Published online Aug 8, 2020. doi: 10.4292/wjgpt.v11.i3.59
Peer-review started: December 29, 2019
First decision: March 27, 2020
Revised: May 17, 2020
Accepted: May 21, 2020
Article in press: May 21, 2020
Published online: August 8, 2020
Processing time: 219 Days and 20.8 Hours
Malnutrition affects 40%-90% of patients with cirrhosis of the liver. L3 skeletal muscle index (L3SMI) is presently accepted as the most objective and quantitative measure available for sarcopenia, a surrogate marker of malnutrition. L3SMI application is, however, limited by non-availability of computed tomography scanning in remote areas, cost, need for extensive training, and the risk of exposure to radiation. Therefore, an alternative dependable measure with wider availability is needed. Malnutrition causes sarcopenia not only in skeletal muscles but also in other muscular structures such as the psoas muscle, diaphragm and tongue. We therefore hypothesised that the tongue, being easily accessible for inspection and for measurement of thickness using ultrasonography, may be used to document sarcopenia.
To measure and compare tongue thickness in healthy individuals and in patients with cirrhosis of the liver and to study its correlation with conventional prognostic scores for patients with cirrhosis of the liver.
Tongue thickness was measured using ultrasonography. One hundred twenty subjects of either gender aged 18 to 65 years were studied, with 30 subjects in each group. The tongue thickness was compared between groups based on “Child Turcotte Pugh” (CTP) scores. The correlations between measured tongue thickness and “Model for end stage liver disease” (MELD) score and between age and measured tongue thickness were also assessed.
Mean tongue thickness (mean ± SD) in patients with CTP class A, B and C was 4.39 ± 0.39 cm, 4.19 ± 0.53 cm, and 3.87 ± 0.42, respectively, and was 4.33 ± 0.49 cm in normal healthy individuals. Significant differences were seen in tongue thickness between patients with CTP class C and those with CTP class A and B (P < 0.05). Patients with CTP class C also had a significantly reduced tongue thickness than normal individuals (P < 0.05). However, no significant difference was seen in tongue thickness between patients with CTP class A and B and normal individuals. A statistically significant, negative correlation was found between MELD score and tongue thickness (r = -0.331) (P < 0.001). No correlation was observed between L3SMI and MELD score (r = 0.074, P = 0.424). L3SMI (mean ± SD) in healthy subjects was 39.66 ± 6.8 and was 38.26 ± 8.88 in patients with CTP class C, and the difference was not significant. No significant correlation was found between age of the patients and tongue thickness. Intra-class correlation coefficient was used to determine the reliability of the tongue thickness measurements. The intra-class correlation coefficient was 0.984 (95%CI: 0.979-0.989) and was indicative of good reliability.
Tongue thickness measured by ultrasonography, correlates significantly with the severity of liver disease, as assessed by CTP and MELD scores. The patients with a CTP score ≥ 10 have significantly reduced tongue thickness as compared to normal individuals and those with less severe liver disease and CTP scores of 5-9. No significant difference in tongue thickness was found between healthy individuals and CTP class A and B patients.
Core tip: Sarcopenia has implications for the management and outcome of patients with cirrhosis of the liver and is therefore included in prognostication. However, the only objective and reproducible measure for sarcopenia using computed tomography (CT) scanning which measures skeletal muscle thickness, is the L3 skeletal muscle index (L3SMI). However, the application of L3SMI is restricted by the need for CT scanning. Compared with CT scan measured L3SMI, tongue thickness can easily be measured in a reproducible manner and accurately with minimal training using ultrasonography and as suggested by this study, is a more sensitive indicator of sarcopenia. Further studies are required to validate these findings and propose tongue thickness as a tool to diagnose sarcopenia.
- Citation: Tandon M, Singh H, Singla N, Jain P, Pandey CK. Tongue thickness in health vs cirrhosis of the liver: Prospective observational study. World J Gastrointest Pharmacol Ther 2020; 11(3): 59-68
- URL: https://www.wjgnet.com/2150-5349/full/v11/i3/59.htm
- DOI: https://dx.doi.org/10.4292/wjgpt.v11.i3.59
Malnutrition has been estimated to affect 50%-90% of patients with cirrhosis of the liver[1]. However, malnutrition is frequently overlooked, in part because nutritional assessment can be difficult in patients with cirrhosis due to fluid retention and because patients with cirrhosis may develop simultaneous loss of skeletal muscle and gain of adipose tissue, culminating in the condition of “sarcopenic obesity”[1]. Sarcopenia is characterised by the loss of muscle mass and is a surrogate marker of malnutrition[2]. Severity of liver disease is assessed using “Model for end stage liver disease” (MELD) score, which is calculated by an online calculator using values for serum creatinine, bilirubin and the international normalised value for prothrombin time. The MELD score is also used to decide upon organ allocation for liver transplant[3], and currently does not include a measure of sarcopenia. Researchers have suggested changes in the MELD score calculation to include a measure of sarcopenia, considering its prognostic implications[4]. Similarly, the “Child Turcotte Pugh” (CTP) score, a conventional scoring system for severity of liver disease based on values of serum bilirubin, albumin, international normalised value for prothrombin time, and measures of encephalopathy and ascites, do not include any measure of sarcopenia[5]. L3 skeletal muscle index (L3SMI) is presently accepted as the most objective and quantitative measure of sarcopenia[6]. However, non-availability of computed tomography (CT) scanning in remote areas, cost, and exposure to radiation are limitations to the use of L3SMI; besides, the use of CT for documenting and quantifying sarcopenia can be justified only in patients who have an indication for CT as part of their standard medical care due to risks of radiation exposure. An optimal index for sarcopenia in terms of availability, reproducibility, practicality, and of prognostic significance is therefore needed and remains a challenging issue.
Malnutrition causes sarcopenia in several muscular structures besides the much-studied lumbar muscles, such as the diaphragm, psoas muscle and tongue[7-9]. In the quest for ultrasonography (USG)-based, bedside targets for documenting sarcopenia, we hypothesised that the tongue being a muscular structure affected by sarcopenia with easy access for inspection and measurement, may also be used to quantify and document sarcopenia. Therefore, a prospective study was conducted with the primary objective to measure and compare tongue thickness in healthy individuals and in patients with cirrhosis of the liver. The secondary objective was to determine the correlation between tongue thickness and conventional prognostic scores for patients with cirrhosis of the liver.
The study was performed at a tertiary care institution after approval by the institutional ethics committee. Consent for the study protocol was obtained from the study subjects. To study 30% difference with power of 80 and type II error of 5%, we needed to study 30 subjects in each group. A total of 120 subjects, who satisfied the inclusion criteria were enrolled and studied from May 2017 to October 2018.
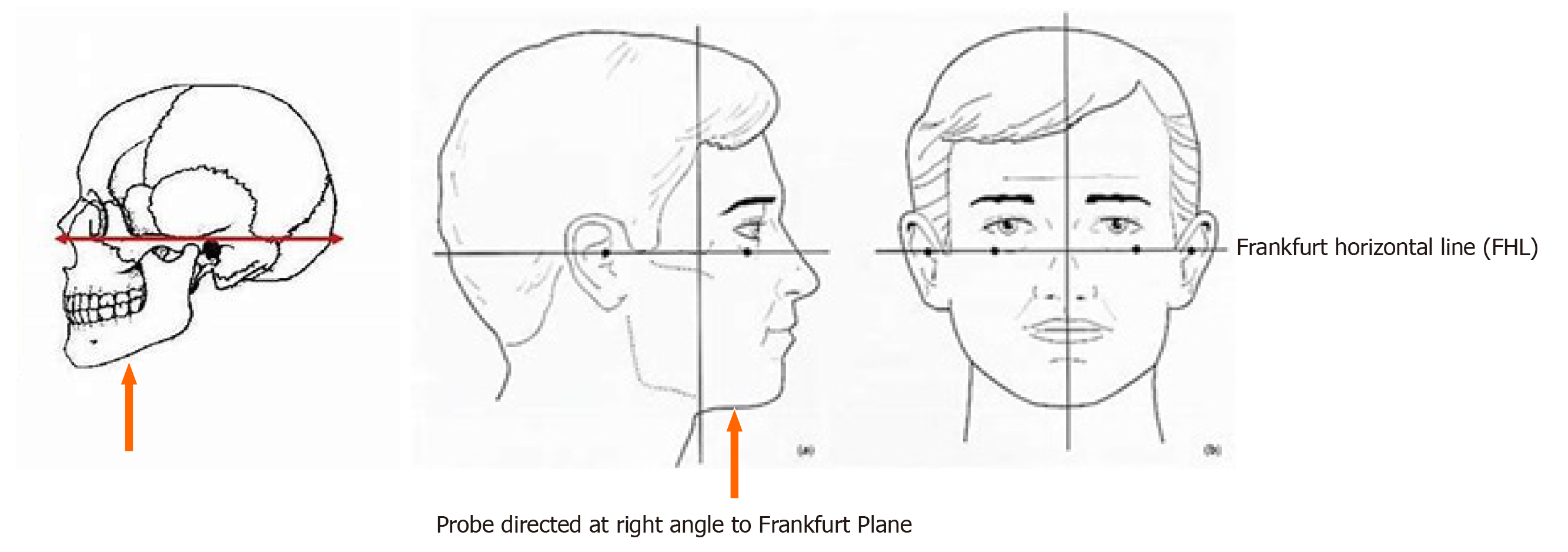
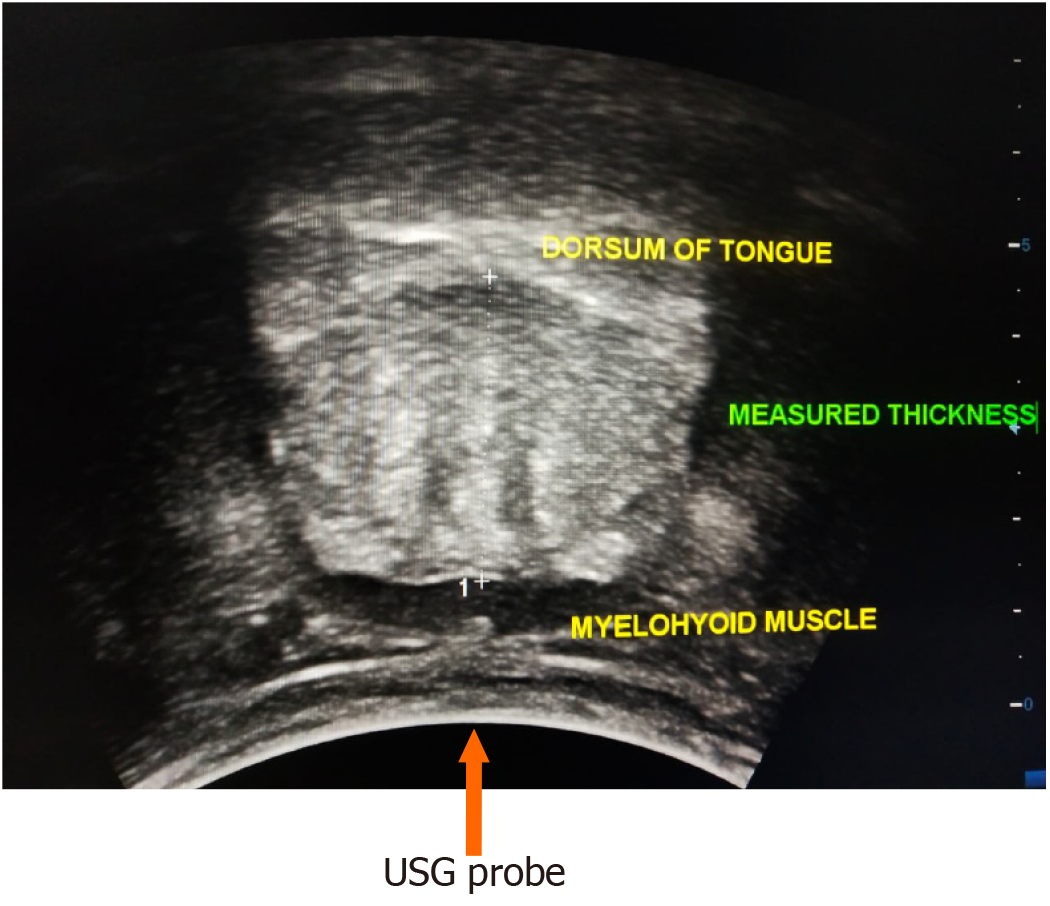
Patients with cirrhosis of the liver due to any aetiology and healthy individuals aged 18-65 years and a body mass index (BMI) > 18 and < 30, visiting the hospital for reasons other than illness, were included in the study. Individuals aged less than 18 years and more than 65 years, patients with acute liver failure and those with glossitis were not included in the study. Tongue thickness was measured using USG with a 3.75 MHz convex probe while the subjects were seated in an upright position. The subjects were instructed to swallow their saliva several times to set the tongue at the resting position, and then the ultrasonic measurements were carried out. The measurement points were determined on the upper and lower surfaces of the lingual muscles in the centre of the plane, perpendicular to the ‘Frankfurt horizontal plane’ in a frontal section (Figure 1). The Frankfurt horizontal plane is formed by drawing a straight horizontal line from the top of the ear canal to the bottom border of the eye along either side of the human skull. This line is called the Frankfurt horizontal line[10]. The vertical distance was measured from the surface of the mylohyoid muscle to the tongue dorsum (Figure 2). Measurements were performed thrice in freeze-frame when the tongue was restored to the resting position after swallowing saliva, and the mean values were obtained. Tongue thickness was measured for all the study subjects, but the L3SMI was calculated from CT scans only for patients with CTP class C who were being investigated for liver transplant and for healthy individuals who were evaluated as possible organ donors. MELD scores were calculated using the online calculator at https://www.mdcalc.com/meld-score-model-end-stage-liver-disease-12-older. Tongue thickness was compared between the groups based on CTP scores. Correlations were also determined between measured tongue thickness and MELD score, and between age and measured tongue thickness.
Data are presented as mean ± SD or frequencies (percentage) and were analysed using SPSS 23.0 software. One-way ANOVA was used to test the significance of parametric data and the Kruskal-Wallis test for non-parametric data. Comparison of categorical data was carried out using the chi square test/Fisher’s exact test. Continuous data were compared by the student t-test/Mann-Whitney test, as applicable. A P value less than 0.05 was considered significant. Intra-class correlation coefficient (ICC) was used to determine the reliability and agreement of the tongue thickness measurements.
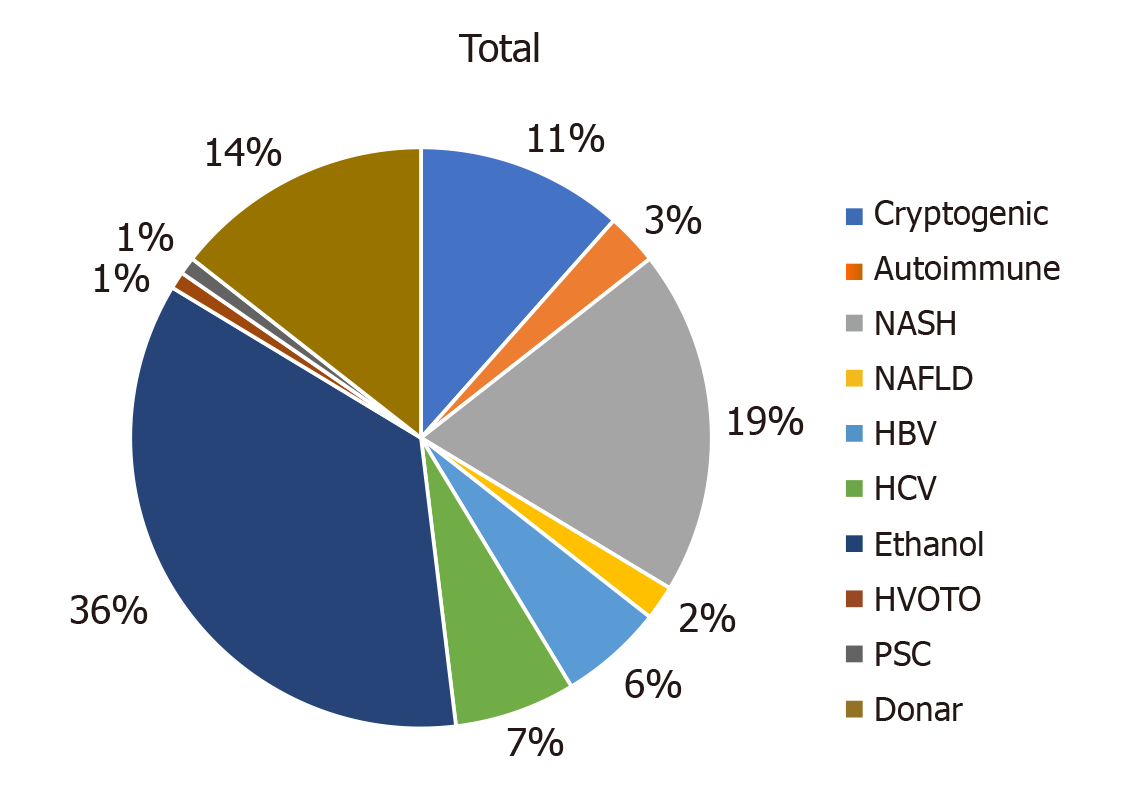
Of 120 patients, 96 were males and 24 were females with a mean age of 47.12 years. The various aetiologies of cirrhosis were ethanol-related, non-alcoholic steato-hepatitis, cryptogenic, hepatitis B virus, hepatitis C virus, autoimmune, hepatic vein outflow tract obstruction, non-alcoholic fatty liver disease, and primary sclerosing cholangitis (Figure 3).
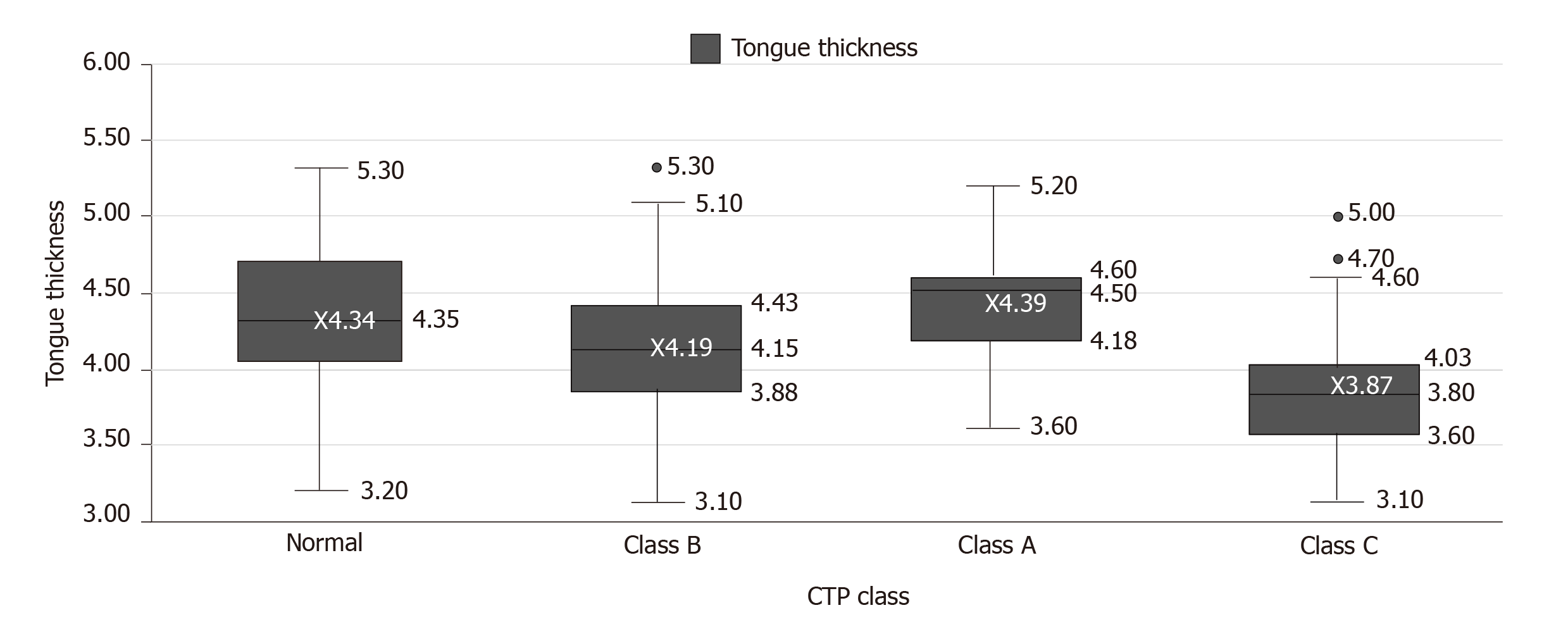
Mean tongue thickness in patients with CTP class A was 4.39 ± 0.39 cm (range 4.25-4.53), in patients with CTP class B was 4.19 ± 0.53 cm (range 3.99-4.39), in patients with CTP class C was 3.87 ± 0.42 cm (range 3.71- 4.02) and in normal healthy individuals was 4.33 ± 0.49 cm (range 4.15-4.51) (Table 1; Figure 4).
| Study group | mean ± SD | Median (range) |
| Child class A | 4.39 ± 0.39 cm | 4.50 cm (range 4.25-4.53) |
| Child class B | 4.19 ± 0.53 cm | 4.15 cm (range 3.99-4.39) |
| Child class C | 3.87 ± 0.42 cm | 3.80 cm (range 3.71-4.02) |
| Normal (healthy) subjects | 4.33 ± 0.49 cm | 4.35 cm (range 4.15-4.51) |
A significant difference was seen in tongue thickness between patients with CTP class C and those with CTP class A and B (P < 0.05). Patients with CTP class C also had significantly reduced tongue thickness than normal individuals (P < 0.05). However, no significant difference was observed in tongue thickness between patients with CTP class A and B and normal individuals (Table 2).
| (I) CTP class | (J) CTP class | Mean difference (I-J) | Std. error | Significance | 95%CI | |
| Lower bound | Upper bound | |||||
| Class A | Class B | 0.2000 | 0.1186 | 0.335 | −0.109 | 0.509 |
| Class C | 0.5233a | 0.1186 | 0.000 | 0.214 | 0.832 | |
| Normal | 0.0567 | 0.1186 | 0.964 | −0.252 | 0.366 | |
| Class B | Class A | −0.2000 | 0.1186 | 0.335 | −0.509 | 0.109 |
| Class C | 0.3233a | 0.1186 | 0.037 | 0.014 | 0.632 | |
| Normal | −0.1433 | 0.1186 | 0.623 | −0.452 | 0.166 | |
| Class C | Class A | −0.5233a | 0.1186 | 0.000 | −0.832 | -0.214 |
| Class B | −0.3233a | 0.1186 | 0.037 | −0.632 | -0.014 | |
| Normal | −0.4667a | 0.1186 | 0.001 | −0.776 | -0.158 | |
| Normal | Class A | −0.0567 | 0.1186 | 0.964 | −0.366 | 0.252 |
| Class B | 0.1433 | 0.1186 | 0.623 | −0.166 | 0.452 | |
| Class C | 0.4667a | 0.1186 | 0.001 | 0.158 | 0.776 | |
mean (± SD) MELD score in patients with CTP class A, B and C was 9.63 ± 2.24, 13.90 ± 2.96 and 25.37 ± 7.92, respectively.
A statistically significant, negative correlation was found between MELD score and tongue thickness (r: −0.331) (P < 0.001) (Table 3).
No significant correlation was found between age of the patients and tongue thickness by USG (Table 4).
| Tongue thickness | Age | ||
| Tongue thickness | Pearson correlation | 1 | −0.081 |
| Significance (2-tailed) | 0.382 | ||
| n | 120 | 120 | |
| Age | Pearson correlation | −0.081 | 1 |
| Significance (2-tailed) | 0.382 | ||
| n | 120 | 120 | |
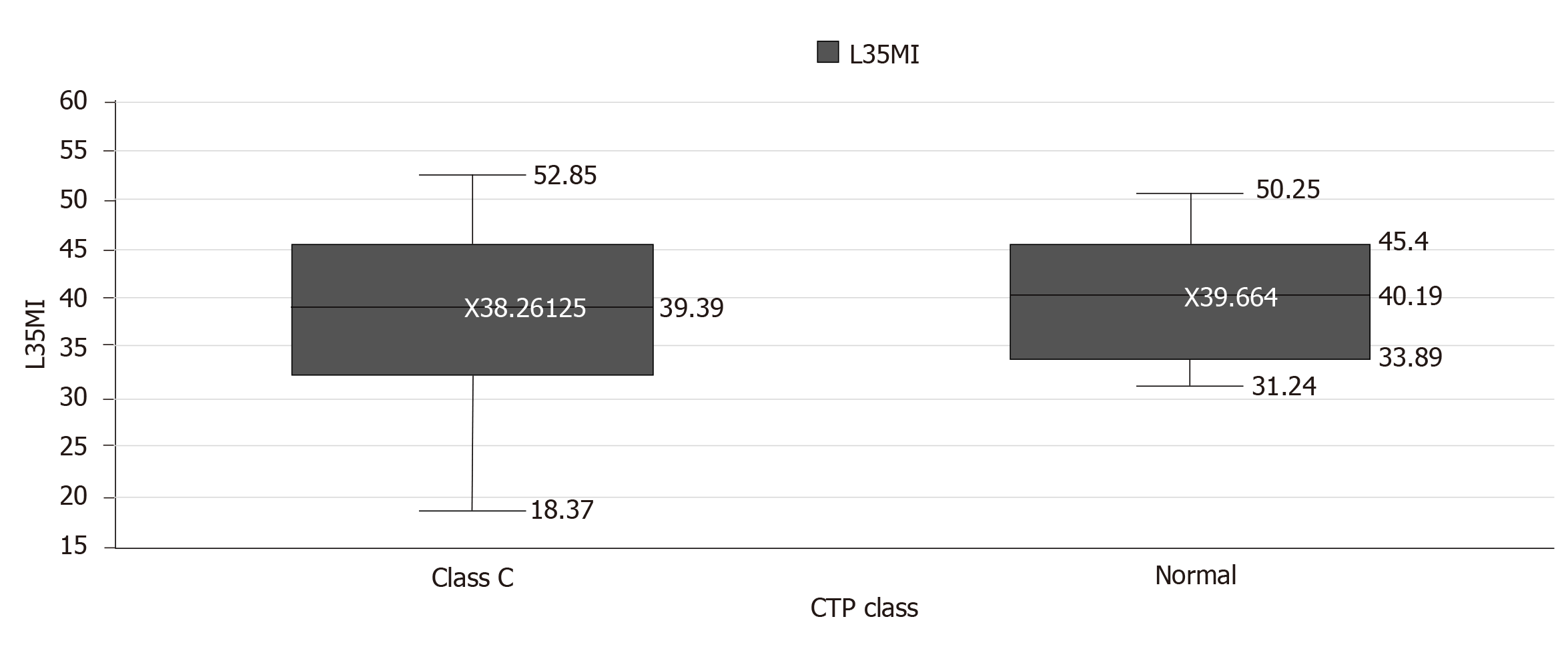
No significant correlation was found between tongue thickness and L3SMI (P = 0.83) (Table 5). In healthy subjects, the mean (± SD) L3SMI value was 39.66 ± 6.8, and was 38.26 ± 8.88 in patients of CTP class C. The difference was not significant (P = 0.63) (Table 6). Barring the outliers, in 2 healthy subjects and in 2 CTP class C patients, the median L3SMI was 40.19 (33.89-45.4) and 39.39 (32.68-45.35), respectively (Figure 5). ICC was used to determine the reliability of tongue thickness measurements. The ICC value was 0.984 (95%CI: 0.979-0.989) and was indicative of good reliability (Table 7).
| Tongue thickness | L3SMI | ||
| Tongue thickness | Pearson correlation | 1 | -0.074 |
| Significance (2-tailed) | 0.424 | ||
| n | 120 | 120 | |
| L3SMI | Pearson Correlation | -0.074 | 1 |
| Significance (2-tailed) | 0.424 | ||
| n | 120 | 120 | |
| CTP class | L3SMI | P value | ||||
| Mean | Standard deviation | Median | Minimum | Maximum | ||
| Class C | 38.2613 | 8.88428 | 39.3900 | 18.37 | 52.85 | 0.63 |
| Normal | 39.6640 | 6.80565 | 40.1900 | 31.24 | 50.25 | |
Our study indicates that tongue thickness measurement by USG correlates significantly with the severity of liver disease, as assessed by CTP scores. The study established that patients with a CTP score ≥ 10 have significantly reduced tongue thickness as compared to normal individuals and those with less severe liver disease with CTP scores of 5-9. Studies have shown that sarcopenia also affects other muscles besides the much studied L3SMI[7-9]. Tongue thickness has even been examined as a bedside measure of sarcopenia in patients with critical illness and has also been correlated with clinical outcome[9].
Malnutrition in cirrhosis is secondary to a multifactorial process and is seen more often in patients with more severe liver disease. We studied the correlation between tongue thickness and MELD score and found a significant negative correlation between the two (r: -0.331, P < 0.01), indicating that as the MELD score increased, tongue thickness decreased and may be interpreted as worsening of sarcopenia with worsening of liver disease. We also found a significant difference in tongue thickness between CTP class C patients and healthy individuals and between CTP class C patients compared to CTP class A and B patients. However, we did not find any significant difference in tongue thickness between healthy individuals and CTP class A and B patients. Apparently, an appreciable degree of sarcopenia manifests only later in the course of liver cirrhosis when a patient qualifies for CTP class C categorisation. Similar to our findings, Montano-Loza et al[11] studied 248 patients and found that sarcopenia was more prevalent in patients with CTP class C (P < 0.05) and in patients with higher MELD scores (P < 0.02). In other studies, Thandassery et al[6] and Tandon et al[12] also found a correlation between the prevalence of sarcopenia and disease severity, as measured by L3SMI and CTP.
However, in the present study, we did not find a correlation between tongue thickness and L3SMI. We also did not find any significant difference in L3SMI between healthy subjects and CTP class C patients. Measuring the mass of a muscle or group of muscles that predominantly have dynamic or postural functions, we believe, is flawed and the paravertebral muscles being postural muscles, are apparently only affected to an appreciable degree late in the disease course when patients may be critically ill and become bedridden. None of the patients included in this study were critically ill and/or admitted to the intensive care unit or were bedridden, and this could possibly be the reason why no difference was found between L3SMI in healthy subjects and CTP class C patients. This reasoning may also be inferred from the study of 116 patients with cirrhosis and hepatocellular carcinoma by Meza-Junco et al[13]. In their study, similar to our findings, the degree of sarcopenia measured using L3SMI did not correlate with CTP or MELD scores. However, in our study, tongue thickness was consistently and significantly decreased in CTP class C patients. Tongue thickness is probably a more sensitive marker of sarcopenia compared to L3SMI and requires further investigation.
A significant number of people more than 65 years old have decreased muscle mass[14]. In this study, we did not include patients more than 65 years, but we did not find a significant correlation between tongue thickness and age of the subjects included (Table 4). Sarcopenia in cirrhotic patients has been associated with increased mortality, sepsis, hyperammonemia, overt hepatic encephalopathy, and increased length of stay after liver transplantation[6]. The literature also suggests that patients with cirrhosis, poor nutritional status and sarcopenia have a higher risk of mortality, independent of the CTP and MELD scores[15]. It is therefore important to diagnose, quantify and perhaps classify the degree of sarcopenia for the medical management of patients with cirrhosis of the liver, for outcome prognosis and for planning interventions such as liver transplantation. This could be greatly helped by having a readily available and reproducible method for the diagnosis and quantification of sarcopenia.
The tongue has the advantages of direct inspection and ease of bedside measurement of thickness using USG, which is more readily available than CT scanning and is without the risk of radiation exposure, and unlike CT does not require extensive training. Tongue thickness measurement, as suggested by our findings, in addition to being objective, reproducible and easy, could be a more sensitive index for detecting sarcopenia than L3SMI. Our study was limited due to it being located at a single centre, and directed at patients with only a single disease, namely cirrhosis of the liver. Further studies exploring USG-measured tongue thickness in people of diverse ethnicity and different age groups in health and in disease, should be carried out to validate our findings and to establish this as a convenient bedside tool for diagnosing sarcopenia. In view of our study findings, we propose that tongue thickness measurement using USG should be considered for the diagnosis and quantification of sarcopenia.
Sarcopenia in patients with chronic liver disease has prognostic implications. L3 skeletal muscle index (L3SMI) calculated from computed tomography (CT) images is currently the only objective and reproducible method accepted for the quantification of sarcopenia. This study aims to determine tongue thickness measured using ultrasonography as an alternative method for diagnosing sarcopenia.
Sarcopenia in patients with chronic liver disease has prognostic implications. Wider application of L3SMI calculated from CT images is limited by cost, the need for extensive training, limited availability and due to the risk of radiation exposure. Clinical researchers have suggested the inclusion of a measure of sarcopenia in established prognostic models for patients with liver disease. A dependable and reproducible method with wider availability is therefore needed.
This study aimed to examine tongue thickness measured using ultrasonography as a dependable bedside tool for the diagnosis of sarcopenia. Significant differences were seen in tongue thickness between healthy individuals and individuals with less severe liver disease compared to patients with more severe chronic liver disease.
Patients with chronic liver disease and healthy individuals who satisfied the inclusion criteria underwent tongue thickness measurement using ultrasonography. The study was observational in nature and no intervention was planned on the basis of observations made. Tongue thickness measurements were compared between healthy individuals and patients with liver disease of different severity. The imaging technique used was ultrasonography, which has wider availability and does not involve radiation exposure unlike CT scanning used to measure L3SMI.
Significant differences were seen in tongue thickness between healthy subjects and patients with less severe liver disease compared to patients with more severe liver disease. Tongue thickness measured using ultrasonography is therefore proposed as a bedside measure of sarcopenia. However, its application requires further validation in studies involving subjects of different ethnicity, in health and in disease.
This study established consistent and significantly reduced tongue thickness in patients with severe liver disease compared to healthy individuals and patients with less severe liver disease. Tongue thickness measured using ultrasonography may therefore be used as a bedside tool for the diagnosis of sarcopenia, an application with wide availability and no risk of radiation exposure compared to CT-based measurement of L3SMI.
The findings in this study require validation in a similar study of tongue thickness using ultrasonography in people of different ethnicity in health and in disease.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Imazeki F, Sabouri AS S-Editor: Wang J L-Editor: Webster JR E-Editor: Wu YXJ
| 1. | Montano-Loza AJ. Clinical relevance of sarcopenia in patients with cirrhosis. World J Gastroenterol. 2014;20:8061-8071. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 171] [Cited by in RCA: 174] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 2. | Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinková E, Vandewoude M, Zamboni M; European Working Group on Sarcopenia in Older People. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412-423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6987] [Cited by in RCA: 8427] [Article Influence: 561.8] [Reference Citation Analysis (0)] |
| 3. | Kamath PS, Kim WR; Advanced Liver Disease Study Group. The model for end-stage liver disease (MELD). Hepatology. 2007;45:797-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1075] [Cited by in RCA: 1223] [Article Influence: 67.9] [Reference Citation Analysis (0)] |
| 4. | Montano-Loza AJ, Duarte-Rojo A, Meza-Junco J, Baracos VE, Sawyer MB, Pang JX, Beaumont C, Esfandiari N, Myers RP. Inclusion of Sarcopenia Within MELD (MELD-Sarcopenia) and the Prediction of Mortality in Patients With Cirrhosis. Clin Transl Gastroenterol. 2015;6:e102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 263] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 5. | Cholongitas E, Papatheodoridis GV, Vangeli M, Terreni N, Patch D, Burroughs AK. Systematic review: The model for end-stage liver disease--should it replace Child-Pugh's classification for assessing prognosis in cirrhosis? Aliment Pharmacol Ther. 2005;22:1079-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 292] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 6. | Thandassery RB, Montano-Loza AJ. Role of Nutrition and Muscle in Cirrhosis. Curr Treat Options Gastroenterol. 2016;14:257-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (2)] |
| 7. | Sharma A, Karna ST, Tandon M, Pandey CK, Chaturvedi R, Vyas V, Goel AD. Use of ultrasound-guided preoperative diaphragmatic thickness as a predictor of postoperative weaning failure in recipients and donors scheduled for living donor liver transplant surgery. Saudi J Anaesth. 2018;12:406-411. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Gu DH, Kim MY, Seo YS, Kim SG, Lee HA, Kim TH, Jung YK, Kandemir A, Kim JH, An H, Yim HJ, Yeon JE, Byun KS, Um SH. Clinical usefulness of psoas muscle thickness for the diagnosis of sarcopenia in patients with liver cirrhosis. Clin Mol Hepatol. 2018;24:319-330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 89] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 9. | Tamura F, Kikutani T, Tohara T, Yoshida M, Yaegaki K. Tongue thickness relates to nutritional status in the elderly. Dysphagia. 2012;27:556-561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 10. | Oh S, Ahn J, Nam KU, Paeng JY, Hong J. Frankfort horizontal plane is an appropriate three-dimensinal reference in the evaluation of clinical and skeletal cant. J Korean Assoc Oral Maxillofac Surg. 2013;39:71-76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (1)] |
| 11. | Montano-Loza AJ, Meza-Junco J, Baracos VE, Prado CM, Ma M, Meeberg G, Beaumont C, Tandon P, Esfandiari N, Sawyer MB, Kneteman N. Severe muscle depletion predicts postoperative length of stay but is not associated with survival after liver transplantation. Liver Transpl. 2014;20:640-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 235] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 12. | Tandon P, Ney M, Irwin I, Ma MM, Gramlich L, Bain VG, Esfandiari N, Baracos V, Montano-Loza AJ, Myers RP. Severe muscle depletion in patients on the liver transplant wait list: its prevalence and independent prognostic value. Liver Transpl. 2012;18:1209-1216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 432] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 13. | Meza-Junco J, Montano-Loza AJ, Baracos VE, Prado CM, Bain VG, Beaumont C, Esfandiari N, Lieffers JR, Sawyer MB. Sarcopenia as a prognostic index of nutritional status in concurrent cirrhosis and hepatocellular carcinoma. J Clin Gastroenterol. 2013;47:861-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 201] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 14. | Mitchell WK, Williams J, Atherton P, Larvin M, Lund J, Narici M. Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength; a quantitative review. Front Physiol. 2012;3:260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 635] [Cited by in RCA: 875] [Article Influence: 67.3] [Reference Citation Analysis (0)] |
| 15. | Montano-Loza AJ, Meza-Junco J, Prado CM, Lieffers JR, Baracos VE, Bain VG, Sawyer MB. Muscle wasting is associated with mortality in patients with cirrhosis. Clin Gastroenterol Hepatol. 2012;10:166-173, 173.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 537] [Cited by in RCA: 607] [Article Influence: 46.7] [Reference Citation Analysis (0)] |