Published online Aug 8, 2020. doi: 10.4292/wjgpt.v11.i3.48
Peer-review started: January 14, 2020
First decision: April 18, 2020
Revised: May 28, 2020
Accepted: July 1, 2020
Article in press: July 1, 2020
Published online: August 8, 2020
Processing time: 204 Days and 4 Hours
There has been a worldwide increase in the reported incidence of inflammatory bowel disease (IBD) in children over the past 2-3 decades. The hepatobiliary (HB) manifestations of IBD have been well-studied in children in industrialized and developed countries but are infrequently reported in low- and middle-income countries (LMIC) such as Egypt.
To determine the prevalence of the HB manifestations in a cohort of Egyptian children with IBD.
This cross-sectional observational study was carried out over a period of 6 mo (between June 2013 to December 2013) at the Paediatric Hepatology and Gastroenterology Units of Cairo University Children's Hospital, which is the largest paediatric tertiary care centre in the country.
The study included 48 patients with confirmed IBD based upon clinical, laboratory, endoscopic and histopathological features, 29 (60.4%) were male. Twenty-four patients (50%) had ulcerative colitis (UC), 11 (22.9%) had Crohn's disease (CD) and 13 (27.1%) had unclassified-IBD (IBD-U), which was formerly known as indeterminate colitis. The mean age of the patients at the time of presentation was 8.14 (± SD 4.02) years and the mean age at the time of study enrolment was 10.16 (± SD 4.19) years. All patients were screened for HB manifestations by physical examination, liver function tests, imaging and liver biopsy when indicated. HB disorders were confirmed in 13 patients (27.1%). Transaminases were elevated in 3 patients (6.3%). Two patients (4.2%) had elevated biliary enzymes (one was diagnosed as primary sclerosing cholangitis (PSC) and the other was diagnosed with PSC/autoimmune hepatitis overlap syndrome and the third patient had hepatitis C virus infection. Ten patients (20.8%) had bright echogenic liver on ultrasound suggesting fatty infiltration as a sequel of malnutrition or medication toxicity.
The commonest HB disorders in Egyptian children with IBD were abnormal liver function tests, fatty infiltration and PSC. These HB manifestations in paediatric patients in LMIC may be relatively more common than in industrialized countries. Therefore, IBD patients in LMIC should be meticulously screened for liver disease to allow prompt diagnosis and management.
Core tip: The incidence of inflammatory bowel disease (IBD) in children has increased recently worldwide. Similarly, the reported incidence of the hepatobiliary (HB) manifestations of IBD in developed countries is rising, while in low- and middle-income countries, there are no much available reports especially in the paediatric age group. In this cohort of Egyptian children with IBD, all patients were screened for HB disorders by physical examination, liver function tests, imaging and liver biopsy when indicated. The most frequently reported HB disorders in these children with IBD were abnormal liver function tests, fatty infiltration and primary sclerosing cholangitis.
- Citation: El-Shabrawi MH, Tarek S, Abou-Zekri M, Meshaal S, Enayet A, Mogahed EA. Hepatobiliary manifestations in children with inflammatory bowel disease: A single-center experience in a low/middle income country. World J Gastrointest Pharmacol Ther 2020; 11(3): 48-58
- URL: https://www.wjgnet.com/2150-5349/full/v11/i3/48.htm
- DOI: https://dx.doi.org/10.4292/wjgpt.v11.i3.48
Inflammatory bowel disease (IBD) in childhood includes Crohn’s disease (CD), ulcerative colitis (UC) and unclassified-IBD (IBD-U), which was formerly known as indeterminate colitis[1]. In the past few years, the incidence of IBD in children in industrialized and developed countries has increased[2-4]. However, although in the low- and middle-income countries (LMICs) there have been few reports, the incidence of IBD in children may also be increasing[5].
The characteristics of IBD in the Egyptian population are similar to the patterns seen in Asian and African populations, and the disease behaviour is milder than that reported in Western countries[6]. Extra-intestinal manifestations (EIMs) of IBD involving the skin, eyes and joints usually parallel the disease activity in the gut; however, diseases involving the hepatobiliary (HB) and pulmonary systems typically do not correlate with the disease activity of bowel inflammation[7]. The reported prevalence of liver and biliary tree disorders in IBD ranges from 3% to more than 50%, depending on whether the definition of the disease includes only definite and persistent conditions or transient alterations of liver function[8,9].
HB complications can be a result of the primary IBD process, medication toxicity, or due to an underlying primary hepatic disorder unrelated to IBD, such as hepatitis caused by viruses, drugs or toxins which are more prevalent in LMICs[10,11]. Some HB manifestations, such as cholelithiasis and portal vein thrombosis, can occur due to the effect of chronic inflammation and the severity of bowel disease with bouts of severe diarrhoea and dehydration[7]. Non-specific hepatomegaly is commonly reported in IBD and is strictly related to fatty infiltration in more than 30% of patients[12], and it does not seem to be related to the patient’s sex or type of IBD[8].
The management of these disorders often requires the expertise of a multidisciplinary team to achieve the best outcome[13].
The aim of the present study was to assess the prevalence and aetiology of HB manifestations in children and adolescents with IBD at a single centre in Egypt (which is classified as a LMIC) and compare them to those in some of the industrialized nations.
This observational study was carried out at the Paediatric Hepatology and Gastroenterology Units of Cairo University Children's Hospital, Cairo, Egypt, over a 6-mo-period (between June 2013 and December 2013). Forty-eight children with an established diagnosis of IBD were included in the study. The diagnosis of IBD was based upon clinical, laboratory, endoscopic and histopathological features.
The study protocol was approved by the Research Ethics Committee of the Paediatrics Department, Faculty of Medicine, Cairo University, Egypt. The research was conducted in accordance to the Helsinki Declaration. All patients were enrolled in the study after an informed consent was obtained from their parents/guardians.
All patients < 18 years of age of both sexes with an established diagnosis of IBD were included. The clinical and laboratory data were retrospectively retrieved from 44 patients’ files, and 4 newly diagnosed patients were prospectively enrolled during the study period. Patients were excluded if any crucial data was missing in their files.
Detailed history taking was carried out, including: (1) Age at the time of presentation; (2) Age at the time of IBD diagnosis; (3) Meticulous family history of IBD in other siblings or parents; (4) History of comorbid conditions other than IBD; (5) Symptoms suggestive of HB manifestations [jaundice, abdominal distension, pruritus, gastrointestinal (GI) bleeding]; (6) Age at the start of treatment and age at the time of onset of HB manifestations; and (7) Medication history [aminosalicylate, azathioprine, corticosteroids, methotrexate, infliximab and/or drugs for associated diseases such as colchicine for Familial Mediterranean fever (FMF)].
The patients’ anthropometric measurements (weight and height) were plotted on Egyptian growth curves (Standard Egyptian Growth, 2008)[14], and the corresponding Z-scores were obtained.
All patients were subjected to meticulous clinical examination focusing on the signs of hepatic complications (jaundice, palmar erythema, spider nevi, lower limb oedema, and abdominal examination for organomegaly and ascites).
All patients were evaluated by liver chemistry, including the following: (1) Total and direct serum bilirubin; (2) Alanine aminotransferase (ALT); (3) Aspartate aminotransferase (AST); (4) Alkaline phosphatase (ALP); (5) Gamma glutamyl transpeptidase (GGT); (6) Serum albumin; and (7) Prothrombin time (PT) and concentration and international normalized ratio.
Abdominal ultrasound was performed in all patients using a greyscale device (FUKUDA-DENSHI; FF Sonic-400 Tokyo, Japan) to assess the liver size, texture, and echogenicity, dilatation of the intra-hepatic biliary radicals, gallbladder size, content and wall thickness, focal lesions, hepatic vasculature, and the presence of ascites. Splenic size was also assessed.
Serum immunoglobulin G (IgG), antinuclear antibody (ANA), smooth muscle antibody (SMA), anti-liver kidney microsomal 1, anti-neutrophil cytoplasmic antibody and anti-Saccharomyces cerevisiae antibody testing was performed in selected cases suspected of having autoimmune hepatitis (AIH) and/or primary sclerosing cholangitis (PSC). Viral markers were assessed for patients suspected of having hepatitis B virus (HBV) or hepatitis C virus (HCV) infections.
Diagnostic and follow-up upper and lower GI endoscopic examinations were performed for all patients to confirm the diagnosis of IBD and to monitor the treatment response. The gross endoscopic appearance as well as the histopathological examination of mucosal biopsy specimens withdrawn at the time of GI endoscopy was the cornerstone for IBD diagnosis in our patients. Endoscopic retrograde cholangiopancreatography (ERCP) or magnetic resonance cholangiopancreatography (MRCP) was performed in cases suspected of having PSC or overlap syndrome (AIH and PSC).
The data were tabulated and statistically analysed. Qualitative data were expressed as numbers and percentages, and were compared by the chi-square test or Fisher's exact test when appropriate. Quantitative data were expressed as the mean ± SD, median and interquartile range, minimum and maximum, and were compared by the Student’s t-test or the Mann-Whitney U test. In all tests, a probability (P) value was considered significant if less than 0.05.
Based on the classic clinical findings, gross endoscopic appearance and microscopic histopathological features, we recruited 48 patients with IBD during this relatively short period from a large tertiary referral centre for all of Egypt. Twenty-nine (60.4%) patients were male; 24 (50%) had UC, 11 (22.9%) had CD, and 13 had IBD-U. The mean age at the time of presentation was 8.14 ± 4.02 years, while the mean age at the time of study enrolment was 10.16 ± 4.19 years.
The most common clinical presentation was recurrent abdominal pain in 47 patients (97.9%), followed by chronic diarrhoea with tenesmus and rectal bleeding (Table 1). None of our patients presented with ulcerating perianal disease. There was a positive family history of IBD in 9 patients (18.75%), representing other affected siblings. Twelve patients (25%) had other associated diseases: 11 had FMF, and 1 patient had systemic lupus erythematosus (SLE).
| Complaint | n | Percentage |
| Abdominal pain | 47 | 97.9 |
| Diarrhoea | 43 | 89.6 |
| Tenesmus | 36 | 75 |
| Bleeding per rectum | 35 | 72.9 |
| Chronic fatigue | 19 | 39.6 |
| Weight loss | 13 | 27.1 |
| Low grade fever | 13 | 27.1 |
Regarding the symptoms suggestive of HB manifestations of IBD, 2 patients had jaundice associated with dark-coloured urine. Eighteen patients (37.5%) presented with abdominal distension, which may also have been attributed to IBD itself. One of our patients presented with pruritus, and none had manifestations of portal hypertension. Upper GI endoscopy confirmed the absence of oesophageal varices. The mean age at the onset of hepatic complications in our patients diagnosed with IBD was 12.10 ± 3.51 years.
The median (range) weight and height by Z score were -1.0 (-5.0-3.0) and -1.2 (-6.6-1.7), respectively. Sixteen patients (33.4%) were on the 3rd percentile or lower in weight, while 13 (27.1%) had short stature.
Abdominal examination showed that 16 patients (33.3%) had abdominal distension, 6 of whom (12.5%) had only hepatomegaly, with mean ± SD size of the liver in the right midclavicular line and midline of 4.83 ± 1.72 and 5.33 ± 2.94 cm, respectively. Two patients (4.2%) had splenomegaly and none had ascites.
With regard to administered medications for the treatment of IBD at the time of study enrolment: (1) Thirty-five patients (72.9%) were receiving mesalamine; (2) Twelve (25%) received salazopyrine; (3) Twenty-six patients (54.2%) were receiving corticosteroids; (4) Twenty patients (41.7%) received azathioprine; (5) Four patients (8.35%) received methotrexate; and (6) Only one patient received infliximab.
In addition, 11 patients (22.9%) who were diagnosed with FMF received colchicine.
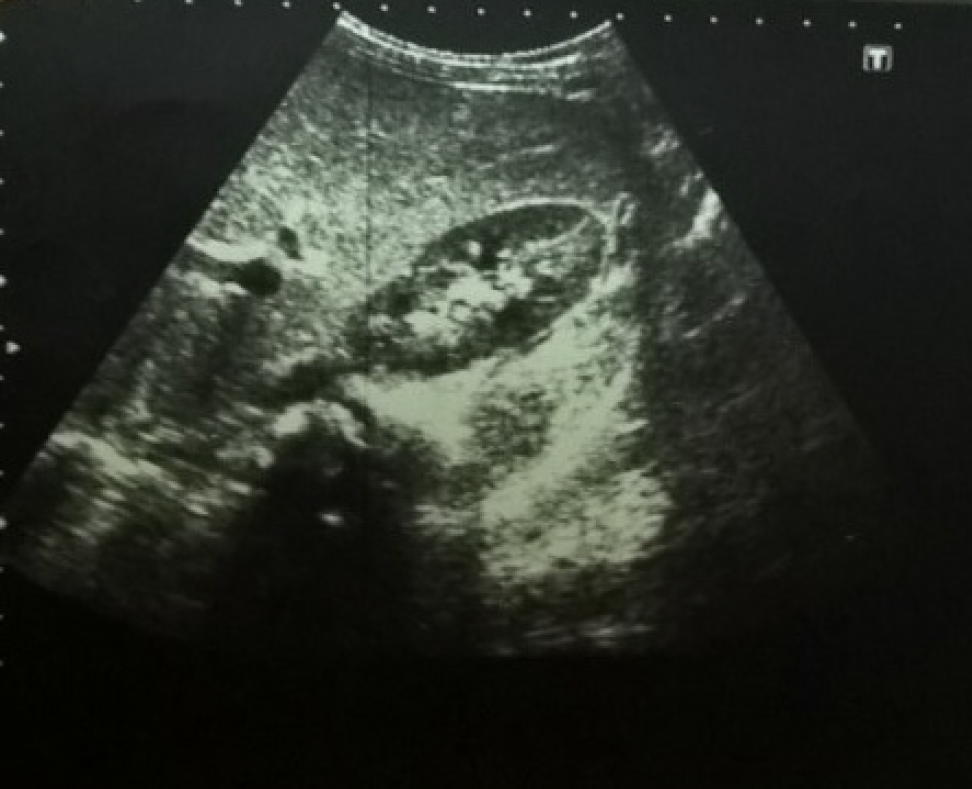
Liver chemistry revealed that 3 patients (6.25%) had elevated liver enzymes, 2 patients (4.2%) had direct hyperbilirubinaemia, and 10 patients (20.8%) had hypoalbuminaemia that may also have been attributed to IBD, as none of the patients had coagulopathy (Table 2). Abdominal ultrasonography revealed that 10 patients (22.7%) had a bright echogenic liver (Figure 1), which may suggest fatty infiltration. Two patients (4.5%) had dilated intrahepatic biliary radicals. Two patients (4.5%) had thickened gallbladder walls, while 4 patients (9.1%) had splenomegaly. None had ascites.
| Parameter | Result | Range |
| ALT in median (IQR) (up to 45 U/L) | 16.5 (13-24.8) | 8-184 |
| AST in median (IQR) (up to 75 U/L) | 27 (20.5-34) | 6-226 |
| TB in median (IQR) (up to 1.4 mg/dL) | 0.4 (0.3-0.5) | 0.1-3.4 |
| DB in median (IQR) (up to 1.4 mg/dL) | 0.1 (0.1-0.1) | 0-1.7 |
| ALP in median (IQR) (up to 640 U/L) | 161 (88-239) | 50-439 |
| GGT in median (IQR) (up to 50 U/L) | 15 (12.3-19.8) | 8-331 |
| Albumin in mean ± SD (3.5-4.5 g/dL) | 3.75 ± 0.78 | 1.3-5.3 |
| PT in mean ± SD (s) | 13.1 ± 0.59 | 11.7-14.8 |
| PC in mean ± SD | 92.8 ± 7.41 | 76-100 |
| INR in mean ± SD | 1.02 ± 0.05 | 1-1.2 |
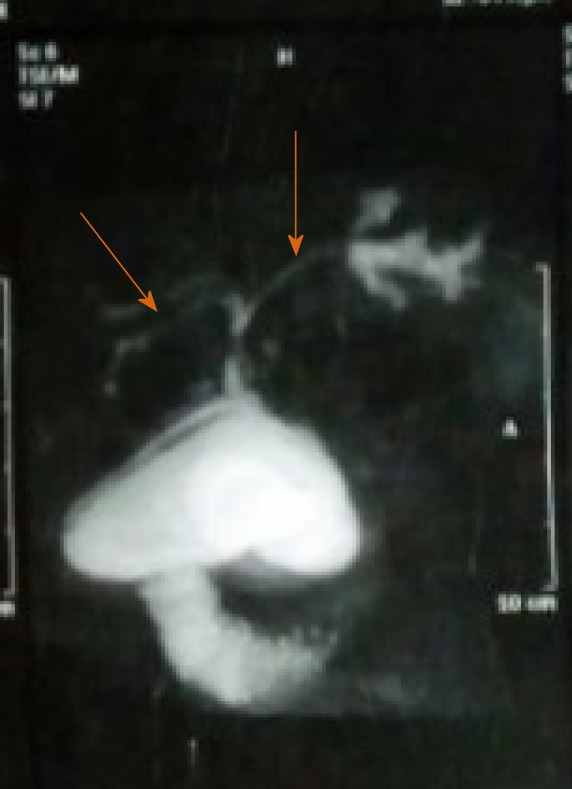
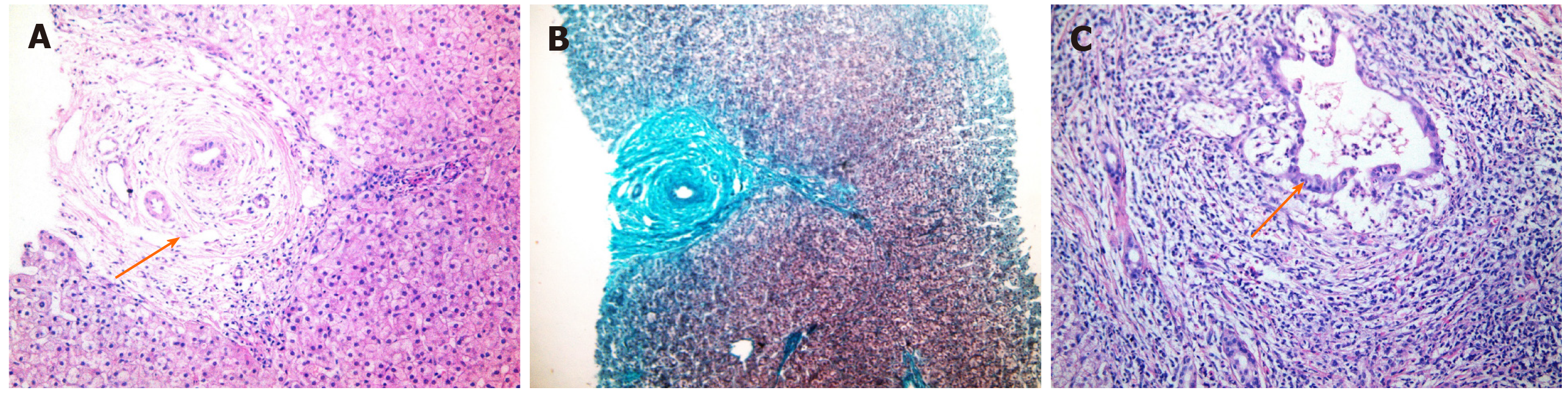
Therefore, the overall frequency of HB manifestations in this study was 27.1% (13 patients). Of these 13 patients, 2 had cholestatic jaundice, elevated transaminases and elevated ALP and GGT, and both were confirmed to have PSC by MRCP (Figure 2) and percutaneous liver biopsy (Figure 3). One of these 2 patients had elevated serum IgG levels, positive ANA and positive SMA, and was diagnosed with PSC/AIH overlap syndrome. Another patient with elevated liver enzymes was diagnosed with SLE and had a concomitant HCV infection. He was recently treated for HCV with oral direct acting antivirals (sofosbuvir/ledipasvir) and achieved sustained virological response followed by normalization of his liver functions. Ten patients had echogenic liver by ultrasound: 3 of them were receiving steroids, 3 patients had malnutrition with poor growth, and 4 patients had both risk factors.
Regarding the 6 patients (12.5%) who had hepatomegaly, two had PSC, 3 had fatty liver, and the remaining patient had HCV infection.
IBDs, including CD, UC, and IBD-U, are chronic and relapsing intestinal disorders[15]. In the Asia–Pacific region, a marked increase in the number of cases of IBD was found, which was compatible with a similar trend worldwide[16-18]. Our current study included 48 Egyptian children with IBD. The most common clinical presentation in our patients was recurrent abdominal pain, which was present in 97.9% of patients, followed by chronic diarrhoea. These findings are consistent with those of other studies where the most common presenting symptoms of IBD in children were abdominal pain (87.5%) and diarrhoea (75.0%)[14].
A positive family history remains the strongest recognizable risk factor for the development of IBD and is reported in approximately 8%-12% of IBD patients[19]. Genetic factors, such as nucleotide-binding oligomerization domain containing 2 (NOD2) and other autophagy-related genes, such as ATG16L1 (autophagy-related 16-like 1), have been implicated in the aetiopathogenesis of IBD[20]. NOD2, a cytosolic protein expressed in monocytes, functions as an intracellular receptor for a bacterial product and is also a component of the innate immune system. CD is associated with frameshift mutations in NOD2, resulting in a truncated and non-functioning protein[21]. Autophagy, the major lysosomal pathway for degrading and recycling cytoplasmic material, constitutes an important homeostatic cellular process. Of interest, single-nucleotide polymorphisms of ATG16L1, a key component in the autophagic response to invading pathogens), have been associated with an increased risk of developing CD[22]. In the current study, nine patients had a positive history of IBD in their siblings. This relatively higher prevalence of positive family history in our study is mostly related to the deeply rooted Egyptian cultural traditions of consanguineous marriages and a large family size[23]. Therefore, we recommend meticulous genetic studies in our patients in Egypt and other LMICs who have a high prevalence of consanguinity.
The most common extra-intestinal manifestation of IBDs, particularly CD, in childhood and adolescence is reported to be impaired physical growth[24]. This was found in the present study, where almost one-third of our patients was on the 3rd percentile or lower for body weight, while 27.1% had short stature[25].
In the present study, 11 patients (22.9%) were also diagnosed with FMF confirmed by genetic testing. This observation agrees with the study by Salah et al[26] conducted at our institute, which reported a significant association between MEFV gene mutation and IBD in Egyptian children, especially in patients with indeterminate colitis (IBD-U)[26].
HB disorders are common in patients with IBD. Persistently elevated transaminases are observed in approximately 20%-30% of these patients[10]. An Italian study reported that abnormal liver chemistry was detected in 20.9% of IBD patients[12]. In this study, the frequency of overall HB manifestations was 27.1% (13 patients). Liver chemistry revealed that only 3 patients (6.25%) had elevated liver enzymes. Of the two patients who had cholestatic jaundice, one was diagnosed with PSC, and the other was diagnosed with PSC/AIH overlap syndrome. This finding was similar to that reported by Broomé et al[27], who found that 1.4%-7.5% of patients with IBD eventually developed PSC during the course of their disease. Patients with PSC usually present with jaundice[28], which was the case in our patients. The disease onset of PSC is typically insidious, but it has been increasingly diagnosed at the asymptomatic stage, likely because of the widespread availability of ERCP and MRCP for evaluating elevated serum ALP levels[10].
AIH/PSC overlap must be considered in any patient with IBD, particularly UC, and PSC exhibits elevated transaminases, polyclonal hyper-gamma-globulinaemia and a liver biopsy suggestive of periportal hepatitis[28]. Autoimmune liver disease is reported in up to 7.8% of children with IBD[29]. In the present study, only one patient suffered from AIH/PSC overlap syndrome.
Corticosteroids may alter the hepatic lipid metabolism and induce hepatic steatosis[30]. Malnutrition is another factor that is highly associated with hepatocyte fatty infiltration. Furthermore, protein synthesis is decreased during protein-calorie malnutrition, which plays a role in triglyceride export into the blood[31]. Thus, triglycerides are retained within the hepatocytes resulting in fatty liver. Increased import of free fatty acids into the liver as a result of abdominal lipolysis, which develops during the weight-loss stages, may add to this process[32]. This finding is in agreement with our study, in which 10 patients had echogenic liver by ultrasound; 3 of them were receiving steroids, 3 patients had malnutrition with poor growth according to the Egyptian growth curves[25], and 4 patients had both risk factors. Although the diagnosis of fatty liver needs to be confirmed histologically, screening can be accomplished via analysis of serum aminotransferases, GGT, triglyceride levels and ultrasonographic appearance of a bright echotexture of the liver[8]. On the other hand, a previous study suggested a complex, multifactorial relationship between the IBDs and the development of non-alcoholic fatty liver disease (NAFLD) beyond the scope of current pharmacological intervention[33].
As immunosuppressive drugs are being used more frequently in IBD, concerns about viral reactivation are increasing. The impact of immunosuppressive therapy for IBD on HCV remains controversial, as steroids can promote viral replication. Egypt has the highest prevalence of HCV infection in the world[34]. The prevalence of HCV infection has increased in IBD patients in comparison to the general population both in Spain and France in studies over the last decade of the 20th century[35]. Similarly, one of the patients (2%) in the current study had associated chronic HCV infection. This patient had multiple risk factors for HCV acquisition, including blood transfusion, recurrent hospitalizations, and repeated endoscopies with intestinal biopsies. However, other studies have demonstrated a similar prevalence in the general population[36,37].
The drug armamentarium for IBD is very wide and might be frequently complicated by hepatotoxicity, adding to the spectrum of liver disease in IBD. However, drug therapy for the associated liver disease itself might be limited, and appropriate timely diagnosis might be all that is offered to the patients, but that issue is beyond the scope of this study.
The prognosis of IBD patients with liver disease is worsening, and close collaboration between IBD clinicians, paediatric hepatologists/gastroenterologists, and primary care providers should be encouraged for early diagnosis, shared decision making and optimal outcome before end-stage liver disease sets in[10]. Liver transplantation (LTx) is not readily available in LMICs. Moreover, recipients with comorbidities at the time of LTx, including those with IBD, have increased morbidity and mortality. Due to the association between IBD and PSC, the frequency of LTx in patients with IBD has increased[10]. Patients with a longer duration of UC and extensive colonic involvement are known to have an increased risk of colorectal cancer. Routine colorectal cancer surveillance should continue for patients with IBD who have had LTx, even in the setting of clinically quiescent disease[37].
In conclusion, the prevalence of HB manifestations in paediatric patients with IBD in Egypt, as one of the LMICs, is relatively high compared to industrialized countries. Investment in research for confounding factors in LMICs might be justifiable, including family genetic studies that might diagnose early cases of IBD and work-up for very early-onset IBD. These patients should be screened for liver disease to allow prompt diagnosis and the offer of available treatment. The most common HB disorders in children with IBD are abnormal liver tests, fatty infiltration and PSC.
Screening for HB manifestations of IBD is mandatory, as many of these complications could be initially asymptomatic. HBV and HCV should not be overlooked and should be screened for in patients with IBD, as these patients have multiple risk factors for HCV acquisition including endoscopies, blood transfusion and recurrent hospitalizations. Therefore, it is necessary to implement mass screening for HCV in these high-risk groups especially in an endemic country such as Egypt. Health education for parents and patients should be carried out to increase knowledge on the nature of the disease and treatment options. Genetic research on the diagnosis of very early-onset IBD and family screening should be emphasized.
Inflammatory bowel disease (IBD) is a systemic illness that can present with multiple extra-intestinal manifestations. Hepatobiliary (HB) disorders associated with IBD are not uncommon. From an aetiopathogenic perspective, there are multiple layers of evidence suggesting that these disorders are not isolated entities and may share mechanistic pathways. All of these disorders usually manifest with abnormal hepatic biochemical tests. Of the HB complications of IBD, primary sclerosing cholangitis (PSC) carries the most significant clinical implications and remains a highly challenging disease to manage.
Recently, the reported incidence of IBD in paediatrics has increased dramatically. The HB manifestations of IBD have been well studied in children in the industrialized and developed countries. On the other hand, there is a paucity of studies on IBD in low- and middle-income countries (LMICs) such as Egypt.
The main objective was to determine the frequency of HB manifestations in Egyptian paediatric patients with IBDs, to achieve an early diagnosis in order to obtain prompt treatment. Moreover, the risk factors associated with the occurrence of HB complications in patients with IBD were determined.
This study was carried out at the Paediatric Hepatology and Gastroenterology Units of Cairo University Children's Hospital over a 6-mo-period. Patients younger than 18 years of age of both sexes were included. The available data were retrieved from the files of patients diagnosed with IBD as well as newly diagnosed patients. The collected data included a full medical history such as name, age, sex, residence, meticulous family history of IBD and other associated autoimmune illnesses; history of jaundice; abdominal distention; pruritus; bleeding; a history of blood transfusion; and a detailed medication history. A meticulous clinical examination was performed, including the assessment of anthropometric measures and a general and detailed abdominal examination. Laboratory investigations, such as CBC, complete liver functions (ALT, AST, total and direct bilirubin, ALP, GGT, albumin and PT) were performed in all patients. In addition, serum immunoglobulin G, antinuclear antibody, smooth muscle antibody, anti-liver kidney microsomal 1, anti-neutrophil cytoplasmic antibody and anti-Saccharomyces cerevisiae antibody were performed for selected cases suspected of having autoimmune hepatitis (AIH) and/or PSC. Viral markers were assessed in patients suspected of having hepatitis B virus (HBV) or hepatitis C virus (HCV) infections. Abdominal ultrasound was performed in all patients. Endoscopic retrograde cholangiopancreatography (ERCP) or magnetic resonance cholangiopancreatography was performed for cases suspected of having PSC. Percutaneous liver biopsy was performed when indicated.
We recruited 48 paediatric patients with IBD. Twenty-nine (60.4%) patients were male. Twenty-four (50%) had UC, 11 (22.9%) had CD, and 13 patients had IBD-U. The median (range) of the weight and height by Z score were -1.0 (-5.0-3.0) and -1.2 (-6.6-1.7), respectively. Sixteen patients (33.4%) were on the 3rd percentile or lower for weight, while 13 (27.1%) had short stature. The most common clinical presentation was recurrent abdominal pain, which was present in 47 patients (97.9%), followed by chronic diarrhoea with tenesmus and rectal bleeding. None of our patients presented with ulcerating perianal disease. There was a positive family history of IBD in 9 patients (18.75%). Twelve patients (25%) had other associated diseases: 11 had FMF, and one patient had systemic lupus erythematosus. Therefore, the overall frequency of HB disorders in this study was 27.1% (13 patients). Two patients had cholestatic jaundice, one of them was diagnosed with PSC, and the other patient had PSC/AIH overlap syndrome. Three (6.3%) patients had elevated liver enzymes. Ten (20.8%) patients had echogenic liver suggesting fatty changes.
HB manifestations in paediatric patients with IBD in LMICs are more common than those in the industrialized countries. Investment in research for confounding factors in LMICs is cost-effective, including family genetic studies that can offer an early diagnosis of IBDs in general and specifically for very early-onset IBD. The importance of awareness of the implications and causes of abnormal hepatic biochemical tests in IBD patients is due to the wide range of possible complications and risks associated with the medications used to treat and manage them. Hepatic biochemical tests should be routinely performed in these patients. When abnormalities are detected, a prompt step by step diagnostic approach should be followed until an aetiology is reached. The frequency of HB manifestations in our patients was not low and may be affected by the type of treatment modality and malnutrition. The highest proportion was found in those suffering from an echogenic liver by abdominal ultrasound, and many of these patients received corticosteroids.
Screening for HB manifestations of IBD is mandatory, as many of these complications may be initially asymptomatic. Complete liver function tests and abdominal ultrasound should be performed on a regular basis. A high index of suspicion of PSC should be raised in patients who present with an unexplained cholestatic jaundice or elevated ALP levels. An echogenic liver detected by abdominal ultrasound requires thorough assessment of the patient’s growth and medication history. Liver biopsy may be needed in selected cases for the definitive diagnosis of NAFLD. Drugs used to treat IBD should be administered cautiously, as a majority of them can potentially cause hepatotoxicity. HBV and HCV should be screened for in patients with IBD especially in endemic areas such as Egypt. Patients with IBD have multiple risk factors for HCV and HBV acquisition, including repeated endoscopies, transfusion of blood products and recurrent hospitalizations. Genetic research should include the diagnosis of very early-onset IBD and screening of similar cases in families.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Egypt
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): E
P-Reviewer: Christodoulou DK, Day AS, Serban ED, Zhang X S-Editor: Dou Y L-Editor: Webster JR E-Editor: Li JH
| 1. | Levine A, Koletzko S, Turner D, Escher JC, Cucchiara S, de Ridder L, Kolho KL, Veres G, Russell RK, Paerregaard A, Buderus S, Greer ML, Dias JA, Veereman-Wauters G, Lionetti P, Sladek M, Martin de Carpi J, Staiano A, Ruemmele FM, Wilson DC; European Society of Pediatric Gastroenterology, Hepatology, and Nutrition. ESPGHAN revised porto criteria for the diagnosis of inflammatory bowel disease in children and adolescents. J Pediatr Gastroenterol Nutr. 2014;58:795-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 822] [Cited by in RCA: 982] [Article Influence: 89.3] [Reference Citation Analysis (0)] |
| 2. | Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW, Kaplan GG. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46-54.e42; quiz e30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3789] [Cited by in RCA: 3515] [Article Influence: 270.4] [Reference Citation Analysis (5)] |
| 3. | Roberts SE, Thorne K, Thapar N, Broekaert I, Benninga MA, Dolinsek J, Mas E, Miele E, Orel R, Pienar C, Ribes-Koninckx C, Thomson M, Tzivinikos C, Morrison-Rees S, John A, Williams JG. A systematic review and meta analysis of paediatric inflammatory bowel disease incidence and prevalence across Europe. J Crohns Colitis. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 74] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 4. | Sýkora J, Pomahačová R, Kreslová M, Cvalínová D, Štych P, Schwarz J. Current global trends in the incidence of pediatric-onset inflammatory bowel disease. World J Gastroenterol. 2018;24:2741-2763. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 271] [Cited by in RCA: 260] [Article Influence: 37.1] [Reference Citation Analysis (6)] |
| 5. | Ng WK, Wong SH, Ng SC. Changing epidemiological trends of inflammatory bowel disease in Asia. Intest Res. 2016;14:111-119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 202] [Cited by in RCA: 238] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 6. | Esmat S, El Nady M, Elfekki M, Elsherif Y, Naga M. Epidemiological and clinical characteristics of inflammatory bowel diseases in Cairo, Egypt. World J Gastroenterol. 2014;20:814-821. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 38] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | Navaneethan U, Shen B. Hepatopancreatobiliary manifestations and complications associated with inflammatory bowel disease. Inflamm Bowel Dis. 2010;16:1598-1619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 151] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 8. | Loftus EV, Sandborn WJ, Lindor KD, LaRusso NF. Interactions between chronic liver disease and inflammatory bowel disease. Inflamm Bowel Dis. 1997;3:288-302. [RCA] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 45] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Fousekis FS, Katsanos KH, Theopistos VI, Baltayiannis G, Kosmidou M, Glantzounis G, Christou L, Tsianos EV, Christodoulou DK. Hepatobiliary and pancreatic manifestations in inflammatory bowel diseases: a referral center study. BMC Gastroenterol. 2019;19:48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 10. | Cakir M, Sag E, Dogan G, Unal F, Kasirga E. Clinical significance of low transaminase levels in children with inflammatory bowel disease. World J Pediatr. 2019;15:143-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Huang ML, Xu XT, Shen J, Qiao YQ, Dai ZH, Ran ZH. Prevalence and factors related to hepatitis B and C infection in inflammatory bowel disease patients in China: a retrospective study. J Crohns Colitis. 2014;8:282-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 12. | Cappello M, Randazzo C, Bravatà I, Licata A, Peralta S, Craxì A, Almasio PL. Liver Function Test Abnormalities in Patients with Inflammatory Bowel Diseases: A Hospital-based Survey. Clin Med Insights Gastroenterol. 2014;7:25-31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (2)] |
| 13. | Mahfouz M, Martin P, Carrion AF. Hepatic Complications of Inflammatory Bowel Disease. Clin Liver Dis. 2019;23:191-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Chu HP, Logarajah V, Tan N, Phua KB. Paediatric inflammatory bowel disease in a multiracial Asian country. Singapore Med J. 2013;54:201-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Miele E, Shamir R, Aloi M, Assa A, Braegger C, Bronsky J, de Ridder L, Escher JC, Hojsak I, Kolaček S, Koletzko S, Levine A, Lionetti P, Martinelli M, Ruemmele F, Russell RK, Boneh RS, van Limbergen J, Veereman G, Staiano A. Nutrition in Pediatric Inflammatory Bowel Disease: A Position Paper on Behalf of the Porto Inflammatory Bowel Disease Group of the European Society of Pediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr. 2018;66:687-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 120] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 16. | Ahuja V, Tandon RK. Inflammatory bowel disease in the Asia-Pacific area: a comparison with developed countries and regional differences. J Dig Dis. 2010;11:134-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 147] [Article Influence: 9.8] [Reference Citation Analysis (1)] |
| 17. | Foster A, Jacobson K. Changing incidence of inflammatory bowel disease: environmental influences and lessons learnt from the South asian population. Front Pediatr. 2013;1:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Yamamoto-Furusho JK, Sarmiento-Aguilar A, Toledo-Mauriño JJ, Bozada-Gutiérrez KE, Bosques-Padilla FJ, Martínez-Vázquez MA, Marroquín-Jiménez V, García-Figueroa R, Jaramillo-Buendía C, Miranda-Cordero RM, Valenzuela-Pérez JA, Cortes-Aguilar Y, Jacobo-Karam JS, Bermudez-Villegas EF; EPIMEX Study Group. Incidence and prevalence of inflammatory bowel disease in Mexico from a nationwide cohort study in a period of 15 years (2000-2017). Medicine (Baltimore). 2019;98:e16291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 19. | Santos MPC, Gomes C, Torres J. Familial and ethnic risk in inflammatory bowel disease. Ann Gastroenterol. 2018;31:14-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 20. | Loddo I, Romano C. Inflammatory Bowel Disease: Genetics, Epigenetics, and Pathogenesis. Front Immunol. 2015;6:551. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 210] [Cited by in RCA: 295] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 21. | Girardin SE, Boneca IG, Viala J, Chamaillard M, Labigne A, Thomas G, Philpott DJ, Sansonetti PJ. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem. 2003;278:8869-8872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1755] [Cited by in RCA: 1766] [Article Influence: 80.3] [Reference Citation Analysis (0)] |
| 22. | Salem M, Ammitzboell M, Nys K, Seidelin JB, Nielsen OH. ATG16L1: A multifunctional susceptibility factor in Crohn disease. Autophagy. 2015;11:585-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 104] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 23. | Al-Mousa H, Al-Saud B. Primary Immunodeficiency Diseases in Highly Consanguineous Populations from Middle East and North Africa: Epidemiology, Diagnosis, and Care. Front Immunol. 2017;8:678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 24. | Däbritz J, Gerner P, Enninger A, Claßen M, Radke M. Inflammatory Bowel Disease in Childhood and Adolescence. Dtsch Arztebl Int. 2017;114:331-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 25. | Ghalli I, Salah N, Hussien F, Erfan M, ElRuby M, Mazen I, Sabry M, Abd El-Razik M, Saad M, Hossney L, Ismaail S, Abd El-Dayem S. In: Proceedings of the 1st National Congress for Egyptian Growth Curves, Cairo University, 11 December 2003. Cairo, Recently published in: Sartorio A, Buckler JMH, Marazzi N, editors. Egyptian Growth Curves 2002; for Infants, Childrenand Adolescents. Crescere nel mondo, Ferring Publisher, 2008. |
| 26. | Salah S, El-Shabrawi M, Lotfy HM, Shiba HF, Abou-Zekri M, Farag Y. Detection of Mediterranean fever gene mutations in Egyptian children with inflammatory bowel disease. Int J Rheum Dis. 2016;19:806-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Broomé U, Glaumann H, Lindstöm E, Lööf L, Almer S, Prytz H, Sandberg-Gertzén H, Lindgren S, Fork FT, Järnerot G, Olsson R. Natural history and outcome in 32 Swedish patients with small duct primary sclerosing cholangitis (PSC). J Hepatol. 2002;36:586-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 83] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 28. | Samarasena JB, Hu KQ. Hepatobiliary manifestations of gastrointestinal and nutritional disorders. Clin Liver Dis. 2011;15:89-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 29. | Bramuzzo M, Martelossi S, Torre G, Cardile S, Arrigo S, Vignola S, Ferrari F, Zuin G, Illiceto MT, Gasparetto M, Pellegrino S, Romano C, Maggiore G, Montico M, Aloi M; SIGENP IBD Group. Clinical Features and Risk Factors of Autoimmune Liver Involvement in Pediatric Inflammatory Bowel Disease. J Pediatr Gastroenterol Nutr. 2016;63:259-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Rogler G. Gastrointestinal and liver adverse effects of drugs used for treating IBD. Best Pract Res Clin Gastroenterol. 2010;24:157-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 121] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 31. | Zou ZY, Shen B, Fan JG. Systematic Review With Meta-analysis: Epidemiology of Nonalcoholic Fatty Liver Disease in Patients With Inflammatory Bowel Disease. Inflamm Bowel Dis. 2019;25:1764-1772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 32. | Solís Herruzo JA, Solís-Muñoz P. Manifestaciones hepatobiliaresen la enfermedad inflamatoria intestinal. Revista Española de Enfermedades Digestivas. 2007;99:525-542. |
| 33. | Lapumnuaypol K, Kanjanahattakij N, Pisarcik D, Thongprayoon C, Wijarnpreecha K, Cheungpasitporn W. Effects of inflammatory bowel disease treatment on the risk of nonalcoholic fatty liver disease: a meta-analysis. Eur J Gastroenterol Hepatol. 2018;30:854-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 34. | El-Gindy EM, Mostafa EF, Montasser IM, Ismail FM. Comparative Study between Different Modalities of Treatment of HCV in New Era of Direct Acting Antiviral Drugs (DAAs) in Aswan Governorate. Egyptian J Hospital Med. 2018;71:3591-3600. [DOI] [Full Text] |
| 35. | Rojas-Feria M, Castro M, Suárez E, Ampuero J, Romero-Gómez M. Hepatobiliary manifestations in inflammatory bowel disease: the gut, the drugs and the liver. World J Gastroenterol. 2013;19:7327-7340. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 84] [Cited by in RCA: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 36. | Sansone S, Guarino M, Castiglione F, Rispo A, Auriemma F, Loperto I, Rea M, Caporaso N, Morisco F. Hepatitis B and C virus reactivation in immunosuppressed patients with inflammatory bowel disease. World J Gastroenterol. 2014;20:3516-3524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 37. | Singh S, Loftus EV, Talwalkar JA. Inflammatory bowel disease after liver transplantation for primary sclerosing cholangitis. Am J Gastroenterol. 2013;108:1417-1425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |