Published online Nov 5, 2021. doi: 10.4292/wjgpt.v12.i6.103
Peer-review started: March 1, 2021
First decision: April 18, 2021
Revised: May 2, 2021
Accepted: September 3, 2021
Article in press: September 3, 2021
Published online: November 5, 2021
Processing time: 245 Days and 20.2 Hours
Incomplete excretion rates are problematic for colon capsule endoscopy (CCE). Widely available booster regimens are suboptimal. Recently published data on one day preparation CCE protocol using castor oil appeared effective.
To assess the impact of adding castor oil to a standard split-dose (2-d) preparation in an unselected Western patient cohort.
All patients aged 18 or more referred to our unit for a CCE over a 5-mo period were prospectively recruited. Controls were retrospectively identified from our CCE database. All patients received split bowel preparation with Moviprep® [polyethylene glycol (PEG)-3350, sodium sulphate, sodium chloride, potassium chloride, sodium ascorbate and ascorbic acid for oral solution; Norgine B. V, United States], a PEG-based solution used predominantly in our colonoscopy practice. Control booster regimen included Moviprep® with 750 mL of water (booster 1) on reaching the small bowel. A further dose of Moviprep® with 250 mL of water was given 3 h later and a bisacodyl suppository (Dulcolax®) 10 mg after 8 h, if the capsule was not excreted. In addition to our standard booster regimen, cases received an additional 15 mL of castor oil given at the time of booster 1. A nested case control design with 2:1 ratio (control:case) was employed. Basic demographics, completion rates, image quality, colonic transit time, diagnostic yield and polyp detection were compared between groups, using a student t or chi-square tests as appropriate.
One hundred and eighty-six CCEs [mean age 60 years (18-97), 56% females, n = 104], including 62 cases have been analysed. Indication breakdown included 96 polyp surveillance (51.6%), 42 lower gastrointestinal symptoms (22.6%), 28 due to incomplete colonoscopy (15%), 18 anaemia (9.7%) and 2 inflammatory bowel disease surveillance (1.1%). Overall, CCE completion was 77% (144/186), image quality was adequate/diagnostic in 91% (170/186), mean colonic transit time was 3.5 h (0.25-13), and the polyp detection rate was 57% (106/186). Completion rates were significantly higher with castor oil, 87% cases (54/62) vs 73% controls (90/124), P = 0.01. The number needed to treat with castor oil to result in an additional complete CCE study was 7, absolute risk reduction = 14.52%, 95% confidence interval (CI): 3.06- 25.97. This effect of castor oil on excretion rates was more significant in the over 60 s, P < 0.03, and in females, P < 0.025. Similarly, polyp detection rates were higher in cases 82% (51/62) vs controls 44% (55/124), P = 0.0001, odds ratio 5.8, 95%CI: 2.77-12.21. Colonic transit times were similar, 3.2 h and 3.8 h, respectively. Image quality was similar, reported as adequate/ diagnostic in 90% (56/62) vs 92% (114/124).
In our capsule endoscopy centre, castor oil addition as a CCE booster significantly improved completion rates and polyp detection in an unselected Western cohort.
Core Tip: This is the largest prospective study to date, assessing the impact of castor oil and its novel use as a colon capsule endoscopy (CCE) booster in an unselected cohort. Our study shows that adding castor oil to a simple split-dose CCE bowel preparation regime has a significant impact on capsule excretion rates and polyp detection.
- Citation: Semenov S, Ismail MS, O'Hara F, Sihag S, Ryan B, O'Connor A, O'Donnell S, McNamara D. Addition of castor oil as a booster in colon capsule regimens significantly improves completion rates and polyp detection. World J Gastrointest Pharmacol Ther 2021; 12(6): 103-112
- URL: https://www.wjgnet.com/2150-5349/full/v12/i6/103.htm
- DOI: https://dx.doi.org/10.4292/wjgpt.v12.i6.103
Colon capsule endoscopy (CCE), as a diagnostic tool, has emerged as a viable alternative to colonoscopy. Patient preference, incomplete colonoscopy and contraindications to colonoscopy represent the majority of current CCE indications. There is growing international data to validate its use in colonic polyp screening where it has been shown to outperform colonoscopy in polyp detection[1], and in colonic polyp surveillance where it has been shown to reduce colonoscopy burden in patients with normal CCE[2]. Given its ability to assess the small bowel, CCE also offers a unique non-invasive option for inflammatory bowel disease (IBD) surveillance[3,4].
Allowing for variation between sites, CCE bowel preparation regimens are predominantly polyethylene glycol (PEG) based[5]. Patients are initially given PEG to cleanse the colon and this is followed by boosters to ensure CCE excretion. A complete study requires continuous image capture from the caecum to the haemorrhoidal plexus within the battery life of the capsule. Unfortunately, incomplete CCE remains problematic with significant variation in reported excretion rates ranging from as low as 70% to as high as 88%[6]. A further potential drawback of CCE is inadequate bowel cleansing. A recent meta-analysis reported median rates of adequate cleansing of 78% and 81% with CCE-1 and CCE-2, respectively[7]. This can be explained by its inability to insufflate the colon, aspirate liquids, control its transit speed, and clean the mucosal surface[6]. Despite its technical limitations, CCE appears to have similar bowel preparation rates to colonoscopy[8].
Multiple booster and cleansing agents have been proposed in the literature in an attempt to improve CCE excretion and bowel preparation rates. Among these, a novel use of castor oil as an additional booster agent in CCE practice has been studied. Castor oil is a pale-yellow vegetable oil pressed from castor beans, produced by the Ricinus plant found mainly in tropical regions. Aside from its other medicinal uses, which include skin care, castor oil has been used as a laxative in traditional medicine for hundreds of years[9].
More recently, the use of castor oil in CCE has been described in several studies. In 2016, the addition of castor oil to CCE boosters has been trialled in a small number of dialysis patients with the aim of reducing liquid loading and resulted in 100% excretion rates (20/20)[6]. A further study looked at the addition of castor oil to a one day bowel preparation protocol developed by a Japanese study group for an Ulcerative Colitis cohort, which yielded excretion rates of 93.9% (31/33)[10]. Finally, a multicentre retrospective study in Japan selecting 319 patients receiving a one-day PEG-based CCE regimen in a mixed cohort of faecal immunochemical test positive, screening and lower gastrointestinal (GI) symptom patients, assessed excretion rates with and without castor oil. Of 152 patients receiving castor oil as a CCE booster, 97% excreted the capsule within the life of its battery compared to 81% (136/167) without castor oil[6]. Given this promising data, we aimed to prospectively assess the effectiveness of adding castor oil as an additional booster to our CCE protocol in an unselected patient cohort. Historically our CCE bowel preparation has been based on a 2 Litre split-dose PEG solution (Moviprep®, Norgine, Denmark) followed by boosters made of the same solution, which has been shown to be effective in the available literature[11].
This was a prospective open-label single-centre pilot study assessing the impact of castor oil on CCE performance. This study was approved as a service evaluation project by the process improvement department which is part of the quality safety and risk management directorate in our hospital. All patients referred routinely for CCE over a 5-mo period (November 2019 to March 2020) received an additional 15 mL of castor oil in conjunction to our standard booster regime. All patients were 18 years or older and had no contraindications to CCE or bowel preparation regimens. All patients with a history of IBD, chronic nonsteroidal anti-inflammatory drug use, previous bowel surgery or any other risk for capsule retention, completed a capsule patency test prior to CCE. The outcome of this pilot was then compared to a control (non-castor oil) cohort identified retrospectively from our CCE database.
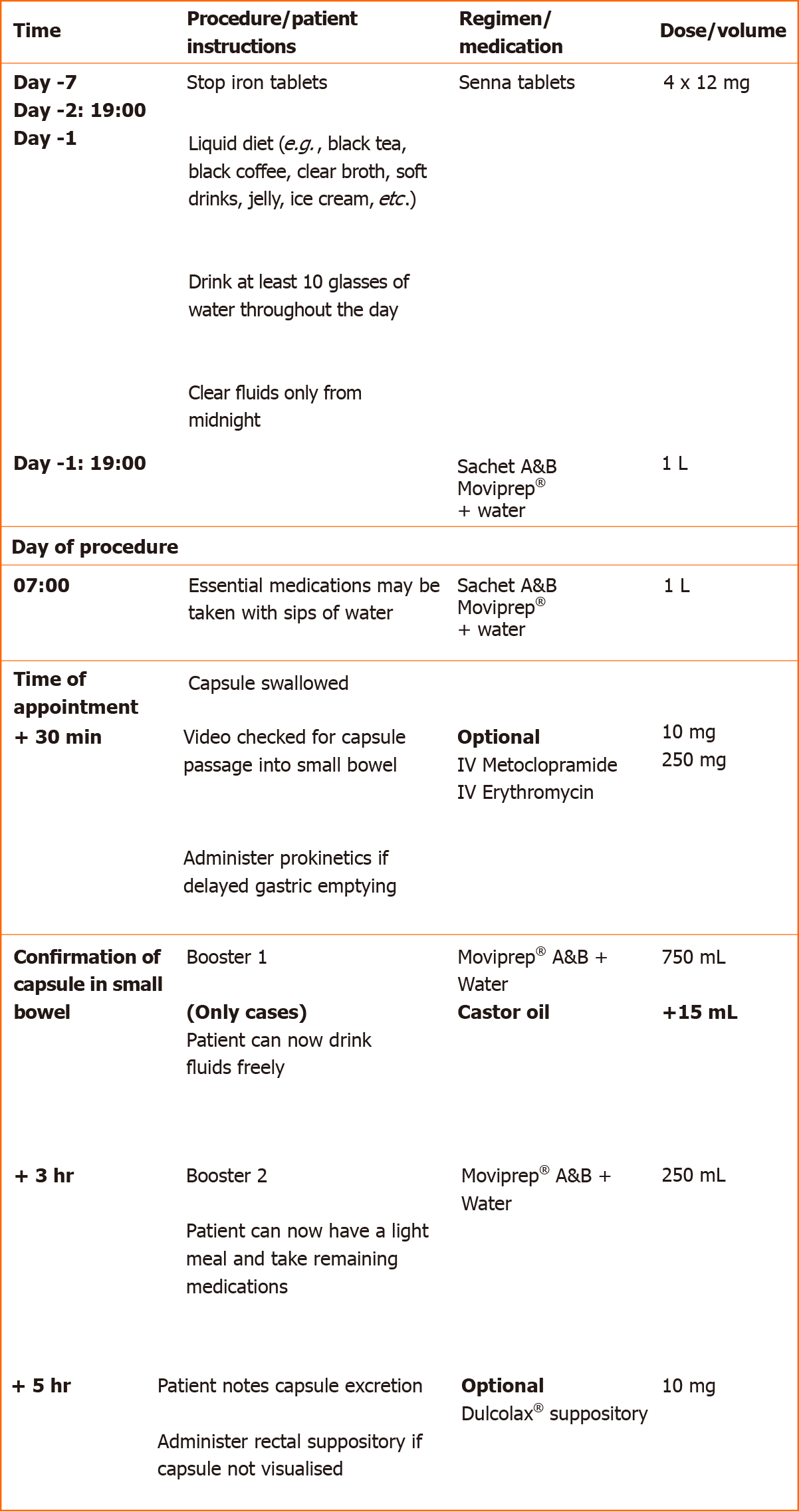
CCE was carried out using the PillCamTM COLON2 (Medtronic, Minneapolis, MN, United States). Figure 1 outlines the bowel preparation protocol used for each CCE procedure.
Two days prior to attending the capsule department for a CCE, all patients received four 12 mg Senna tablets. This was followed by a two-litre split-dose bowel preparation with Moviprep® (PEG-3350, sodium sulphate, sodium chloride, potassium chloride, sodium ascorbate and ascorbic acid for oral solution; Norgine B. V, United States), a PEG-based solution used predominantly in our colonoscopy practice. The patients were instructed to ingest the 1st litre on the evening before, and the 2nd litre on the morning of the procedure. In the event of delayed gastric emptying, recorded as presence of capsule in the stomach 30 min post ingestion, all patients without contraindications received intravenous prokinetics; 10mg of metoclopramide followed by 250 mg erythromycin, if unsuccessful.
Control booster regimen included Moviprep® with 750 mL of water (booster 1) on reaching the small bowel. A further dose of Moviprep® with 250 mL of water was given 3 h later and a bisacodyl suppository (Dulcolax®) 10 mg after 8 h, if the capsule was not excreted. Cases followed the same regimen with the addition of 15 mL of castor oil given with booster 1. The studies were all read by trained CCE readers, unblinded to bowel preparation, and the final reports were reviewed and signed off at our local departmental capsule review board.
A nested case control design was employed with a 2:1 ratio (2 controls:1 case) whereby controls were taken from our capsule database in chronological order without any selection bias.
We recorded patient demographics including age, gender and indication for CCE. CCE excretion/completion was defined as uninterrupted image capture from the caecum to the dentate line within its battery life. In the event of failed capsule excretion, CCE was considered complete if images of the haemorrhoidal plexus were recorded.
Colonic image quality was based on the reader’s overall impression of the bowel preparation and recorded as either “adequate” or “inadequate” at the time of reporting. All CCE procedures were read by trained capsule endoscopists and reports reviewed at weekly capsule review meetings with at least one CCE expert reader present. The cleansing level was evaluated based on a previously validated scale and classified as poor (large amount of faecal residue), fair (enough residue to preclude a completely reliable examination), good (small amount of residue, not enough to interfere with examination) and excellent (no more than small amounts of adherent faeces) for each colonic segment. Examinations scored as ‘poor’ or ‘fair’ in any segment were considered ‘inadequate’, whereas those scored as ‘good’ or ‘excellent’ in all segments were considered ‘adequate’[12,13].
Colonic transit time was automatically generated by the PILLCAM™ SOFTWARE V9 and recorded directly from the CCE report. Findings were recorded and clinically significant findings included: Colonic polyps, cancers, inflammation and bleeding. Extra colonic findings were also documented where present. A CCE positivity rate was calculated by including studies with significant colonic findings as outline above. Adverse events and complications were documented.
Results were compared between the two groups of patients. Statistical analysis employed a student t test and chi-square tests as appropriate, utilising the GraphPad online software. A P value of less than 0.05 was considered statistically significant. Odds ratios (OR), number needed to treat (NNT) and absolute risk reduction were calculated as required. Per protocol analysis was undertaken including patients only who were able to swallow the capsule and took at least some of the study medication.
A total of 186 CCEs have been analysed; 124 controls and 62 cases receiving castor oil with booster 1. In all, the mean age was 60 years of age and 56% were females (104/186). The age and gender breakdown did not statistically differ between the two populations. The following were indications for CCE in order of prevalence; 96 polyp surveillance (51.6%), 42 lower GI symptoms (22.6%), 28 due to incomplete colonoscopy (15%), 18 anaemia (9.7%) and 2 IBD surveillance (1.1%). Allowing for a slightly larger proportion of castor oil patients referred for anaemia work up; the indication breakdown did not significantly vary. Table 1 outlines the breakdown of demo
| Total | With castor oil | Without castor oil | P value | |
| 186 | 62 | 124 | ||
| Age | 0.2365 | |||
| Mean | 60.0 | 62.0 | 59.0 | |
| Range | 18-97 | 22-97 | 18-86 | |
| Gender | 0.8357 | |||
| Male | 82 (44%) | 28 (45.2) | 54 (43.5) | |
| Female | 104 (56%) | 34 (54.8) | 70 (56.5) | |
| Indications | ||||
| Polyp surveillance | 96 | 30/62 (48.4) | 66/124 (53.2) | 0.5362 |
| Lower gastrointestinal symptoms | 42 | 10/62 (16.1) | 32/124 (25.8) | 0.1382 |
| Incomplete colonoscopy | 28 | 11/62 (17.7) | 17/124 (13.7) | 0.4712 |
| Anaemia | 18 | 10/62 (16.1) | 8/124 (6.5) | 0.0355 |
| IBD surveillance | 2 | 1/62 (1.6) | 1/124 (0.8) | 0.6174 |
Overall CCE completion was 77% (144/186). Image quality was adequate and/or diagnostic in 91% (170/186). Mean colonic transit time was 3.5 h with a range of 0.25-13. Overall CCE positivity (presence of significant colonic findings) was 59% (109/186) and the polyp detection rate was 57% (106/186). Additional pathology including colonic diverticulae, small bowel findings and gastric findings were found in 63% (78/124), 22% (27/124) and 12% (15/124) of the overall studies, respectively. There were no cases of colorectal cancer recorded in this study.
Completion rates were significantly higher with castor oil, 87% (54/62) compared with 73% controls (90/124), (P = 0.01). The NNT with castor oil to result in an additional complete CCE study was 7, absolute risk reduction = 14.52%, [95% confidence interval (CI): 3.06- 25.97]. Polyp detection rates were also higher in the castor oil group 82% (51/62) vs 44% (55/124), (P ≤ 0.0001), with an OR of 5.8, (95%CI: 2.77-12.21). Similarly, overall positivity rates, which include studies with polyps, colitis and bleeding, were higher with castor oil, 84% (52/62) vs 46% (57/124), (P ≤ 0.0001), OR of 6.1, (95%CI: 2.85 to 13.11).
Transit times were similar, 3.2 h and 3.8 h, with and without castor oil, respectively. Castor oil did not contribute to poorer image quality as rates were similar between the two groups; reported as adequate and/or diagnostic in 90% (56/62) vs 92% (114/124). Table 2 outlines comparisons between cases and controls.
| Variables | Overall | With castor oil | Without castor oil | P value |
| Capsule completion | 144/186 (77) | 54/62 (87) | 90/124 (73) | 0.0128 |
| Image quality(adequate/diagnostic) | 170/186 (91) | 56/62 (90) | 114/124 (92) | 0.3558 |
| Colonic transit time (hr) | ||||
| Mean: | 3.5 | 3.2 | 3.8 | 0.1779 |
| 95%CI: -2.90 to 0.54 | ||||
| Range: | 0.25-13 | 0.25-13 | 0.5-13 | |
| CCE positivity | 109/186 (59) | 52/62 (84) | 57/124 (46) | < 0.0001 |
| CI: 2.85 to 13.11 | ||||
| OR 6.1 | ||||
| Polyp detection rate | 106/186 (57) | 51/62 (82) | 55/124 (44) | < 0.0001 |
| CI: 2.77 to 12.21 | ||||
| OR 5.8 |
Castor oil appears to improve completion rates. This effect is more significant in the over 60 s, (P < 0.03). Similarly, the effect of the addition of castor oil is more pronounced in females, (P < 0.025). This is shown in Table 3. The NNT with castor oil to have one more complete study was 6 for both female gender (absolute risk reduction 18.5%, 95%CI: 1.94-34.36) and older age (absolute risk reduction 18%, 95%CI: 1.65-34.46). The NNT with castor oil to have one more complete study was 5 for older females (absolute risk reduction 24.36%, 95%CI: 1.23 to 47.48).
| Variable | Total | With castor oil | Without castor oil | P value |
| Overall capsule completion | 144/186 (77) | 54/62 (87) | 90/124 (73) | 0.0128 |
| Age: | ||||
| ≤ 60 | 67/78 (86) | 24/26 (92) | 43/52 (83) | 0.1250 |
| > 60 | 77/108 (71) | 30/36 (83) | 47/72 (65) | 0.0253 |
| Gender: | ||||
| Male | 68/82 (83) | 25/28 (89) | 43/54 (80) | 0.1352 |
| Female | 76/104 (73) | 29/34 (85) | 47/70 (67) | 0.0251 |
| Indication: | ||||
| Polyp surveillance | 75/96 (78) | 28/30 (93) | 47/66 (71) | 0.0075 |
| Lower gastrointestinal symptoms | 36/42 (86) | 10/10 (100) | 26/32 (81) | 0.0450 |
| Incomplete colonoscopy | 19/28 (68) | 7/11 (63) | 12/17 (7) | 0.3502 |
| Anaemia | 12/18 (67) | 8/10 (80) | 4/8 (50) | 0.0899 |
| IBD surveillance | 2/2 (100) | 1/1 (100) | 1/1 (100) | 1.0 |
The male gender appears to be a predictor of higher excretion rates (83% vs 73%), however this does not reach statistical significance, (P = 0.0553). Unsurprisingly, younger age is a significant predictive factor of higher excretion rates (86% vs 71%), (P = 0.0094).
Allowing for low incidence, castor oil did not appear to influence excretion rates in patients referred following an incomplete colonoscopy, anaemia work-up and IBD surveillance.
There were no reported significant adverse events with castor oil and no documented events of patients refusing castor oil. There were also no significant complications associated with CCE procedure or the remainder of bowel preparation regimens, including capsule retention, bowel obstruction, severe abdominal pain, IBD flare and anaphylaxis to medications.
With the increasing demand for solutions in tackling long colonoscopy waiting lists, CCE has become an attractive alternative. Given its potential, the importance of maximising CCE’s performance has been recognised in the literature with a growing body of work looking into improving capsule excretion, image quality, detection of pathology and patient acceptance. Our study is the largest European study to date prospectively assessing the use of castor oil as an addition to a CCE booster regimen in an unselected cohort. Our data suggests small volumes of cheap and readily available castor oil (15 mL) can significantly increase excretion rates (87%) without compromising image quality or colonic transit times. This effect appears more significant in an older population and in females.
The significance of castor oil in completion rates is matched by other studies including the largest to date multicentre retrospective study from Japan, reporting rates as high as 97%[6]. Of note, the authors used a very different and complex preparation regimen comprising of 7 different agents [magnesium citrate, sodium picosulphate (MCSP), Senna, Moviprep®, Mosapride, metoclopramide, Daikenchuto®] and up to 3 L of bowel preparation in one day. This contrasts with our simple split-dose regimen requiring less bowel preparation volumes on the day of the procedure. Our protocol is based on evidence from Denmark showing no added value in adding gastrografin or magnesium citrate in a split-dose regimen[11]. This study included MCSP, a preparation highlighted in recent European guidelines for its safety concerns. Because of hyperosmolarity and magnesium content, solutions containing MCSP are contraindicated in patients with congestive heart disease, hypermagnesemia, rhabdomyolisis, GI ulcerations, and severe impairment of renal function, which can lead to magnesium accumulation[14]. This could be one of the factors contributing to a lower excretion rate in our study (87% vs 97%). A further factor worth noting is that male gender has been identified as a significant predictor for capsule excretion in both studies and could be responsible for higher excretion rates in the Japanese study, which reports a male majority in its castor oil group of 66% (101 vs 51) as opposed to a female majority in our study of 54% (29 vs 25). Similar findings have been reported for standard colonoscopy[15]. Our study reveals that excretion rates also vary by indication, with polyp surveillance and lower GI symptom cohorts doing better, with 93% and 100% excretion rates, respectively.
Unlike other proposed booster agents like sodium phosphate which has been associated with nephropathy and electrolyte disturbances[16], castor oil appears safe and acceptable to patients with no significant side effects reported during the study period. Indeed, unlike other proposed booster regimens, castor oil has been used for thousands of years and is only contraindicated in pregnancy as it is known to induce uterine contractions[17]. Given its lower volume, castor oil has an advantage over larger volume ascorbic acid-based, magnesium-based, sulphate-based, or gastrografin-based booster preparations[11,18,19] as this is more likely to be acceptable to patients. It is important to note, our protocol added 15 mL of castor oil to booster 1, contrasting with some other studies which have utilised higher doses of 30-60 mL with variable efficacy. The excretion rate in our study remains suboptimal, < 90%, which is the minimum standard for adequate bowel preparation in colonoscopy as recommended in recent European guidelines[14]. Whether increasing the dose of castor oil leads to further improvement in completion rates is unclear and warrants further investigation.
Oral ingestion of castor oil stimulates lipases in the small intestine to produce ricinoleic acid which in turn produces a strong laxative effect[9]. Reassuringly, this agent has not had an effect on the overall colonic transit rates as seen in our study. This finding is consistent with previous studies suggesting this effect is selective to small bowel mucosa, by activating intestinal EP3 receptors, and not the colon[17], in turn preserving the diagnostic value of CCE. It is also important to note that overall image quality appears to be unchanged despite castor oil’s effect on small bowel transit which can result in the capsule reaching the colon prematurely, i.e., before colonic cleansing is complete with a split dose PEG regimen.
The authors acknowledge limitations of this being a single centre study. This can, however, also be viewed as a strength as this ensured that all patients received a high quality and uniform CCE procedure in accordance with our departmental protocol. Secondly, this study incorporates a retrospective control cohort which can contribute to a selection bias. Thirdly, due to a departmental polyp surveillance initiative which overlapped with the period of this study, our patient cohort was skewed by a large proportion (52%) of CCE patients referred for polyp surveillance. This resulted in a particularly high overall polyp detection rate of 57%. Correlation with colonoscopy could be of benefit but this data was not available as most of CCE patients did not require a short-term follow up colonoscopy within the period of the study. This does not affect the validity of our data as cases and controls did not vary significantly by age, gender or CCE indication. Surprisingly, despite a smaller proportion of castor oil CCEs referred for polyp surveillance compared to the non-castor oil group (48.4% vs 53.2%), polyp detection rates were almost twice as high (44% vs 82%). One potential reason for this is the higher completion rates leading to more frequent visualisation of the entire colonic mucosa and increased detection of left sided lesions. This highlights the value of castor oil in CCE bowel preparation and its potential as an alternative tool in polyp screening or surveillance.
In our capsule endoscopy centre, the addition of a single 15 mL dose of castor oil to booster 1, as part of a simple split dose Moviprep® CCE protocol, appears safe, acceptable by patients and significantly improves completion rates and polyp detection in an unselected cohort.
Colon capsule endoscopy (CCE) has emerged as a valuable tool in gastroenterology. There remains significant variation in bowel preparation and booster preparations between capsule endoscopy centres. Currently, there is limited data available on the use of castor oil as an additional agent in booster regimens for CCE. Our study is the largest study to date that assesses the use of castor oil in CCE procedures prospectively in a western population.
Our capsule endoscopy centre recognises the suboptimal completion rates of CCE, in our centre and worldwide, and investigates the addition of castor oil in improving this. With this study, we aim to add to the limited available data on castor oil in CCE preparation regimens and highlight the need for further research.
Our main objective was to assess the impact of adding castor oil to a standard split-dose (2-d) preparation in an unselected Western patient cohort in our CCE practice. Our secondary objectives included studying the impact of castor oil on diagnostic yield and identifying patient factors associated with CCE completion and/or more likely to benefit from castor oil. Our study suggests that adding castor oil is significantly associated with higher capsule completion rates and in turn, higher diagnostic yield. This highlights the need for further research in this field, as completion rates is a recognised limitation of this diagnostic test.
Our study identified a retrospective “control” arm (without castor oil) and collected data on a prospective “cases” arm (with castor oil), employing a 2:1 nested control: case design, in assessing the benefit of adding castor oil to a 2-d bowel preparation regimen in our CCE practice. We utilised student t and chi-square tests when comparing basic demographics, completion rates, image quality, colonic transit time, diagnostic yield and polyp detection between the two groups. This was a novel study methodology, with respect to castor oil use, yet to be replicated in other centres.
Our study evaluated 186 CCE procedures (62 cases and 124 controls). We found that overall CCE completion was 77% and was significantly higher in the castor oil group with 87% vs 73%. This effect of castor oil appears to be more effective in older populations and females. Interestingly, positivity rates and polyp detection rates also increased with the addition of castor oil, 84% vs 46% and 82% vs 44%, respectively. Reassuringly, adding castor oil did not reduce image quality or colonic transit time.
What are the new theories that this study proposes? – Castor oil not only improves completion rates but also has potential to improve diagnostic yield of CCE. Castor oil appears safe and acceptable by patients and can be used in an unselected cohort with little to no adverse events. What are the new methods that this study proposed? – This study proposes the addition of low dose castor oil as a booster agent to a standard split-dose CCE bowel preparation.
What is the direction of the future research? - There is a need to explore and expand on research of using castor oil in CCE in different populations, alternative doses and in combination with other bowel preparation regimens, with the aim of improving CCE performance parameters.
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: Irish Society of Gastroenterology.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Ireland
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Chen HB, Gweon TG, Nakaji K S-Editor: Liu M L-Editor: A P-Editor: Liu JH
| 1. | Blanes-Vidal V, Nadimi ES, Buijs MM, Baatrup G. Capsule endoscopy vs. colonoscopy vs. histopathology in colorectal cancer screening: matched analyses of polyp size, morphology, and location estimates. Int J Colorectal Dis. 2018;33:1309-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 2. | Kroijer R, Kobaek-Larsen M, Qvist N, Knudsen T, Baatrup G. Colon capsule endoscopy for colonic surveillance. Colorectal Dis. 2019;21:532-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 3. | Hilmi I, Kobayashi T. Capsule endoscopy in inflammatory bowel disease: when and how. Intest Res. 2020;18:265-274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Bruining DH, Oliva S, Fleisher MR, Fischer M, Fletcher JG; BLINK study group. Panenteric capsule endoscopy versus ileocolonoscopy plus magnetic resonance enterography in Crohn's disease: a multicentre, prospective study. BMJ Open Gastroenterol. 2020;7:e000365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 5. | Kastenberg D, Burch WC Jr, Romeo DP, Kashyap PK, Pound DC, Papageorgiou N, Sainz IF, Sokach CE, Rex DK. Multicenter, randomized study to optimize bowel preparation for colon capsule endoscopy. World J Gastroenterol. 2017;23:8615-8625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Ohmiya N, Hotta N, Mitsufuji S, Nakamura M, Omori T, Maeda K, Okuda K, Yatsuya H, Tajiri H. Multicenter feasibility study of bowel preparation with castor oil for colon capsule endoscopy. Dig Endosc. 2019;31:164-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 7. | Spada C, Pasha SF, Gross SA, Leighton JA, Schnoll-Sussman F, Correale L, González Suárez B, Costamagna G, Hassan C. Accuracy of First- and Second-Generation Colon Capsules in Endoscopic Detection of Colorectal Polyps: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2016;14:1533-1543.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 8. | Kastenberg D, Bertiger G, Brogadir S. Bowel preparation quality scales for colonoscopy. World J Gastroenterol. 2018;24:2833-2843. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 104] [Cited by in RCA: 160] [Article Influence: 22.9] [Reference Citation Analysis (10)] |
| 9. | WATSON WC, GORDON RS Jr. Studies on the digestion, absorption and metabolism of castor oil. Biochem Pharmacol. 1962;11:229-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 50] [Article Influence: 1.9] [Reference Citation Analysis (1)] |
| 10. | Okabayashi S, Kobayashi T, Nakano M, Toyonaga T, Ozaki R, Tablante MC, Kuronuma S, Takeuchi O, Hibi T. A Simple 1-Day Colon Capsule Endoscopy Procedure Demonstrated to be a Highly Acceptable Monitoring Tool for Ulcerative Colitis. Inflamm Bowel Dis. 2018;24:2404-2412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Kroijer R, Dyrvig AK, Kobaek-Larsen M, Støvring JO, Qvist N, Baatrup G. Booster medication to achieve capsule excretion in colon capsule endoscopy: a randomized controlled trial of three regimens. Endosc Int Open. 2018;6:E1363-E1368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Calderwood AH, Lai EJ, Fix OK, Jacobson BC. An endoscopist-blinded, randomized, controlled trial of a simple visual aid to improve bowel preparation for screening colonoscopy. Gastrointest Endosc. 2011;73:307-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 13. | González-Suárez B, Pagés M, Araujo IK, Romero C, Rodríguez de Miguel C, Ayuso JR, Pozo À, Vila-Casadesús M, Serradesanferm A, Ginès À, Fernández-Esparrach G, Pellisé M, López-Cerón M, Flores D, Córdova H, Sendino O, Grau J, Llach J, Serra-Burriel M, Cárdenas A, Balaguer F, Castells A. Colon capsule endoscopy versus CT colonography in FIT-positive colorectal cancer screening subjects: a prospective randomised trial-the VICOCA study. BMC Med. 2020;18:255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 14. | Hassan C, East J, Radaelli F, Spada C, Benamouzig R, Bisschops R, Bretthauer M, Dekker E, Dinis-Ribeiro M, Ferlitsch M, Fuccio L, Awadie H, Gralnek I, Jover R, Kaminski MF, Pellisé M, Triantafyllou K, Vanella G, Mangas-Sanjuan C, Frazzoni L, Van Hooft JE, Dumonceau JM. Bowel preparation for colonoscopy: European Society of Gastrointestinal Endoscopy (ESGE) Guideline - Update 2019. Endoscopy. 2019;51:775-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 407] [Cited by in RCA: 351] [Article Influence: 58.5] [Reference Citation Analysis (4)] |
| 15. | Aljarallah B, Alshammari B. Colonoscopy completion rates and reasons for incompletion. Int J Health Sci (Qassim). 2011;5:102-107. [PubMed] |
| 16. | Ehrenpreis ED, Parakkal D, Semer R, Du H. Renal risks of sodium phosphate tablets for colonoscopy preparation: a review of adverse drug reactions reported to the US Food and Drug Administration. Colorectal Dis. 2011;13:e270-e275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Tunaru S, Althoff TF, Nüsing RM, Diener M, Offermanns S. Castor oil induces laxation and uterus contraction via ricinoleic acid activating prostaglandin EP3 receptors. Proc Natl Acad Sci U S A. 2012;109:9179-9184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 124] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 18. | Kakugawa Y, Saito Y, Saito S, Watanabe K, Ohmiya N, Murano M, Oka S, Arakawa T, Goto H, Higuchi K, Tanaka S, Ishikawa H, Tajiri H. New reduced volume preparation regimen in colon capsule endoscopy. World J Gastroenterol. 2012;18:2092-2098. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 40] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 19. | Hartmann D, Keuchel M, Philipper M, Gralnek IM, Jakobs R, Hagenmüller F, Neuhaus H, Riemann JF. A pilot study evaluating a new low-volume colon cleansing procedure for capsule colonoscopy. Endoscopy. 2012;44:482-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |