ROS production is strongly related to induction of ER stress[6]. Our findings suggest the presence of ER stress in the animals from the CCl4 group, since there was an increase in the expression of ATF6 and GRP78/BiP. The transcription of these chaperones is increased in response to various stimuli that disturb or overload ER function, including exposure to xenobiotics[29]. A significant decrease in the expression of proteins that predict ER stress was observed in animals receiving MLT. Our data are consistent with recent findings showing that MLT reduces ER stress in different models of cell injury[24]. For example, treatment with MLT reduced ER stress and modulated UPR in rabbits with fulminant hepatitis of viral origin, an effect that was associated with a reduction in apoptosis, cell death, and liver damage[24]. MLT also showed a neuroprotective effect through the reduction of ER stress in neuronal cells of newborn rats after hypoxia-ischemia[63]. Typically, ROS are controlled by intracellular antioxidants such as SOD; however, the excess demand in protein folding can overload antioxidant response. In support of this theory, the use of antioxidants has been shown to enhance protein folding and reduce apoptosis in response to ER stress[29].
Another highly regulatory mechanism essential for cellular redox homeostasis and protein folding involves HSPs and the nuclear factor HSF1[29]. Among HSPs, HSP70 is one of the protein families that has been more conserved in evolution, being expressed in the cell both constitutively and inductively. Our data show that animals exposed to CCl4 showed higher expression of HSP70 and HSF1. One of the cellular responses to stress is HSP activation[64]. Several lines of evidence highlight the deleterious effects of HSPs on various human diseases, including cancer, in which case it promotes survival and proliferation of tumor cells and drug resistance[65]. Members of the HSP70 family have been particularly implicated in the pathophysiology and pathogenesis of several liver diseases such as hepatitis B and C, non-alcoholic steatohepatitis, autoimmune hepatitis, primary biliary cirrhosis, and others[26]. Treatment with MLT reduced HSP70 and HSF1 expression.
The exact mechanism leading to xenobiotic-induced cellular stress is still not well understood and may involve multiple factors, such as xenobiotic concentration, time of exposure, mechanism of action, cell type affected, among others. The combination of different cell damages, including oxidative stress, ER stress, UPR, and cytosolic responses, may lead to cell apoptosis[29].
We conclude that MLT acts as a potent antioxidant, promoting the activation of the nuclear factor Nrf2, which allows the reduction in oxidative stress. Concomitantly, we observed the restoration of serum zinc levels, which contributes to numerous hepatic cytoprotective processes. With the reduction of oxidative stress, it is possible to attenuate the ER stress and the unfolded protein response, as well as the cell damage caused by the toxic agent CCl4.
The evaluation of new markers in this model may contribute to a better understanding of other pathophysiological mechanisms of cirrhosis and its complications. MLT may offer a promising therapeutic approach to liver diseases, given its effectiveness in the attenuation of oxidative stress. A major limitation in the application of MLT is that most of the studies were designed especially on rats. More research is needed in clinical trials to approve the potentials of this indolamine in humans.
Chronic liver diseases are characterized by a multistep process that involves several molecules and cellular events to transform a normal parenchyma into a parenchyma with steatosis, increased collagen deposition, fibrosis, and cirrhosis. Melatonin (MLT) is a potent antioxidant molecule that is shown to have a beneficial effect in various pathological situations, due to its action against free radicals.
To reduce the generation of reactive oxygen species would be a way to slow the progression of cell damage observed in liver diseases.
To study the effects of MLT on biochemical analysis, on serum zinc levels, and on oxidative stress in rats exposed to carbon tetrachloride (CCl4), and to evaluate the expression of proteins involved in cell damage, endoplasmic reticular stress, and unfolded protein response in animals with liver injury induced by CCl4 and treated with MLT.
Twenty male Wistar rats (230-250 g) were divided into four groups: Control rats, rats treated with MLT alone, rats treated with CCl4 alone, and rats treated with CCl4 plus MLT. CCl4 was administered as follows: Ten doses every 5 d, ten every 4 d, and 7 every 3 d. MLT was administered intraperitoneally at a dose of 20 mg/kg from the 10th wk to the end of the experiment (16th wk).
Administration of CCl4 caused an increase in liver enzyme levels, a reduction in serum zinc levels, and greater oxidative stress and ER stress. MLT treatment was able to reverse the changes promoted by the toxic agent CCl4.
We conclude that MLT acts as a potent antioxidant, promoting activation of the nuclear factor Nrf2, which allows the reduction in oxidative stress. Concomitantly, we observed the restoration of serum zinc levels, which contributes to numerous hepatic cytoprotective processes. With the reduction of oxidative stress, it is possible to attenuate the ER stress and the unfolded protein response, as well as the cell damage caused by the toxic agent CCl4.
MLT may offer a promising therapeutic approach to liver diseases, given its effectiveness in the attenuation of oxidative stress. A major limitation in the application of MLT is that most of the studies were designed especially on rats. More research is needed in clinical trials to approve the potentials of this indolamine in humans.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Wan CC S-Editor: Wang LL L-Editor: Wang TQ P-Editor: Wang LL
| 1. | Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655-1669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2139] [Cited by in RCA: 2164] [Article Influence: 127.3] [Reference Citation Analysis (0)] |
| 2. | Klaunig JE, Kamendulis LM, Hocevar BA. Oxidative stress and oxidative damage in carcinogenesis. Toxicol Pathol. 2010;38:96-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 607] [Cited by in RCA: 636] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 3. | Klaunig JE, Wang Z, Pu X, Zhou S. Oxidative stress and oxidative damage in chemical carcinogenesis. Toxicol Appl Pharmacol. 2011;254:86-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 308] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 4. | Gong Y, Yang Y. Activation of Nrf2/AREs-mediated antioxidant signalling, and suppression of profibrotic TGF-β1/Smad3 pathway: a promising therapeutic strategy for hepatic fibrosis-A review. Life Sci. 2020;256:117909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 5. | Rashid K, Sinha K, Sil PC. An update on oxidative stress-mediated organ pathophysiology. Food Chem Toxicol. 2013;62:584-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 94] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 6. | Brookheart RT, Michel CI, Listenberger LL, Ory DS, Schaffer JE. The non-coding RNA gadd7 is a regulator of lipid-induced oxidative and endoplasmic reticulum stress. J Biol Chem. 2009;284:7446-7454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 7. | Grüngreiff K, Reinhold D, Wedemeyer H. The role of zinc in liver cirrhosis. Ann Hepatol. 2016;15:7-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 117] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 8. | Brenner C, Galluzzi L, Kepp O, Kroemer G. Decoding cell death signals in liver inflammation. J Hepatol. 2013;59:583-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 583] [Cited by in RCA: 776] [Article Influence: 64.7] [Reference Citation Analysis (0)] |
| 9. | Lenna S, Han R, Trojanowska M. Endoplasmic reticulum stress and endothelial dysfunction. IUBMB Life. 2014;66:530-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 124] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 10. | Touitou Y, Fevre-Montange M, Proust J, Klinger E, Nakache JP. Age- and sex-associated modification of plasma melatonin concentrations in man. Relationship to pathology, malignant or not, and autopsy findings. Acta Endocrinol (Copenh). 1985;108:135-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 50] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Paredes SD, Korkmaz A, Manchester LC, Tan DX, Reiter RJ. Phytomelatonin: a review. J Exp Bot. 2009;60:57-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 216] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 12. | Reiter RJ, Tan DX, Sainz RM, Mayo JC, Lopez-Burillo S. Melatonin: reducing the toxicity and increasing the efficacy of drugs. J Pharm Pharmacol. 2002;54:1299-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 296] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 13. | Reiter RJ, Tan DX, Terron MP, Flores LJ, Czarnocki Z. Melatonin and its metabolites: new findings regarding their production and their radical scavenging actions. Acta Biochim Pol. 2007;54:1-9. [PubMed] |
| 14. | Wasser S, Lim GY, Ong CN, Tan CE. Anti-oxidant ebselen causes the resolution of experimentally induced hepatic fibrosis in rats. J Gastroenterol Hepatol. 2001;16:1244-1253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Mills E, Wu P, Seely D, Guyatt G. Melatonin in the treatment of cancer: a systematic review of randomized controlled trials and meta-analysis. J Pineal Res. 2005;39:360-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 174] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 16. | Siegel D, Bolton EM, Burr JA, Liebler DC, Ross D. The reduction of alpha-tocopherolquinone by human NAD(P)H: quinone oxidoreductase: the role of alpha-tocopherolhydroquinone as a cellular antioxidant. Mol Pharmacol. 1997;52:300-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 189] [Reference Citation Analysis (0)] |
| 17. | Carbajo-Pescador S, García-Palomo A, Martín-Renedo J, Piva M, González-Gallego J, Mauriz JL. Melatonin modulation of intracellular signaling pathways in hepatocarcinoma HepG2 cell line: role of the MT1 receptor. J Pineal Res. 2011;51:463-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 18. | Pavanato A, Tuñón MJ, Sánchez-Campos S, Marroni CA, Llesuy S, González-Gallego J, Marroni N. Effects of quercetin on liver damage in rats with carbon tetrachloride-induced cirrhosis. Dig Dis Sci. 2003;48:824-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 94] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 19. | Cremonese RV, Pereira-Filho AA, Magalhães R, de Mattos AA, Marroni CA, Zettler CG, Marroni NP. [Experimental cirrhosis induced by carbon tetrachloride inhalation: adaptation of the technique and evaluation of lipid peroxidation]. Arq Gastroenterol. 2001;38:40-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Rosa DP, Bona S, Simonetto D, Zettler C, Marroni CA, Marroni NP. Melatonin protects the liver and erythrocytes against oxidative stress in cirrhotic rats. Arq Gastroenterol. 2010;47:72-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8015] [Cited by in RCA: 40251] [Article Influence: 821.4] [Reference Citation Analysis (0)] |
| 22. | Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7899] [Cited by in RCA: 7984] [Article Influence: 169.9] [Reference Citation Analysis (0)] |
| 23. | Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170-3175. [PubMed] |
| 24. | Tuñón MJ, San-Miguel B, Crespo I, Laliena A, Vallejo D, Álvarez M, Prieto J, González-Gallego J. Melatonin treatment reduces endoplasmic reticulum stress and modulates the unfolded protein response in rabbits with lethal fulminant hepatitis of viral origin. J Pineal Res. 2013;55:221-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 25. | Mauriz JL, Molpeceres V, García-Mediavilla MV, González P, Barrio JP, González-Gallego J. Melatonin prevents oxidative stress and changes in antioxidant enzyme expression and activity in the liver of aging rats. J Pineal Res. 2007;42:222-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 63] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 26. | Liu T, Daniels CK, Cao S. Comprehensive review on the HSC70 functions, interactions with related molecules and involvement in clinical diseases and therapeutic potential. Pharmacol Ther. 2012;136:354-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 215] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 27. | Basu S. Carbon tetrachloride-induced lipid peroxidation: eicosanoid formation and their regulation by antioxidant nutrients. Toxicology. 2003;189:113-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 278] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 28. | El-Ansary AK, Kotb M, Rizk MZ, Siddiqi NJ. Prooxidant mechanisms in toxicology. Biomed Res Int. 2014;2014:308625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 29. | Lafleur MA, Stevens JL, Lawrence JW. Xenobiotic perturbation of ER stress and the unfolded protein response. Toxicol Pathol. 2013;41:235-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 30. | Mauriz JL, Matilla B, Culebras JM, González P, González-Gallego J. Dietary glycine inhibits activation of nuclear factor kappa B and prevents liver injury in hemorrhagic shock in the rat. Free Radic Biol Med. 2001;31:1236-1244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 64] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 31. | Subramanian P, Dakshayani KB, Pandi-Perumal SR, Trakht I, Cardinali DP. 24-hour rhythms in oxidative stress during hepatocarcinogenesis in rats: effect of melatonin or alpha-ketoglutarate. Redox Rep. 2008;13:78-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 32. | Srinivasan V, Pandi-Perumal SR, Brzezinski A, Bhatnagar KP, Cardinali DP. Melatonin, immune function and cancer. Recent Pat Endocr Metab Immune Drug Discov. 2011;5:109-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 33. | Bona S, Filippin LI, Di Naso FC, de David C, Valiatti B, Isoppo Schaun M, Xavier RM, Marroni NP. Effect of antioxidant treatment on fibrogenesis in rats with carbon tetrachloride-induced cirrhosis. ISRN Gastroenterol. 2012;2012:762920. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 34. | de David C, Rodrigues G, Bona S, Meurer L, González-Gallego J, Tuñón MJ, Marroni NP. Role of quercetin in preventing thioacetamide-induced liver injury in rats. Toxicol Pathol. 2011;39:949-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 35. | Arendt J. Melatonin and the Mammalian Pineal Gland. London: Chapman & Hall, 1995: 331. [DOI] [Full Text] |
| 36. | Reiter RJ, Tan DX, Manchester LC, Simopoulos AP, Maldonado MD, Flores LJ, Terron MP. Melatonin in edible plants (phytomelatonin): Identification, concentrations, bioavailability and proposed functions. World Rev Nutr Diet. 2007;97:211-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 37. | Novo E, Parola M. Redox mechanisms in hepatic chronic wound healing and fibrogenesis. Fibrogenesis Tissue Repair. 2008;1:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 299] [Cited by in RCA: 254] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 38. | Claustrat B, Brun J, Chazot G. The basic physiology and pathophysiology of melatonin. Sleep Med Rev. 2005;9:11-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 567] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 39. | Anisimov VN, Popovich IG, Zabezhinski MA, Anisimov SV, Vesnushkin GM, Vinogradova IA. Melatonin as antioxidant, geroprotector and anticarcinogen. Biochim Biophys Acta. 2006;1757:573-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 170] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 40. | Reiter RJ, Tan DX, Manchester LC, Qi W. Biochemical reactivity of melatonin with reactive oxygen and nitrogen species: a review of the evidence. Cell Biochem Biophys. 2001;34:237-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 502] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 41. | Wang H, Wei W, Wang NP, Gui SY, Wu L, Sun WY, Xu SY. Melatonin ameliorates carbon tetrachloride-induced hepatic fibrogenesis in rats via inhibition of oxidative stress. Life Sci. 2005;77:1902-1915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 276] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 42. | Oleshchuk O, Ivankiv Y, Falfushynska H, Mudra A, Lisnychuk N. Hepatoprotective Effect of Melatonin in Toxic Liver Injury in Rats. Medicina (Kaunas). 2019;55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 43. | Halliwell B. Free radicals and antioxidants: updating a personal view. Nutr Rev. 2012;70:257-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 487] [Cited by in RCA: 488] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 44. |
Magierowski M, Jasnos K, Pawlik M, Krzysiek-Maczka G, Ptak-Belowska A, Olszanecki R, Kwiecien S, Korbut R, and Brzozowski T The Involvement of Mas Receptor, Nitric Oxide, Prostaglandins, and Sensory Neuropeptides.
|
| 45. | Reiter RJ, Rosales-Corral SA, Manchester LC, Liu X, Tan DX. Melatonin in the biliary tract and liver: health implications. Curr Pharm Des. 2014;20:4788-4801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 46. | Belmont-Díaz J, López-Gordillo AP, Molina Garduño E, Serrano-García L, Coballase-Urrutia E, Cárdenas-Rodríguez N, Arellano-Aguilar O, Montero-Montoya RD. Micronuclei in bone marrow and liver in relation to hepatic metabolism and antioxidant response due to coexposure to chloroform, dichloromethane, and toluene in the rat model. Biomed Res Int. 2014;2014:425070. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 47. | Jung KH, Hong SW, Zheng HM, Lee DH, Hong SS. Melatonin downregulates nuclear erythroid 2-related factor 2 and nuclear factor-kappaB during prevention of oxidative liver injury in a dimethylnitrosamine model. J Pineal Res. 2009;47:173-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 96] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 48. | Aleksunes LM, Slitt AL, Maher JM, Dieter MZ, Knight TR, Goedken M, Cherrington NJ, Chan JY, Klaassen CD, Manautou JE. Nuclear factor-E2-related factor 2 expression in liver is critical for induction of NAD(P)H:quinone oxidoreductase 1 during cholestasis. Cell Stress Chaperones. 2006;11:356-363. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 48] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 49. | Kim HJ, Vaziri ND. Contribution of impaired Nrf2-Keap1 pathway to oxidative stress and inflammation in chronic renal failure. Am J Physiol Renal Physiol. 2010;298:F662-F671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 373] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 50. | Lee IC, Kim SH, Baek HS, Moon C, Kang SS, Kim YB, Shin IS, Kim JC. The involvement of Nrf2 in the protective effects of diallyl disulfide on carbon tetrachloride-induced hepatic oxidative damage and inflammatory response in rats. Food Chem Toxicol. 2014;63:174-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 102] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 51. | Li CM, Li L, Wu J, Bai JY, Sun Y, Huang S, Wang GL. Upregulation of heat shock protein 32 with hemin alleviates acute heat-induced hepatic injury in mice. Cell Stress Chaperones. 2014;19:675-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 52. | Oh CJ, Kim JY, Min AK, Park KG, Harris RA, Kim HJ, Lee IK. Sulforaphane attenuates hepatic fibrosis via NF-E2-related factor 2-mediated inhibition of transforming growth factor-β/Smad signaling. Free Radic Biol Med. 2012;52:671-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 122] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 53. | Farombi EO, Shrotriya S, Na HK, Kim SH, Surh YJ. Curcumin attenuates dimethylnitrosamine-induced liver injury in rats through Nrf2-mediated induction of heme oxygenase-1. Food Chem Toxicol. 2008;46:1279-1287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 214] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 54. | Crespo I, Miguel BS, Laliena A, Alvarez M, Culebras JM, González-Gallego J, Tuñón MJ. Melatonin prevents the decreased activity of antioxidant enzymes and activates nuclear erythroid 2-related factor 2 signaling in an animal model of fulminant hepatic failure of viral origin. J Pineal Res. 2010;49:193-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 55. | Kaufman Z, Salvador GA, Liu X, Oteiza PI. Zinc and the modulation of Nrf2 in human neuroblastoma cells. Free Radic Biol Med. 2020;155:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 56. | Lahiri DK, Chen D, Lahiri P, Rogers JT, Greig NH, Bondy S. Melatonin, metals, and gene expression: implications in aging and neurodegenerative disorders. Ann N Y Acad Sci. 2004;1035:216-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 57. | Mocchegiani E, Santarelli L, Tibaldi A, Muzzioli M, Bulian D, Cipriano K, Olivieri F, Fabris N. Presence of links between zinc and melatonin during the circadian cycle in old mice: effects on thymic endocrine activity and on the survival. J Neuroimmunol. 1998;86:111-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 38] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 58. | Mocchegiani E, Bulian D, Santarelli L, Tibaldi A, Muzzioli M, Pierpaoli W, Fabris N. The immuno-reconstituting effect of melatonin or pineal grafting and its relation to zinc pool in aging mice. J Neuroimmunol. 1994;53:189-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 59. | Fernandes SA, Bona S, Cerski CT, Marroni NP, Marroni CA. ALTERATION OF TASTE BUDS IN EXPERIMENTAL CIRRHOSIS. Is there correlation with human hypogeusia? Arq Gastroenterol. 2016;53:278-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 60. | Lee AS. The ER chaperone and signaling regulator GRP78/BiP as a monitor of endoplasmic reticulum stress. Methods. 2005;35:373-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 709] [Cited by in RCA: 800] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 61. | Wang M, Wey S, Zhang Y, Ye R, Lee AS. Role of the unfolded protein response regulator GRP78/BiP in development, cancer, and neurological disorders. Antioxid Redox Signal. 2009;11:2307-2316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 469] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 62. | Zaouali MA, Boncompagni E, Reiter RJ, Bejaoui M, Freitas I, Pantazi E, Folch-Puy E, Abdennebi HB, Garcia-Gil FA, Roselló-Catafau J. AMPK involvement in endoplasmic reticulum stress and autophagy modulation after fatty liver graft preservation: a role for melatonin and trimetazidine cocktail. J Pineal Res. 2013;55:65-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 81] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 63. | Carloni S, Albertini MC, Galluzzi L, Buonocore G, Proietti F, Balduini W. Melatonin reduces endoplasmic reticulum stress and preserves sirtuin 1 expression in neuronal cells of newborn rats after hypoxia-ischemia. J Pineal Res. 2014;57:192-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 96] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 64. | Ashkenazi L, Haim A. Light interference as a possible stressor altering HSP70 and its gene expression levels in brain and hepatic tissues of golden spiny mice. J Exp Biol. 2012;215:4034-4040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 65. | Bellaye PS, Burgy O, Causse S, Garrido C, Bonniaud P. Heat shock proteins in fibrosis and wound healing: good or evil? Pharmacol Ther. 2014;143:119-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |