Published online Jun 9, 2020. doi: 10.4292/wjgpt.v11.i2.25
Peer-review started: December 30, 2019
First decision: February 24, 2020
Revised: March 26, 2020
Accepted: May 12, 2020
Article in press: May 12, 2020
Published online: June 9, 2020
Processing time: 160 Days and 11.9 Hours
In order to improve risk stratification and clinical management of the pancreatic ductal adenocarcinoma (PDAC), the American Joint Committee on Cancer (AJCC) has published its eighth edition staging manual. Some major changes have been introduced in the new staging system for both T and N categories. Given the rarity of resectable disease, distal pancreatic cancer is likely underrepresented in the published clinical studies, and how the impact of the staging system actually reflects on to clinical outcomes remain unclear.
To validate the AJCC 8th edition of TNM staging in distal PDAC.
A retrospective cohort study was performed in seven academic medical centers in the United States. Clinicopathological prognostic factors associated with progression-free survival (PFS) and overall survival (OS) were evaluated through univariate and multivariate analyses.
Overall, 454 patients were enrolled in the study, and were divided into 2 subgroups: Invasive intraductal papillary mucinous neoplasms (IPMN) (115 cases) and non-IPMN associated adenocarcinoma (339 cases). Compared to invasive IPMN, non-IPMN associated adenocarcinomas are more common in relatively younger patients, have larger tumor size, are more likely to have positive lymph nodes, and are associated with a higher tumor (T) stage and nodal (N) stage, lymphovascular invasion, perineural invasion, tumor recurrence, and a worse PFS and OS. The cohort was predominantly categorized as stage 3 per AJCC 7th edition staging manual, and it’s more evenly distributed based on 8th edition staging manual. T and N staging of both 7th and 8th edition sufficiently stratify PFS and OS in the entire cohort, although dividing into N1 and N2 according to the 8th edition does not show additional stratification. For PDAC arising in IPMN, T staging of the 7th edition and N1/N2 staging of the 8th edition appear to further stratify PFS and OS. For PDAC without an IPMN component, T staging from both versions fails to stratify PFS and OS.
The AJCC 8th edition TNM staging system provides even distribution for the T staging, however, it does not provide better risk stratification than previous staging system for distal pancreatic cancer.
Core tip: The American Joint Committee on Cancer 8th edition TNM staging system provides even distribution for the T staging, however, it does not provide better risk stratification than previous staging system for distal pancreatic cancer. This study also demonstrates the significant difference of clinical outcome and risk stratification between invasive intraductal papillary mucinous neoplasms and non-intraductal papillary mucinous neoplasms associated pancreatic ductal adenocarcinoma.
- Citation: Yin F, Saad M, Xie H, Lin J, Jackson CR, Ren B, Lawson C, Karamchandani DM, Bernabeu BQ, Jiang W, Dhir T, Zheng R, Schultz CW, Zhang D, Thomas CL, Zhang X, Lai J, Schild M, Zhang X, Liu X. Validation of American Joint Committee on Cancer 8th edition of TNM staging in resected distal pancreatic cancer. World J Gastrointest Pharmacol Ther 2020; 11(2): 25-39
- URL: https://www.wjgnet.com/2150-5349/full/v11/i2/25.htm
- DOI: https://dx.doi.org/10.4292/wjgpt.v11.i2.25
Pancreatic ductal adenocarcinoma (PDAC) is one of the most aggressive cancers. In the United States, PDAC is the fourth leading cause of cancer related deaths causing approximately 7% of all cancer mortalities, and has a dismal 5-year survival rate of less than 10%[1,2]. A strong contributor to the low overall survival (OS) rate is a lack of early diagnostic symptoms, which results in PDAC frequently presenting at a late stage with locally advanced or metastatic disease[3].
In order to improve risk stratification, staging, and clinical management of PDAC, the American Joint Committee on Cancer (AJCC) published its eighth edition staging manual for pancreatic cancer in October 2016[4]. Some major changes have been introduced in the new staging system for both T and N categories. Of note, tumor size has become the only factor for the T staging except for pT4. pT1 is further subclassified into T1a (tumor 0.5 cm or less), T1b (tumor greater than 0.5 cm and less than 1 cm), and T1c (tumor greater than 1 cm but no more than 2 cm). The most important change was to replace the requirement of extra-pancreatic extension with tumor size (tumor greater than 4 cm) for a pT3 tumor. For the N category, N1 (regional lymph node metastasis) is subclassified into N1 (metastasis in 1-3 nodes) and N2 (metastasis in 4 or more nodes) in 8th edition staging system[5]. Given the rarity of resectable disease, distal pancreatic cancer is likely underrepresented in the published clinical studies, and how the impact of the staging system actually reflects on to clinical outcomes remain unclear.
Intraductal papillary mucinous neoplasm (IPMN) is a pancreatic neoplasm with different epithelial types and histopathological grades[6,7] that has the potential to progress into invasive carcinoma. The invasive IPMN and non-IPMN associated PDAC are likely derived through different molecular pathways[8,9], and reports have suggested improved survival of patients with invasive IMPN as compared to patients with non-IPMN associated PDAC[10,11]. However, whether invasive IPMN and non-IPMN PDAC share similar prognostic factors still remains unclear. So far, there is no specific staging system for invasive IPMN. Conventionally, it is staged using the AJCC system primarily developed and validated for non-IPMN associated PDAC. The size of the invasive component in invasive IPMN is subjective due to variability in sampling and measurement techniques and may not be a reliable parameter for staging.
Until now, the majority of validation studies for AJCC 8th edition were performed in patients with solely or predominantly PDAC in the head of pancreas[5,12-16]. The current study was designed to validate the major T and N staging changes by the AJCC 8th edition of TNM staging manual in a cohort of patients with resected distal pancreatic carcinoma, as well as in a sub-cohort of patients with invasive IPMN or non-IPMN associated PDAC. Univariate and multivariable survival analyses were performed to identify prognostic factors in a multi-centered large-scale study.
After approval from Institutional Review Boards from individual institutions, a retrospective cohort study was performed with cases collected from seven academic medical centers in the United States (University of Florida, Indiana University, Dartmouth-Hitchcock Medical Center, Penn State Health Hershey Medical Center, Thomas Jefferson University Hospital, University of Rochester Medical Center, and Yale University).
Clinicopathological data of resected distal PDAC cases were retrieved from year 2005 to 2018. Other tumor subtypes such as non-invasive IPMN, non-invasive mucinous cystic neoplasm, neuroendocrine neoplasm, and acinar cell carcinoma were excluded. Those cases which underwent preoperative neoadjuvant therapy were excluded from this study.
Histopathological data were collected through reviewing pathology reports and glass slides. Oncological information was collected including tumor size, type, differentiation, margin status, splenic vasculature involvement, and lymph node status. All cases were re-staged based on the AJCC 7th and 8th edition, respectively. Splenic vein involvement was defined as tumor invading through the venous wall. Splenic artery involvement was defined as tumor invading into or through the arterial wall.
Clinical information including patient’s age, gender, radiographic studies, recurrence, metastasis, and survival status was collected through reviewing the medical records from the time of resection until September 2018. The progression free survival (PFS) is defined as the interval between the date of surgery and the date of initial recurrence and/or metastasis of tumor or death. The OS is defined as the interval between the date of surgery and the date of death.
Continuous variables were summarized as median and interquartile range (IQR) and compared with Wilcoxon rank sum test. Categorical variables were summarized as counts and percentages and compared with Fisher’s exact test. PFS and OS were evaluated using Kaplan-Meier curves, and analyzed using log-rank analysis. Cox proportional hazards regression models were used in univariate and multivariable survival analyses to identify factors associated with PFS and OS. The proportionality assumption was assessed graphically using log (-log) plots and quantitatively using the Z statistic. All tests were two-sided and performed in R (version 3.5.3). A P value of < 0.05 was considered statistically significant.
The study enrolled 454 patients with resected distal pancreatic cancer. The age of the patients ranged from 27 to 91 years, with a mean age of 67.1 years, 53.5% of the patients were female (n = 243), and 46.5% were male (n = 211). Pathologic demographics and major pathologic features are summarized in Table 1.
| Feature | Level | Total cohort | Subcohort | ||
| Invasive IPMN | Non-IPMN associated PDAC | P value | |||
| Median (IQR) | |||||
| Age (in yr) | 67.6 (60.0, 74.8) | 70.0 (64.1, 75.5) | 66.7 (59.4, 74.0) | 0.003 | |
| Tumor size (in cm) | 3.5 (2.5, 5.0) | 3.0 (2.0, 4.5) | 3.6 (2.5, 5.2) | 0.012 | |
| Median (95%CI) | |||||
| PFS (in mo) | 21.0 (17.0, 27.0) | NR (47.0, NR) | 17.0 (13.0, 22.0) | < 0.001 | |
| OS (in mo) | 21.0 (19.0, 24.0) | 60.0 (28.0, 69.0) | 19.0 (18.0, 22.0) | < 0.001 | |
| n (%) | |||||
| Gender (male/female) | Female | 243 (53.5) | 61 (53.0) | 182 (53.7) | 0.991 |
| Male | 211 (46.5) | 54 (47.0) | 157 (46.3) | ||
| Tumor differentiation | Well | 41 (9.0) | 15 (13.4) | 26 (7.9) | 0.103 |
| Moderate | 271 (59.7) | 71 (63.4) | 200 (61.0) | ||
| Poor | 128 (28.2) | 26 (23.2) | 102 (31.1) | ||
| AJCC 8th ed staging system | T1 | 76 (16.7) | 32 (27.8) | 44 (13.0) | 0.003 |
| T2 | 202 (44.5) | 46 (40.0) | 156 (46.2) | ||
| T3 | 166 (36.6) | 36 (31.3) | 130 (38.5) | ||
| AJCC 8th ed staging system | N0 | 216 (47.6) | 69 (60.0) | 147 (43.4) | 0.004 |
| N1 | 181 (39.9) | 31 (27.0) | 150 (44.2) | ||
| N2 | 57 (12.6) | 15 (13.0) | 42 (12.4) | ||
| Lymphovascular invasion | Positive | 219 (48.2) | 44 (38.3) | 175 (51.6) | 0.011 |
| Negative | 219 (48.2) | 69 (60.0) | 150 (44.2) | ||
| Indetermi-nant | 16 (3.5) | 2 (1.7) | 14 (4.1) | ||
| Perineural invasion | Positive | 366 (80.6) | 80 (69.6) | 286 (84.4) | 0.001 |
| Negative | 82 (18.1) | 34 (29.6) | 48 (14.2) | ||
| Indetermi-nant | 6 (1.3) | 1 (0.9) | 5 (1.5) | ||
| Recurrence/metastasis | Present | 218 (48.0) | 43 (37.4) | 175 (51.6) | 0.015 |
| Absent | 236 (52.0) | 72 (62.6) | 164 (48.4) | ||
| Splenic parenchymal invasion | Present | 25 (5.5) | 2 (1.7) | 23 (6.9) | 0.067 |
| Absent | 425 (93.6) | 113 (98.3) | 312 (93.1) | ||
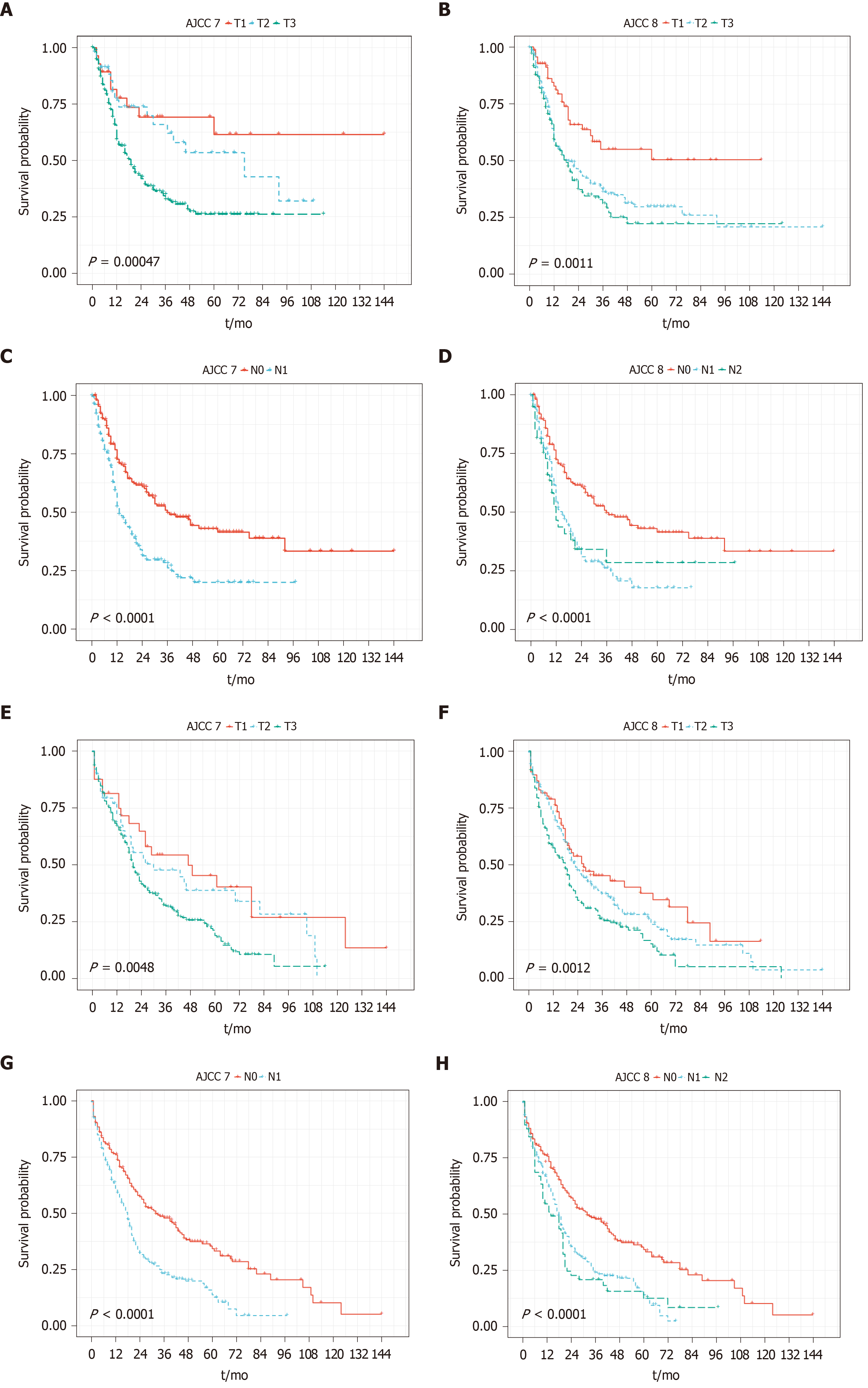
The majority of patients were categorized as stage pT3 per AJCC 7th edition staging manual, with 32 patients (7.0%), 50 patients (11.0%), and 351 patients (77.3%) being classified as pT1, pT2, and pT3, respectively. When categorizing based upon the AJCC 8th edition staging manual, the distribution was relatively more even, with 76 patients (16.7%), 202 patients (44.5%), and 166 patients (36.6%) classified as pT1, pT2, and pT3, respectively. T staging of both 7th and 8th systems sufficiently stratified PFS and OS in the entire cohort (Figure 1A, B, E and F).
Among the 237 patients with positive lymph nodes (pN1 per 7th edition), 180 patients (75.9%) were classified as pN1, and 57 patients (24.1%) were classified as pN2 based on AJCC 8th edition. N staging of both 7th and 8th systems sufficiently stratified PFS and OS in the entire cohort, although dividing into N1 and N2 according to the 8th edition did not show additional stratification (Figure 1C, D, G and H).
The diagnostic group (IPMN vs non-IPMN) is an important prognostic factor in resected distal pancreatic cancer. In the cohort, 115 patients (25.3%) and 339 patients (74.7%) were diagnosed as invasive IPMN and non-IPMN associated PDAC, respectively. There was no gender difference between the 2 groups, with a slight female predominance in both groups (53.0% female in IPMN group vs 53.7% in non-IPMN group). Compare to the invasive IPMN group, non-IPMN associated PDAC are more commonly seen in relatively younger patients (median age 66.7 years in non-IPMN group vs 70.0 years in IPMN group; P = 0.003), and have larger tumor size [median tumor size 3.6 cm (IQR: 2.5-5.2) in non-IPMN group vs median tumor size 3.0 cm (IQR: 2.0-4.5) in IPMN group; P = 0.012]. Non-IPMN associated PDAC are also associated with higher tumor (T) stage and nodal (N) stage, lymphovascular invasion (LVI), perineural invasion, tumor recurrence, as well as worse PFS and OS (Table 1).
A total of 115 patients with invasive IPMN were included in the study. Sixty-one patients (54.0%) were female and fifty-four (47.0%) were male, with the median age of 70.0 years (Table 1).
In univariable analysis for invasive IPMN, tumor size, poor differentiation, pT3 stage (8th edition staging manual), positive lymph node, pN2 stage (8th edition staging manual), and pathologic splenic vein invasion were significantly associated with both poor PFS and poor OS. LVI, splenic artery invasion, and splenic parenchyma invasion were only associated with PFS. Positive resection margin and perineural invasion were only associated with OS. In multivariable analysis, only pT3 (8th edition staging manual) remained as independent prognosticator for OS (Table 2).
| Feature | Level | PFS | OS | ||
| HR (95%CI), P value | |||||
| Gender | 1.67 (0.91-3.05), P = 0.10 | - | 1.33 (0.85-2.08), P = 0.22 | - | |
| Age | 0.99 (0.96-1.02), P = 0.52 | - | 1.01 (0.98-1.03), P = 0.54 | - | |
| Tumor size | 1.18 (1.05-1.32), P = 0.01 | - | 1.10 (1.00-1.21), P = 0.04 | - | |
| Tumor differentiation | Well | - | - | - | - |
| Moderate | 2.71 (0.82-8.91), P = 0.10 | 12290265.82 (0.00-Inf), P = 1.00 | 1.04 (0.53-2.02), P = 0.92 | 1.62 (0.34-7.66), P = 0.55 | |
| Poor | 3.88 (1.06-14.27), P = 0.04 | 2274054.50 (0.00-Inf), P = 1.00 | 2.31 (1.10-4.86), P = 0.03 | 1.78 (0.15-20.67), P = 0.64 | |
| Positive lymph node | 1.19 (1.05-1.35), P = 0.01 | - | 1.19 (1.07-1.31), P < 0.01 | - | |
| T stage (AJCC 8th edition staging manual) | T1 | - | - | - | - |
| T2 | 2.69 (1.14-6.37), P = 0.02 | 0.81 (0.07-9.31), P = 0.87 | 1.65 (0.92-2.97), P = 0.09 | 0.89 (0.09-8.48), P = 0.92 | |
| T3 | 3.48 (1.42-8.51), P = 0.01 | 5.29 (0.44-62.97), P = 0.19 | 2.28 (1.23-4.24), P = 0.01 | 26.22 (2.13-322.77), P = 0.01 | |
| N stage (AJCC 8th edition staging manual) | N0 | - | - | - | - |
| N1 | 2.03 (1.03-4.02), P = 0.04 | 13.78 (1.15-165.63), P = 0.04 | 1.58 (0.93-2.67), P = 0.09 | 1.17 (0.24-5.70), P = 0.85 | |
| N2 | 3.65 (1.60-8.34), P < 0.01 | 29.12 (0.87-979.78), P = 0.06 | 2.52 (1.36-4.65), P < 0.01 | 1.63 (0.10-27.11), P = 0.73 | |
| Lymphovascular invasion | 2.12 (1.16-3.90), P = 0.02 | 0.31 (0.04-2.41), P = 0.26 | 1.43 (0.90-2.28), P = 0.13 | - | |
| Perineural invasion | 1.99 (0.98-4.04), P = 0.06 | - | 2.45 (1.39-4.33), P < 0.01 | 2.67 (0.54-13.22), P = 0.23 | |
| Splenic artery invasion | 5.30 (1.41-19.94), P = 0.01 | 2.44 (0.10-62.16), P = 0.59 | 1.70 (0.59-4.88), P = 0.32 | - | |
| Splenic vein invasion | 18.69 (4.57-76.48), P < 0.01 | 5.28 (0.22-129.25), P = 0.31 | 2.66 (1.08-6.55), P = 0.03 | 5.62 (0.61-51.99), P = 0.13 | |
| Positive margin | 1.25 (0.30-5.19), P = 0.76 | - | 3.14 (1.49-6.62), P < 0.01 | 0.00 (0.00-Inf), P = 1.00 | |
| Splenic parenchyma invasion | 14.93 (1.83-121.81), P = 0.01 | 1.00 (1.00-1.00), P = NaN | 1.23 (0.17-8.87), P = 0.84 | - | |
| Adjuvant radiation therapy | 0.97 (0.52-1.81), P = 0.92 | - | 0.81 (0.48-1.37), P = 0.43 | - | |
| Adjuvant chemotherapy | 2.50 (0.89-7.00), P = 0.08 | - | 0.69 (0.39-1.22), P = 0.20 | - | |
| Radiographic splenic artery invasion | 2.12 (0.85-5.29), P = 0.11 | - | 1.45 (0.59-3.56), P = 0.41 | - | |
| Radiographic splenic vein invasion | 2.27 (1.04-4.94), P = 0.04 | 2.88 (0.33-24.96), P = 0.34 | 1.46 (0.68-3.13), P = 0.33 | - | |
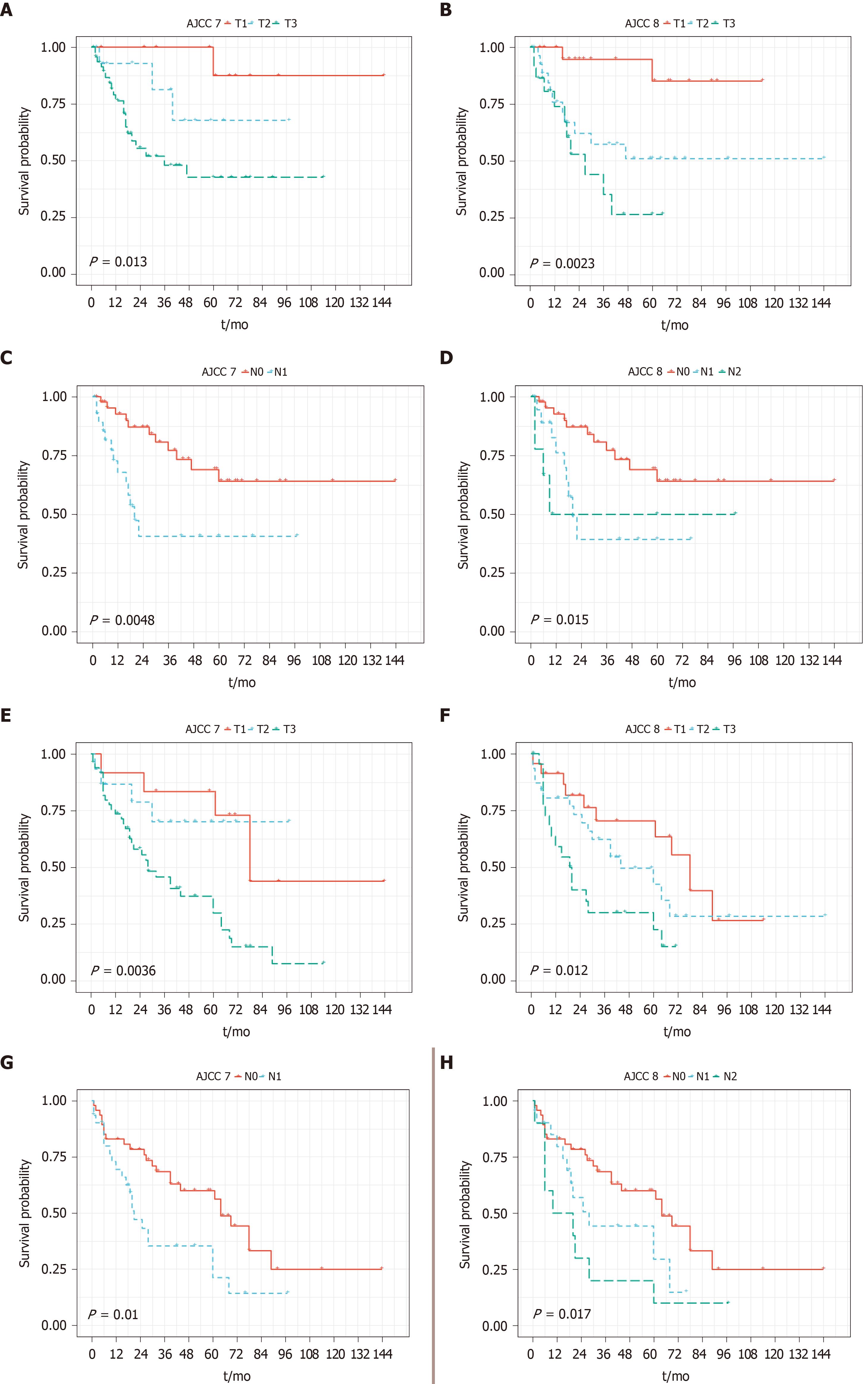
Per AJCC 7th edition staging manual, 13 patients (11.3%), 21 patients (18.3%), and 77 patients (67.0%) were classified as pT1, pT2, and pT3, respectively. Per AJCC 8th edition, 32 patients (27.8%), 46 patients (40.0%), and 36 patients (31.3%) were classified as pT1, pT2 and pT3, respectively. Among 46 patients with lymph node metastasis (pN1 per AJCC 7th edition), 31 patients and 15 patients were further diagnosed with pN1 and pN2 tumor according to AJCC 8th edition. T staging of the 7th system appeared to further stratify PFS and OS, however N1/N2 staging of the 8th edition appeared to further stratify PFS and OS (Figure 2).
A total of 339 patients with non-IPMN associated PDAC were included in the study. One hundred eighty-two patients (53.7%) were female and one hundred fifty-seven (46.3%) were male, with the median age of 66.7 years (Table 1).
In univariable analysis for non-IPMN associated PADC, nodal metastasis, pN1 stage (8th edition), and LVI were significantly associated with both PFS and OS. Tumor size, pathologic or radiographic evidence of splenic vein invasion, and adjuvant chemotherapy were all significantly associated with OS. In multivariable analysis, however, only pN1 (8th edition staging manual) remained as an independent prognosticator for both PFS and OS, while adjuvant chemotherapy remained as independent prognosticator for OS only (Table 3).
| Feature | Level | PFS | OS | ||
| HR (95%CI), P value | |||||
| Gender | 0.89 (0.66-1.20), P = 0.43 | - | 1.05 (0.82-1.35), P = 0.69 | - | |
| Age | 0.99 (0.98-1.01), P = 0.29 | - | 1.01 (1.00-1.03), P = 0.05 | 1.00 (0.98-1.02), P = 0.93 | |
| Tumor size | 1.07 (0.99-1.15), P = 0.09 | - | 1.08 (1.02-1.16), P = 0.01 | - | |
| Tumor differentiation | Well | - | - | - | - |
| Moderate | 1.11 (0.64-1.91), P = 0.71 | - | 1.33 (0.78-2.27), P = 0.29 | 1.56 (0.65-3.78), P = 0.32 | |
| Poor | 1.21 (0.68-2.14), P = 0.52 | - | 1.72 (0.99-2.98), P = 0.05 | 2.23 (0.90-5.53), P = 0.08 | |
| Positive lymph node | 1.06 (1.01-1.12), P = 0.03 | - | 1.08 (1.03-1.13), P < 0.01 | - | |
| T stage (AJCC 8th edition staging manual) | T1 | - | - | - | - |
| T2 | 1.39 (0.83-2.31), P = 0.21 | 1.22 (0.73-2.05), P = 0.45 | 0.97 (0.64-1.46), P = 0.87 | 1.12 (0.57-2.20), P = 0.73 | |
| T3 | 1.56 (0.92-2.62), P = 0.10 | 1.19 (0.68-2.07), P = 0.54 | 1.33 (0.88-2.03), P = 0.18 | 0.80 (0.39-1.64), P = 0.54 | |
| N stage (AJCC 8th edition staging manual) | N0 | - | - | - | - |
| N1 | 1.73 (1.25-2.39), P < 0.01 | 1.59 (1.09-2.32), P = 0.02 | 1.75 (1.33-2.32), P < 0.01 | 2.54 (1.41-4.58), P < 0.01 | |
| N2 | 1.57 (0.96-2.56), P = 0.07 | 1.47 (0.82-2.63), P = 0.20 | 1.83 (1.23-2.72), P < 0.01 | 2.45 (0.92-6.51), P = 0.07 | |
| Lymphovascular invasion | 1.43 (1.05-1.95), P = 0.02 | 1.07 (0.73-1.56), P = 0.74 | 1.46 (1.12-1.89), P < 0.01 | 0.82 (0.45-1.49), P = 0.51 | |
| Perineural invasion | 1.16 (0.76-1.78), P = 0.48 | - | 1.19 (0.83-1.73), P = 0.35 | - | |
| Splenic artery invasion | 1.41 (0.71-2.80), P = 0.33 | - | 1.38 (0.81-2.36), P = 0.24 | - | |
| Splenic vein invasion | 1.35 (0.82-2.23), P = 0.24 | - | 2.09 (1.44-3.03), P < 0.01 | 1.59 (0.83-3.02), P = 0.16 | |
| Positive margin | 0.78 (0.43-1.40), P = 0.40 | - | 1.20 (0.78-1.82), P = 0.41 | - | |
| Splenic invasion | 1.65 (0.95-2.85), P = 0.07 | - | 1.32 (0.82-2.11), P = 0.25 | - | |
| Adjuvant radiation therapy | 0.93 (0.69-1.27), P = 0.66 | - | 0.85 (0.64-1.12), P = 0.25 | - | |
| Adjuvant chemotherapy | 1.45 (0.90-2.34), P = 0.13 | - | 0.51 (0.37-0.71), P < 0.01 | 0.34 (0.20-0.58), P < 0.01 | |
| Radiographic splenic artery invasion | 1.27 (0.87-1.84), P = 0.21 | - | 1.13 (0.81-1.59), P = 0.47 | - | |
| Radiographic splenic vein invasion | 1.26 (0.88-1.80), P = 0.20 | - | 1.51 (1.10-2.07), P = 0.01 | 1.08 (0.65-1.80), P = 0.75 | |
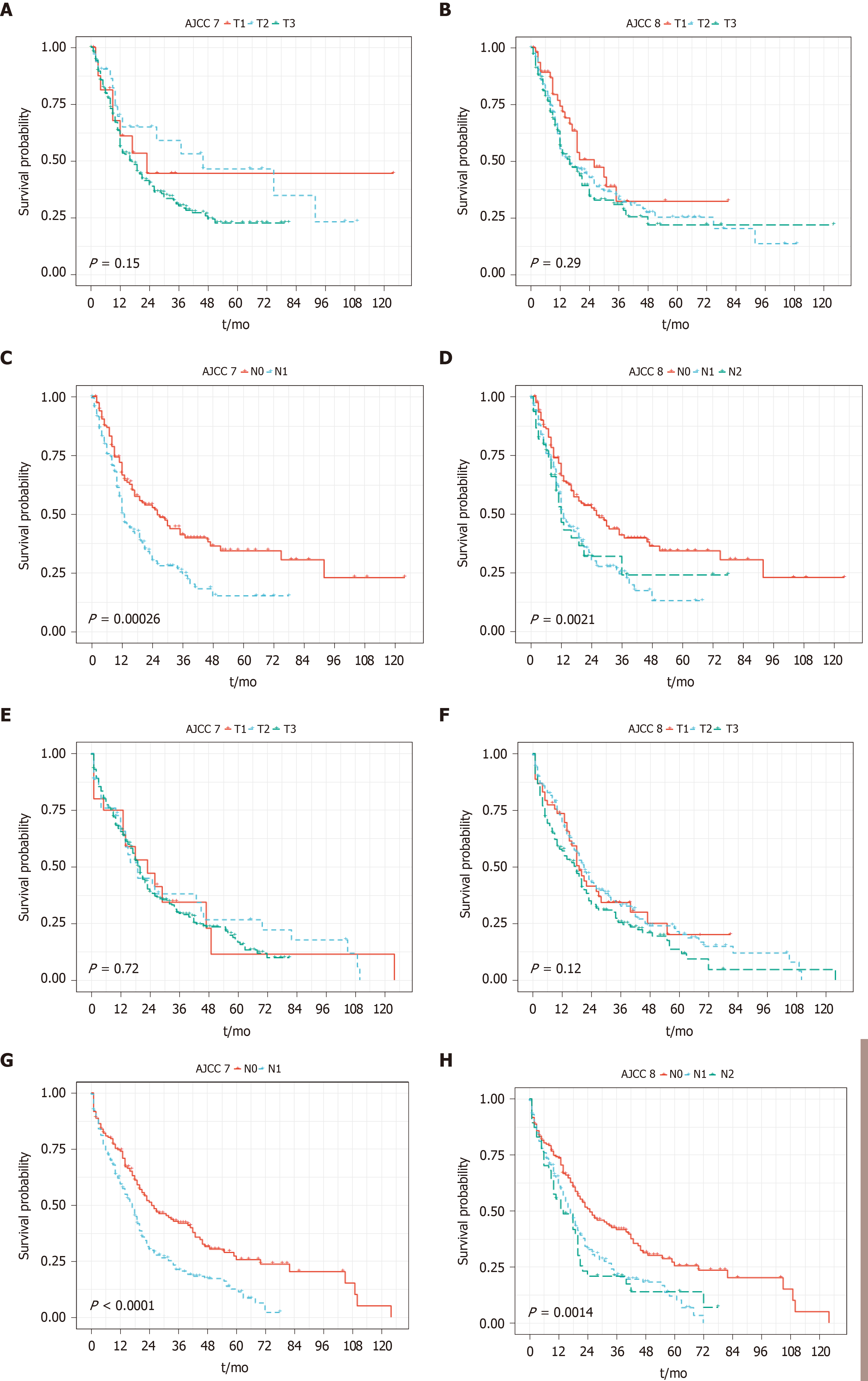
Per AJCC 7th edition staging manual, 19 patients (5.6%), 29 patients (8.6%), and 274 patients (80.8%) were classified as pT1, pT2, and pT3, respectively. Per AJCC 8th edition staging manual, 44 patients (13.0%), 149 patients (44.0%), and 130 patients (38.3%) were classified as pT1, pT2 and pT3, respectively. Among 191 patients with lymph node metastasis (pN1 per AJCC 7th edition staging manual), 149 patients and 42 patients were further sub classified into pN1 and pN2 tumor according to AJCC 8th edition. For PDAC without IPMN component, T staging from both staging systems failed to stratify PFS and OS. N staging of both staging systems could stratify PFS and OS, although dividing N category into pN1 and pN2 as in the 8th edition staging manual did not add further value to this group (Figure 3).
In this study, we evaluated the prognostic relevance of the 8th edition AJCC TNM staging manual in a large United States cohort of distal pancreatic cancer. The new staging system recognizes tumor size as one of the most important prognostic factors for tumor staging. In practice, the vast majority of PDAC cases had spread into the peripancreatic soft tissue and were staged as pT3 even after the introduction of the 5th edition of the AJCC staging manual in 1997. By removing the diagnostic criteria of extra pancreatic extension for pT3, patients with PDAC are distributed more evenly using the new AJCC 8th staging system.
Even with relatively strict selection criteria, wide heterogeneity of diagnostic groups was still inevitable within our study subjects. To simply this issue, we classified the cases as invasive IPMN and non-IPMN-associated adenocarcinoma. Consistent with previous reports[10,11], invasive IPMN is associated with lower T stage and N stage, as well as better survival in our study. The study revealed multiple independent prognostic factors for patients with distal pancreatic cancer, such as poor differentiation, tumor size, pT3 stage (8th edition staging manual), positive lymph node, pN2 stage (8th edition staging manual), LVI, splenic artery or vein invasion, positive resection margin, and splenic parenchymal invasion. Interestingly, very limited overlap was observed among patients with invasive IPMN and non-IPMN associated PDAC. For invasive IPMN patients, pT3 is the only significant prognostic factor for OS upon multivariable analysis. On the other hand, lymph node metastasis (pN1 stage per 8th edition) appears to be the only independent prognosticator for both PFS and OS in non-IPMN-assoiciated adenocarcinoma. The underlying mechanism account for these differences as well as their distinct clinical behavior and survival is not clear, but the molecular pathways underlying tumorigenesis for each entity might provide clues in the future.
Consistent with a recent study[16], our study demonstrates that the AJCC 7th edition T staging better stratifies survival in patients with invasive IPMN. Tumor size alone is not an independent prognostic factor based on multivariable analysis[16]. It’s interesting to identify pT3 (8th edition) as the only significant risk factor for survival in our study. One possible explanation is that majority of the pT3 tumor (> 4 cm per 8th edition staging manual) extend into peripancreatic soft tissue, and would also be staged as pT3 per the 7th edition staging manual[17]. In contrast to the T staging, N1/N2 staging of the 8th edition staging manual appears to further stratify PFS and OS in invasive IPMN.
An unexpected finding in this study is that T staging from both staging systems failed to stratify PFS and OS in resected distal non-IPMN associated PDAC. Multiple studies have demonstrated the clinical relevance, reproducibility, and risk stratification of the AJCC 8th edition staging manual[5,12-15]. However, due to the relative rarity, resected distal PDAC is likely underrepresented among those published studies. It should be noted that the stage-independent OS in distal pancreatic cancer is much worse as compared to its counterpart in the pancreatic head, and tumor location itself has been considered as a prognostic factor for survival in pancreatic cancer patients[18,19]. Delay in diagnosis is likely the major reason for its poor prognosis, although other facts may also play a role. For example, distal pancreatic cancer patients are significantly older at the time of diagnosis, and less dissectible lymph nodes are present at the distal portion of the pancreas[19]. Unlike invasive IPMN, lymph node metastasis is the most important prognostic factor for survival in non-IPMN associated PDAC. N staging of both 7th and 8th edition could stratify PFS and OS, although dividing N category into pN1 and pN2 in the 8th edition staging manual did not add further value in this group. The prognostic value of lymph node involvement has also been reported in a recent large scaled multi-institutional study. Morales-Oyarvide et al[20] demonstrated that the AJCC 8th edition staging system was a practical classification of lymph node involvement. Similar to other related validation studies[5,12-16], predominant patient population in this study (74%) had PDAC in the head of pancreas, and only 14% of the patients had PDAC in the tail of pancreas. Notably, the prognostic value of lymph node involvement was weaker in patients with resected distal pancreatic cancer[20].
This study has several strengths. It was a multicentered large-scale study designed to validate the major changes in the newer AJCC TNM staging system for resected distal pancreatic cancers. This study provides important insights for future revision of the AJCC staging system. Furthermore, all cases were re-staged according to the AJCC 7th or 8th edition and re-reviewed by pathologists subspecialized in gastrointestinal and pancreatic pathology. In addition, we included several potentially prognostic parameters such as radiographic evidence and histologic evidence of splenic vasculature and parenchymal invasion. In our study, we also compared the clinical behavior and risk stratification for invasive IPMN and non-IPMN associated PDAC.
Our study also has some limitations. First, all cases were collected from major academic cancer centers that might have introduced selection bias. Second, the cases were collected from a 13-year period of time (2005-2018) during which multiple different AJCC staging editions (5th to 7th edition) had been applied for pancreatic cancer staging. However, all cases in this study were re-staged according to the 7th and 8th AJCC editions. The size of invasive IPMN was recorded from the surgical pathology report as this parameter is difficult to generate as it depends on the combination of macroscopic examination, sampling, and histology; histology review with measurement on slide alone does not provide an accurate assessment of this parameter.
In conclusion, our study demonstrates that the AJCC 8th edition TNM staging system provides even distribution for the T staging, however, it does not improve risk stratification when compared to previous staging system for resectable distal pancreatic cancers. Our study also demonstrates the significant difference of clinical outcome and risk stratification between invasive IPMN and non-IPMN associated PDAC. Our study indicates that tumor location and subtype are important factors to be considered in future revisions of the AJCC staging system for pancreatic cancer.
Pancreatic ductal adenocarcinoma (PDAC) is one of the leading causes of cancer related death worldwide. For the purpose of better risk stratification and clinical management, the American Joint Committee on Cancer (AJCC) published the eighth edition staging manual for pancreatic cancer that has introduced significant changes for both tumor (T) staging and nodal (N) staging. Notably majority of the validation studies were focused on PDAC in the head of pancreas, and the resected distal pancreatic adenocarcinoma was likely underrepresented due to its clinical rarity. Whether the AJCC 8th edition staging manual provides equal risk stratification for both invasive intraductal papillary mucinous neoplasm (IPMN) and non-IPMN associated PDAC is also unclear.
It’s important to investigate whether the new AJCC staging system provides risk stratification in patients with distal pancreatic cancers. It’s also important to investigate the clinical behavior and risk stratification for invasive IPMN and non-IPMN associated PDAC.
This study aims to validate the AJCC 8th edition staging manual in distal PDAC.
Clinicopathological data of resected distal PDAC cases were retrieved. All cases were re-staged based on the AJCC 7th and 8th edition, respectively. Categorical variables were compared with Fisher’s exact test. Progression-free survival (PFS) and overall survival (OS) were evaluated through Kaplan-Meier curves and univariate/multivariate analyses.
T and N staging of both 7th and 8th edition sufficiently stratify PFS and OS in the entire cohort, although dividing into N1 and N2 according to the 8th edition does not show additional stratification. For PDAC arising in IPMN, T staging of the 7th edition and N1/N2 staging of the 8th edition appear to further stratify PFS and OS. For PDAC without an IPMN component, T staging from both versions fails to stratify PFS and OS.
The AJCC 8th edition TNM staging system provides even distribution for the T staging, however, it does not provide better risk stratification than previous staging system for distal pancreatic cancer. There is significant difference of clinical outcome and risk stratification between invasive IPMN and non-IPMN associated PDAC.
Tumor location and subtype are important factors to be considered in future revisions of the AJCC staging system for pancreatic cancer.
We thank all participating institutions for the support of this study.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): E
P-Reviewer: Liao R, Wang ZF, Zou J S-Editor: Dou Y L-Editor: A E-Editor: Qi LL
| 1. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13300] [Cited by in RCA: 15435] [Article Influence: 2572.5] [Reference Citation Analysis (2)] |
| 2. | Cooperman AM, Bruckner H, Snady H, Hammerman H, Fader A, Feld M, Golier F, Rush T, Siegal J, Kasmin F, Cohen S, Wayne MG, Iskandar ME, Steele JG. Cancer of the Pancreas-Actual 5, 10, and 20+Year Survival: The Lucky and Fortunate Few. Surg Clin North Am. 2018;98:73-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Christein JD, Kendrick ML, Iqbal CW, Nagorney DM, Farnell MB. Distal pancreatectomy for resectable adenocarcinoma of the body and tail of the pancreas. J Gastrointest Surg. 2005;9:922-927. [PubMed] |
| 4. | Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR, Sullivan DC, Jessup JM, Brierley JD, Gaspar LE, Schilsky RL, Balch CM, Winchester DP, Asare EA, Madera M, Gress DM, Meyer LR. American Joint Committee on Cancer. AJCC cancer staging manual. 8th ed. New York, NY: Springer; 2017. |
| 5. | Kamarajah SK, Burns WR, Frankel TL, Cho CS, Nathan H. Validation of the American Joint Commission on Cancer (AJCC) 8th Edition Staging System for Patients with Pancreatic Adenocarcinoma: A Surveillance, Epidemiology and End Results (SEER) Analysis. Ann Surg Oncol. 2017;24:2023-2030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 196] [Article Influence: 24.5] [Reference Citation Analysis (2)] |
| 6. | Adsay V, Mino-Kenudson M, Furukawa T, Basturk O, Zamboni G, Marchegiani G, Bassi C, Salvia R, Malleo G, Paiella S, Wolfgang CL, Matthaei H, Offerhaus GJ, Adham M, Bruno MJ, Reid MD, Krasinskas A, Klöppel G, Ohike N, Tajiri T, Jang KT, Roa JC, Allen P, Fernández-del Castillo C, Jang JY, Klimstra DS, Hruban RH; Members of Verona Consensus Meeting, 2013. Pathologic Evaluation and Reporting of Intraductal Papillary Mucinous Neoplasms of the Pancreas and Other Tumoral Intraepithelial Neoplasms of Pancreatobiliary Tract: Recommendations of Verona Consensus Meeting. Ann Surg. 2016;263:162-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 191] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 7. | Furukawa T, Hatori T, Fujita I, Yamamoto M, Kobayashi M, Ohike N, Morohoshi T, Egawa S, Unno M, Takao S, Osako M, Yonezawa S, Mino-Kenudson M, Lauwers GY, Yamaguchi H, Ban S, Shimizu M. Prognostic relevance of morphological types of intraductal papillary mucinous neoplasms of the pancreas. Gut. 2011;60:509-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 211] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 8. | Fritz S, Fernandez-del Castillo C, Mino-Kenudson M, Crippa S, Deshpande V, Lauwers GY, Warshaw AL, Thayer SP, Iafrate AJ. Global genomic analysis of intraductal papillary mucinous neoplasms of the pancreas reveals significant molecular differences compared to ductal adenocarcinoma. Ann Surg. 2009;249:440-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 81] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 9. | Omori Y, Ono Y, Tanino M, Karasaki H, Yamaguchi H, Furukawa T, Enomoto K, Ueda J, Sumi A, Katayama J, Muraki M, Taniue K, Takahashi K, Ambo Y, Shinohara T, Nishihara H, Sasajima J, Maguchi H, Mizukami Y, Okumura T, Tanaka S. Pathways of Progression From Intraductal Papillary Mucinous Neoplasm to Pancreatic Ductal Adenocarcinoma Based on Molecular Features. Gastroenterology. 2019;156:647-661.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 125] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 10. | Woo SM, Ryu JK, Lee SH, Yoo JW, Park JK, Kim YT, Yoon YB. Survival and prognosis of invasive intraductal papillary mucinous neoplasms of the pancreas: comparison with pancreatic ductal adenocarcinoma. Pancreas. 2008;36:50-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 53] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 11. | Yamada S, Fujii T, Hirakawa A, Takami H, Suenaga M, Hayashi M, Niwa Y, Hattori N, Iwata N, Kanda M, Tanaka C, Kobayashi D, Nakayama G, Koike M, Fujiwara M, Kodera Y. Comparison of the Survival Outcomes of Pancreatic Cancer and Intraductal Papillary Mucinous Neoplasms. Pancreas. 2018;47:974-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 12. | Allen PJ, Kuk D, Castillo CF, Basturk O, Wolfgang CL, Cameron JL, Lillemoe KD, Ferrone CR, Morales-Oyarvide V, He J, Weiss MJ, Hruban RH, Gönen M, Klimstra DS, Mino-Kenudson M. Multi-institutional Validation Study of the American Joint Commission on Cancer (8th Edition) Changes for T and N Staging in Patients With Pancreatic Adenocarcinoma. Ann Surg. 2017;265:185-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 351] [Article Influence: 43.9] [Reference Citation Analysis (0)] |
| 13. | Pu N, Li J, Xu Y, Lee W, Fang Y, Han X, Zhao G, Zhang L, Nuerxiati A, Yin H, Wu W, Lou W. Comparison of prognostic prediction between nomogram based on lymph node ratio and AJCC 8th staging system for patients with resected pancreatic head carcinoma: a SEER analysis. Cancer Manag Res. 2018;10:227-238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 14. | Asano D, Nara S, Kishi Y, Esaki M, Hiraoka N, Tanabe M, Shimada K. A Single-Institution Validation Study of Lymph Node Staging By the AJCC 8th Edition for Patients with Pancreatic Head Cancer: A Proposal to Subdivide the N2 Category. Ann Surg Oncol. 2019;26:2112-2120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Kwon W, He J, Higuchi R, Son D, Lee SY, Kim J, Kim H, Kim SW, Wolfgang CL, Cameron JL, Yamamoto M, Jang JY. Multinational validation of the American Joint Committee on Cancer 8th edition pancreatic cancer staging system in a pancreas head cancer cohort. J Hepatobiliary Pancreat Sci. 2018;25:418-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 16. | Fan Z, Cheng H, Jin K, Gong Y, Huang Q, Xu J, Ni Q, Yu X, Liu C, Luo G. AJCC 7th edition staging classification is more applicable than AJCC 8th edition staging classification for invasive IPMN. World J Surg Oncol. 2019;17:137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Saka B, Balci S, Basturk O, Bagci P, Postlewait LM, Maithel S, Knight J, El-Rayes B, Kooby D, Sarmiento J, Muraki T, Oliva I, Bandyopadhyay S, Akkas G, Goodman M, Reid MD, Krasinskas A, Everett R, Adsay V. Pancreatic Ductal Adenocarcinoma is Spread to the Peripancreatic Soft Tissue in the Majority of Resected Cases, Rendering the AJCC T-Stage Protocol (7th Edition) Inapplicable and Insignificant: A Size-Based Staging System (pT1: ≤2, pT2: >2-≤4, pT3: >4 cm) is More Valid and Clinically Relevant. Ann Surg Oncol. 2016;23:2010-2018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 94] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 18. | Birnbaum DJ, Bertucci F, Finetti P, Birnbaum D, Mamessier E. Head and Body/Tail Pancreatic Carcinomas Are Not the Same Tumors. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 19. | Artinyan A, Soriano PA, Prendergast C, Low T, Ellenhorn JD, Kim J. The anatomic location of pancreatic cancer is a prognostic factor for survival. HPB (Oxford). 2008;10:371-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 185] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 20. | Morales-Oyarvide V, Rubinson DA, Dunne RF, Kozak MM, Bui JL, Yuan C, Qian ZR, Babic A, Da Silva A, Nowak JA, Khalaf N, Brais LK, Welch MW, Zellers CL, Ng K, Chang DT, Miksad RA, Bullock AJ, Tseng JF, Swanson RS, Clancy TE, Linehan DC, Findeis-Hosey JJ, Doyle LA, Hornick JL, Ogino S, Fuchs CS, Hezel AF, Koong AC, Wolpin BM. Lymph node metastases in resected pancreatic ductal adenocarcinoma: predictors of disease recurrence and survival. Br J Cancer. 2017;117:1874-1882. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 81] [Article Influence: 10.1] [Reference Citation Analysis (0)] |