Published online Jan 21, 2019. doi: 10.4292/wjgpt.v10.i1.1
Peer-review started: August 27, 2018
First decision: October 5, 2018
Revised: October 24, 2018
Accepted: December 10, 2018
Article in press: December 11, 2018
Published online: January 21, 2019
Processing time: 147 Days and 5.2 Hours
Cirrhosis of liver is a major problem in the western world. Portal hypertension is a complication of cirrhosis and can lead to a myriad of pathology of which include the development of porto-systemic collaterals. Gastrointestinal varices are dilated submucosal veins, which often develop at sites near the formation of gastroesophageal collateral circulation. The incidence of varices is on the rise due to alcohol and obesity. The most significant complication of portal hypertension is life-threatening bleeding from gastrointestinal varices, which is associated with substantial morbidity and mortality. In addition, this can cause a significant burden on the health care facility. Gastrointestinal varices can happen in esophagus, stomach or ectopic varices. There has been considerable progress made in the understanding of the natural history, pathophysiology and etiology of portal hypertension. Despite the development of endoscopic and medical treatments, early mortality due to variceal bleeding remains high due to significant illness of the patient. Recurrent variceal bleed is common and in some cases, there is refractory variceal bleed. This article aims to provide a comprehensive review of the management of gastrointestinal varices with an emphasis on endoscopic interventions, strategies to handle refractory variceal bleed and newer endoscopic treatment modalities. Early treatment and improved endoscopic techniques can help in improving morbidity and mortality.
Core tip: Cirrhosis of liver can lead to gastrointestinal varices. Gastrointestinal bleed from varices can be debilitating and can cause morbidity and mortality if not well controlled. This is a detailed review on the endoscopic management of variceal bleed and gives an insight into some of the new endoscopic techniques that can be helpful in treating variceal bleed.
- Citation: Boregowda U, Umapathy C, Halim N, Desai M, Nanjappa A, Arekapudi S, Theethira T, Wong H, Roytman M, Saligram S. Update on the management of gastrointestinal varices. World J Gastrointest Pharmacol Ther 2019; 10(1): 1-21
- URL: https://www.wjgnet.com/2150-5349/full/v10/i1/1.htm
- DOI: https://dx.doi.org/10.4292/wjgpt.v10.i1.1
Less than 1% of the United States population have cirrhosis of liver[1]. In the western world, the most common etiology of portal hypertension is cirrhosis due to alcoholic liver disease, nonalcoholic steatohepatitis (NASH), and hepatitis C infection[2]. According to a recent estimate 15 million people in the United States have alcohol abuse disorder, nearly 88000 people die annually due to alcohol, and 10%-15% of people with alcoholism develop cirrhosis[3]. Another 3 million people have chronic hepatitis C infection[4], and 25%-28% of these patients go on to develop cirrhosis[5,6]. Nonalcoholic fatty liver disease (NAFLD) is a spectrum of chronic liver disease consisting of mild to an advanced form of fatty degeneration of the liver described as NASH. Prevalence of NASH is estimated to be around 3%-8% of the general population, and 10%-25% of these patients progress to cirrhosis[7]. Moreover, the rate of NASH is rising due to the increasing prevalence of obesity, insulin resistance, and diabetes. NASH is the second most common cause among patients with cirrhosis who are currently waiting for liver transplant. Recent trends have indicated that NAFLD is expected to overtake hepatitis C and alcohol as the most common etiology of liver cirrhosis and indication for liver transplants in the western countries by year 2030[8,9]. Therefore, in order to reduce morbidity and mortality, as well as the overall burden on healthcare, it is essential to develop cost-effective screening and management strategies for portal hypertension related to cirrhosis.
Gastrointestinal varices are abnormally dilated submucosal veins in the digestive tract due to portal hypertension and can potentially cause life-threatening bleeding. Prevalence of varices increases with the severity of liver disease (Child-Pugh class A 42.7%, class B 70.7% and class C 75.5%)[2,10]. The Child-Pugh score is described in Table 1. The incidence of esophageal varices in cirrhotic patients is around 5% at the end of one year and 28% at the end of three years. Small varices progress to large varices at a rate of 10% to 12% annually[11]. Approximately 50% of all patients with a new diagnosis of cirrhosis have gastrointestinal varices[2]. Annual risk of variceal bleeding among small and large varices is 5% and 15% respectively[12]. The six-week mortality rate among patients with index variceal bleeding is approximately 20%[13]. Risk of rebleeding without endoscopic intervention is almost 60% with an increased mortality rate (33%)[14].
| Child-Pugh scoring | |||
| 1 point | 2 points | 3 points | |
| Hepatic encephalopathy | None | Grade I-II (or suppressed with medication) | Garde III-IV (or refractory) |
| Ascites | None | Mild | Moderate to severe |
| PT/INR | < 1.7 | 1.71-2.30 | > 2.30 |
| Serum albumin (g/L) | > 35 | 28-35 | < 28 |
| Total bilirubin (μmol/L) | < 34 | 34-50 | > 50 |
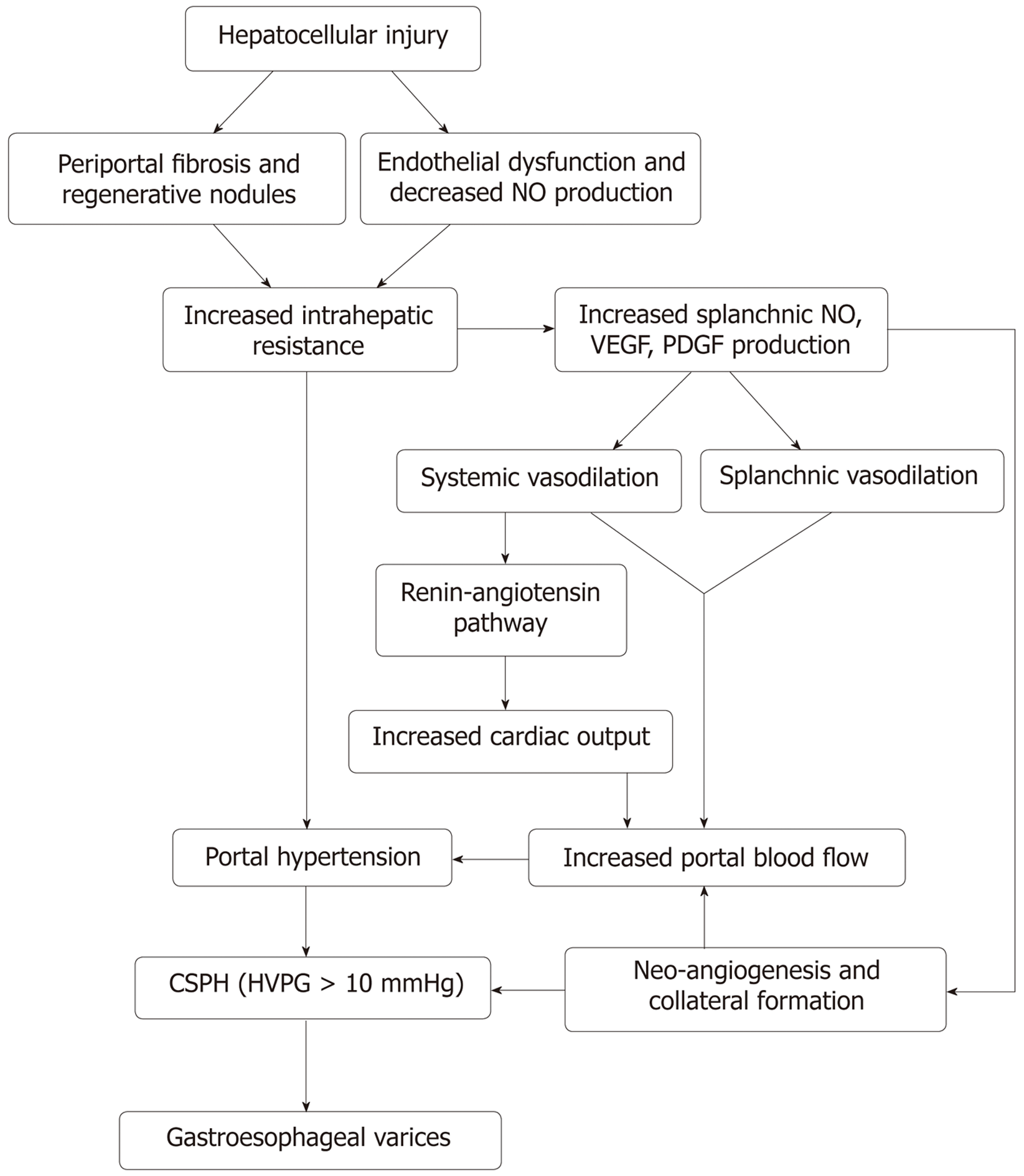
The development of portal hypertension in cirrhosis is a multifactorial process with changes in both the portal and systemic circulation. This is shown in Figure 1. The majority of patients in western countries with portal hypertension have underlying cirrhosis. Non-cirrhotic portal hypertension is typically less common and encompasses a broad range of pathology, typically vascular in origin[15]. Portal hypertension is defined as hepatic vein pressure gradient (HVPG) more than 5 mmHg. The HVPG is a surrogate means to measure pressure in the portal veins. Normal HVPG (= hepatic vein wedge pressure - free hepatic vein pressure) is around 3-5 mmHg. Varices usually develop when patients have HVPG >10 mmHg and presence of HVPG > 12 mmHg is a risk factor for variceal bleeding. Reduction in HVPG to less than 12 mmHg or by ≥ 20% from baseline reduces the risk of initial bleeding, and other complications of portal hypertension (ascites, encephalopathy)[14].
Porto-systemic shunting due to portal hypertension causes diversion of the portal blood into systemic circulation and results in variceal formation. Presence of ongoing liver injury due to alcohol, viral hepatitis (hepatitis B and C), or NASH can lead to increase in the size of the varices, whereas elimination of etiological factor can lead to decrease in the size or disappearance of varices in patients with alcoholic cirrhosis[16,17].
Architectural distortion: Hepatocellular injury causes transformation of hepatic stellate cells into myofibroblasts. Increased expression of pro-inflammatory genes and fibrotic activity, as a result, promotes neoangiogenesis and interstitial collagen deposition resulting in distortion of the hepatic sinusoidal architecture[18,19]. Architectural damage and regenerative nodules are responsible for nearly 2nd/3rd of the increase in intrahepatic resistance.
Increased vascular resistance: In addition to the known anatomical disruption in the sinusoidal architecture, it is now understood that there are changes in the neurohormonal regulation of vascular tone within the portal circulation. The hepatic injury causes increased production of vasoconstrictors (endothelin 1[20,21] and thromboxane A2[22,23]) and reduction in nitric oxide (NO) synthesis due to sinusoidal endothelial dysfunction[24]. The imbalance in the production of vasoconstrictors and vasodilators causes impaired vasomotor control leading to further increase in resistance and is responsible for approximately 1st/3rd of the increase in intrahepatic resistance[25,26].
Portal hypertension induces neurohormonal changes in the splanchnic circulation as well. Overproduction of NO from splanchnic endothelium leads to reduced splanchnic and systemic vascular resistance[27-29]. Furthermore, a compensatory activation of the renin-angiotensin mechanism leads to increased cardiac output and hepatic blood flow. Increased portal pressure is also suspected to result in overproduction of angiogenic factors such as vascular endothelial growth factor, platelet-derived growth factor at the microcirculatory level, contributing to angiogenesis and collateral formation resulting in varices[30,31].
Gastrointestinal varices develop as a consequence of portal hypertension. Most common etiology of portal hypertension in the United States is cirrhosis due to alcohol, NASH, and hepatitis C. The exact prevalence of portal hypertension is not known. Causes of portal hypertension are classified as below.
Extrahepatic: Portal vein thrombosis, splenic vein thrombosis.
Intrahepatic: Schistosomiasis, congenital hepatic fibrosis, and sarcoidosis. (1) Sinusoidal: Cirrhosis due to viral hepatitis (hepatitis B and C), NASH, alcohol, primary biliary cirrhosis, primary sclerosing cholangitis, hemochromatosis, Wilson’s disease, and cytotoxic drugs; and (2) Postsinusoidal: Budd-Chiari syndrome, caval web, constrictive pericarditis, and veno-occlusive disorders.
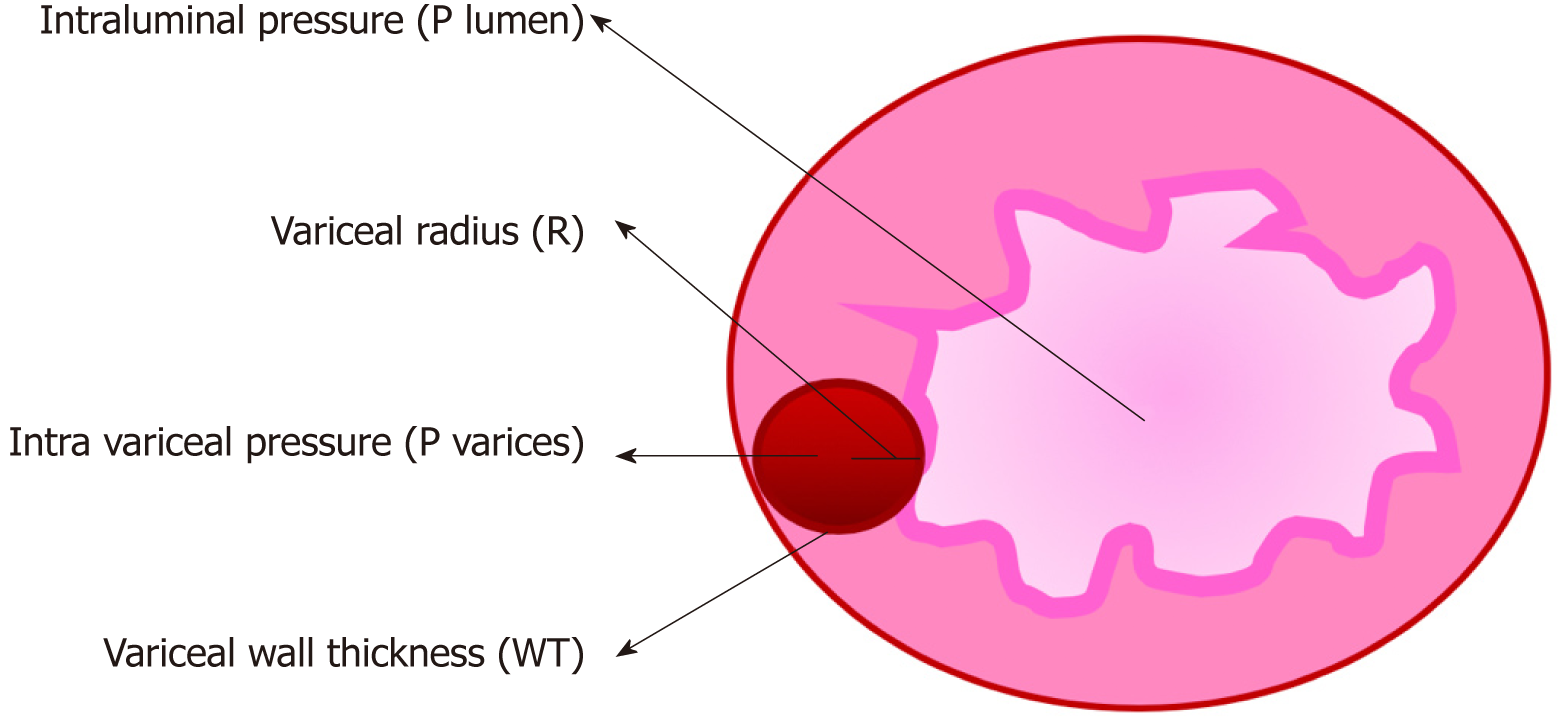
Increased blood flow through the portosystemic collaterals due to portal hypertension causes dilation of the submucosal venous plexus resulting in elevated intravariceal pressure and wall tension. The mechanism of variceal rupture can be explained by Frank’s modified Laplace’s law[32]. This is shown in Figure 2.
Wall tension (T) = [Transmural pressure (Pvarices-Plumen) × variceal radius (R)]/[Variceal wall thickness (WT)].
Rise in portal pressure causes increased flow through the varices and thus increased intravariceal pressure. In a randomized control trial (RCT) patients with HVPG < 12 mmHg did not develop variceal bleeding[33], and presence of HVPG > 20 mmHg was associated with high risk of failed hemostasis and death[34]. Whereas, a decrease in HVPG > 20% from the baseline reduces complications of portal hypertension including bleeding, ascites, encephalopathy, and death[35-37].
Large varices (> 5 mm) have a higher tendency to bleed due to increased wall tension as explained above.
Increased wall tension and the presence of ‘red wale sign’ (dilated capillaries on the variceal wall) indicate a high risk for bleeding.
Presence of coagulopathy, infection, and decompensated cirrhosis are other risk factors for variceal bleeding.
Esophago-gastro duodenoscopy (EGD) is the gold standard procedure used in the diagnosis of gastroesophageal varices (GOVs). Based on the endoscopic assessment, GOVs are classified into small (< 5 mm), and large varices(> 5 mm)[38] for clinical management. Disadvantages of endoscopy include the risk of sedation, higher cost, bleeding and risk of aspiration.
Endoscopic ultrasound (EUS) has been evaluated as a diagnostic tool in assessing GOVs. EUS is better than EGD in detecting gastric varices (GVs), and its ability to evaluate the anatomy of collateral and perforating veins makes it an excellent choice in monitoring treatment response to endoscopic variceal ligation (EVL) and predicting recurrence[39-41]. Currently, EUS is not considered as a primary diagnostic modality due to limited availability of local expertise.
A recent meta-analysis reviewed the use of capsule endoscopy for the diagnosis and grading of esophageal varices and noted a diagnostic accuracy of 90% with a pooled sensitivity and specificity of 83% and 85%, respectively[42]. The inability of capsule endoscopy to detect GVs is a significant drawback. Even though capsule endoscopy is relatively less invasive and does not require sedation, the diagnostic sensitivity is not adequate to advocate for index surveillance. It may be a consideration for a select subgroup of high-risk patients who are unwilling to undergo more invasive traditional endoscopic evaluation[43,44]. One study showed that 97% of the patients preferred capsule endoscopy over endoscopy with or without sedation[44].
Various clinical findings, laboratory tests, and imaging studies have been considered as predictors of clinically significant portal hypertension (CSPH) (HVPG > 12 mmHg); however, they are not accurate enough to either reliably diagnose or exclude CSPH. Specifically, transient elastography, platelet count, spleen size, magnetic resonance elastography, and splenic stiffness are the most commonly used parameters to predict the presence of CSPH and varices in patients with cirrhosis. The presence of portosystemic collaterals on ultrasound, computed tomography, or magnetic resonance imaging is indicative of CSPH and necessitate screening endoscopy[38]. Liver stiffness measured by transient elastography in combination with platelet count can rule out presence high-risk varices[45]. A liver stiffness < 20 kPa and platelet count > 150000/μL indicate < 5% chance of having high-risk varices, and screening endoscopy can be safely deferred as long as ongoing clinical monitoring can be assured[46].
Esophageal varices are the most common type of gastrointestinal varices, and their prevalence in Child-Pugh class A is 42.7%, around 70.7% in class B, and 75.5% in class C[2]. The bleeding risk for small varices and large varices is around 5% and 15% per year respectively. Early mortality rate (6 wk) is approximately 20%[47] in esophageal varices after index bleeding.
Venous drainage from the sub-mucosal venous plexus of the esophagus drains into the collateral veins around the esophagus. The interconnected collateral venous plexus runs longitudinally along the esophagus and communicates with submucosal venous plexus through perforating veins in the palisading area. The cervical esophagus drains into inferior thyroid vein, the thoracic esophagus drains to azygous, hemizygous, intercostal, and bronchial veins, whereas the abdominal portion of the esophagus drains into the left gastric vein, which in turn empties into the portal vein. Portal hypertension leads to shunting of blood from the portal circulation into these low pressure, thin-walled submucosal systemic veins and manifest as varices.
Grade I: Varices extending just above the mucosal level.
Grade II: Varices projecting by one-third of the luminal diameter that cannot be compressed with air insufflation.
Grade III: Varices projecting up to 50% of the luminal diameter and in contact with each other.
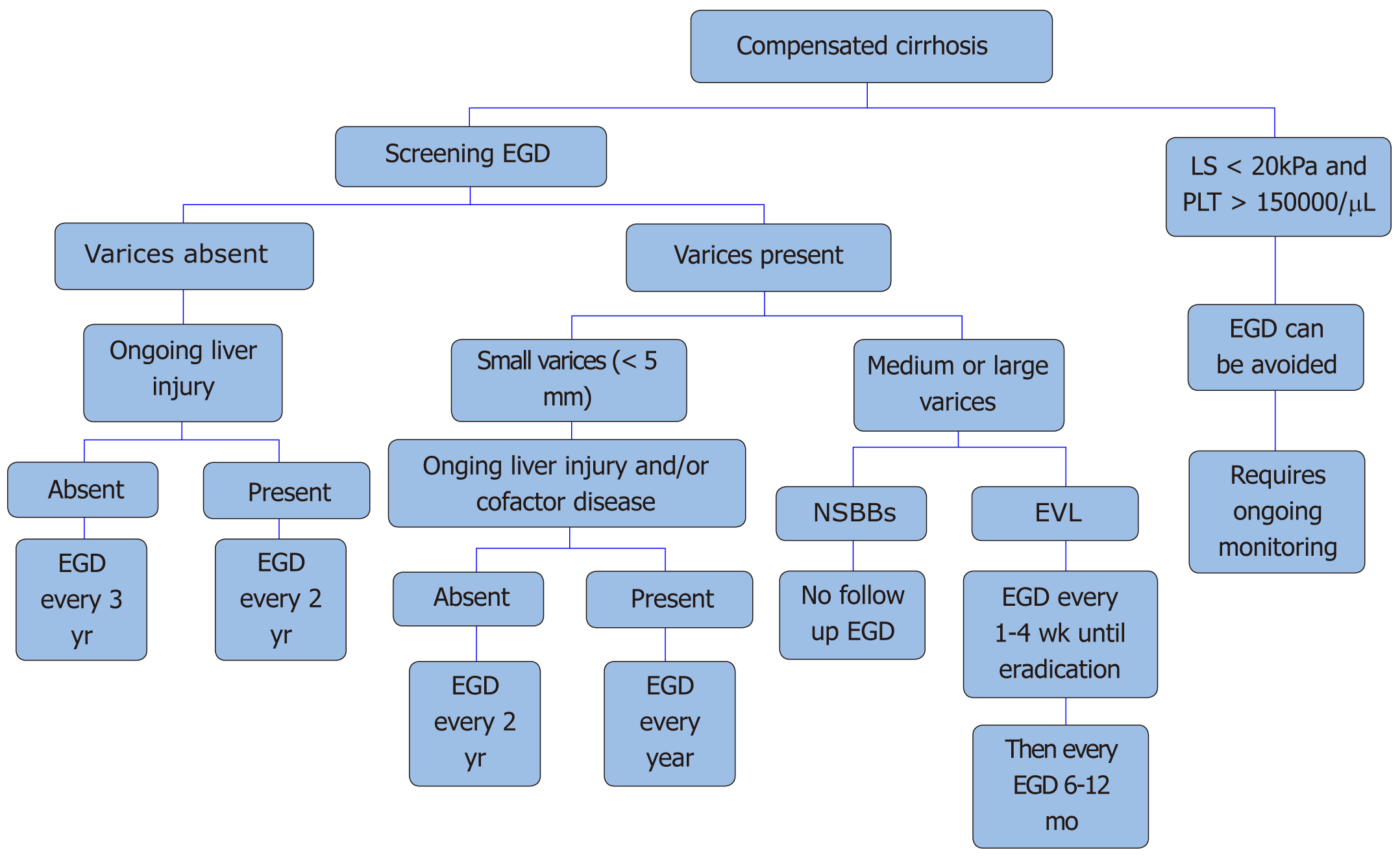
Shown in Figure 3. All patients who are newly diagnosed with cirrhosis should be screened for esophageal varices. Patients with compensated cirrhosis without varices in the absence of ongoing liver injury, endoscopy should be done every three years. Those who have compensated cirrhosis without varices, but have an ongoing liver injury (alcohol abuse, hepatitis C) and/or other cofactor diseases (alcohol/obesity) screening endoscopy should be repeated every two years.
Patients with small varices without ongoing liver injury or cofactor disease endoscopy is recommended every two years, and every year if either ongoing liver injury or cofactor disease is present. Patients with medium and large size varices should be started on nonselective beta-blockers or considered for EVL. If the patient is on nonselective beta blockers, no further surveillance endoscopy is needed.
On the other hand, if EVL is considered for primary prophylaxis endoscopy should be done every 1-2 wk until eradication and then repeated every 6-12 mo.
Either nonselective beta blockers or EVL (Figure 4) can be used as primary prophylaxis of variceal hemorrhage in patients with medium/large esophageal varices. Only approved nonselective beta-blockers are propranolol, nadolol, and carvedilol[38,48-52]. The choice should be made based on the cost, contraindications, availability, and patient preference. Nonselective beta-blockers are preferred over EVL due to their low cost, easy availability, ability to reduce the HVPG. Nonselective beta-blockers reduce the risk of hemorrhage and other complications (ascites, encephalopathy, and death) of portal hypertension[37]. Based on the currently available data, beta-blockers do not prevent the development of varices or their progression from small to large varices, although there is some reported benefit of reduction in risk of bleeding.
The effect of nadolol on the progression of variceal size was studied in a prospective randomized study. A total of 161 patients were randomized into nadolol (n = 83) and placebo (n = 78) groups. All patients had yearly screening endoscopy and with a mean follow up of 36 mo. The cumulative probability of bleeding and progression of small varices was lower in nadolol group (20%) when compared to placebo (51%) (P < 0.001) (absolute risk difference: 31%; 95%CI: 17%-45%)[53]. However, this benefit has not been proven in other studies.
In a recent meta-analysis of 6 RCTs, the effect of nonselective beta-blockers in cirrhotic patients with no or small varices was analyzed. The incidence of large varices (OR = 1.05, 95%CI: 0.25-4.36; P = 0.95), first variceal bleeding (OR = 0.59, 95%CI: 0.24-1.47; P = 0.26) and death (OR = 0.70, 95%CI: 0.45-1.10; P = 0.12) were similar in both nonselective beta-blocker group and placebo group. However, the incidence of adverse events in the nonselective beta-blockers group was significantly higher than the placebo group. Notably, nonselective beta-blockers did not reduce the incidence of large varices or prevent the progression of small varices to large varices[54].
On the other hand, when compared to nonselective beta blockers, EVL has a higher rate of recurrence of varices, lacks the benefit of HVPG reduction, and needs further endoscopic surveillance. EVL has lower but more severe side effects (bleeding, ulcers, and strictures) compared to nonselective beta-blockers (weakness, tiredness, shortness of breath). However, there is no significant difference in the mortality rate between the two[46].
In a prospective randomized study, the combination of EVL and propranolol was compared to EVL alone among high-risk patients. One hundred forty-four patients in total were randomized into EVL + propranolol (n = 72) group and EVL alone (n = 72) group respectively. Addition of propranolol to EVL did not reduce the risk of first variceal bleed (7% vs 11%, P = 0.72) or death (8% vs 15%, P = 0.37). However, the combination group had significant adverse effects due to propranolol in 22% of the patients. Combination of nonselective beta-blockers and EVL is not recommended as primary prophylaxis due to a higher rate of side effects. However, the recurrence of varices was significantly lower when propranolol was added (P = 0.03)[55]. Recent practice society guidelines suggest the use of nonselective beta-blockers as a recommended therapy for primary prophylaxis for small varices with high-risk features (presence of ‘red wale’ signs or decompensated cirrhosis)[38,46].
Isosorbide mononitrate, sclerotherapy, glue injection, and transjugular intrahepatic portosystemic stent (TIPS) shunt are not used as primary prophylaxis due to a higher rate of side effects without mortality benefit.
Use of nonselective beta-blockers among patients who have cirrhosis with refractory ascites is controversial. A prospective case study showed that the use of nonselective beta-blockers in this patient group was associated with increased mortality[56]. Another study also showed the increased risk of renal injury, hospital stay and mortality with the use of nonselective beta-blockers with spontaneous bacterial peritonitis due to post-paracentesis circulatory dysfunction[57]. However, a meta-analysis of 3 RCTs and 13 observational studies (n = 8279) showed no significant difference in mortality or incidence of hepatorenal syndrome and spontaneous bacterial peritonitis among cirrhosis patients with refractory ascites, when treated with nonselective beta blockers[58]. Due to concern for possible deleterious effects in patients with advanced cirrhosis, many physicians now prefer EVL over nonselective beta blockers. Larger RCTs are required before nonselective beta-blockers are considered as a contraindication in this subgroup.
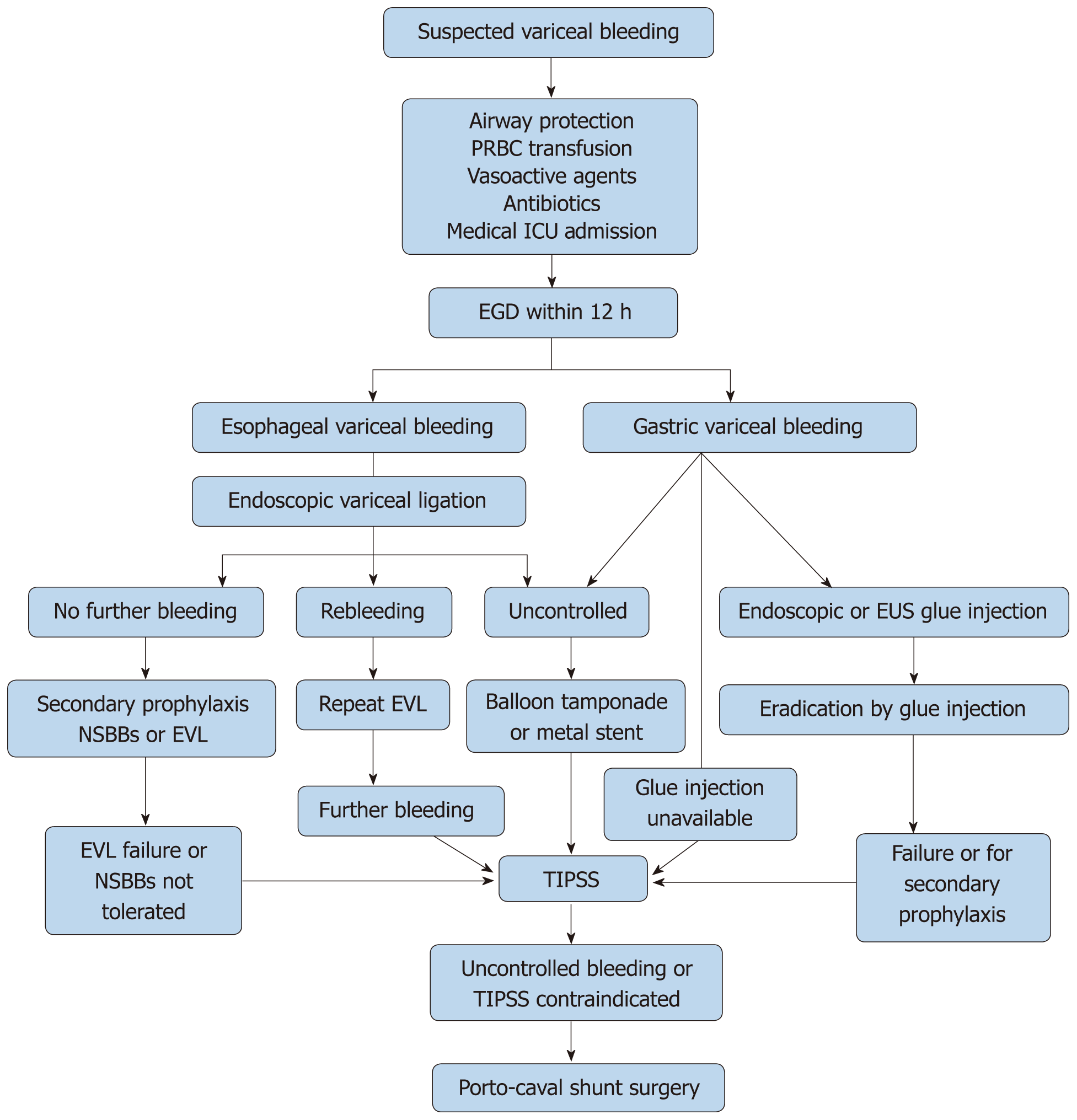
General measures: All patients with acute variceal bleeding should be resuscitated at an early stage to protect the airway and achieve hemodynamic stability, preferably in a medical intensive care unit. Prognostic indicators for early mortality due to acute variceal bleeding are HVPG, Child-Pugh score, and model for end-stage liver disease (MELD) score. A MELD score of > 19 showed 20% mortality due to index variceal bleeding[34,47,59]. When measured within 24 h of acute bleeding, HVPG > 20 mmHg predicts a high risk of early rebleeding and death[38,48,60]. The Child-Pugh score is also a significant predictor of early mortality and can help guide patient risk stratification[61]. Medical management with vasoactive agents, antibiotics, blood transfusion, combined with EVL is the standard of care in treating acute variceal bleeding.
Restrictive transfusion strategy: All patients with Hb ≤ 7 g/dL should get packed red blood cells to maintain hemoglobin at 7-8 g/dL. Previous RCTs have shown a survival benefit, reduced need for blood transfusion, and a lower rate of adverse events with a restrictive strategy when compared to liberal transfusion[62].
Most patients with acute variceal bleeding have elevated HVPG (> 12 mmHg). Further elevation of HVPG due to liberal transfusion can increase the risk of rebleeding. In a recent meta-analysis, the incidence of death (OR = 0.52, 95%CI: 0.31-0.87, P = 0.01), blood transfusion requirement (standard mean difference: -0.74, 95%CI: -1.15--0.32, P = 0.0005) and hospital stay (standard mean difference: -0.17, 95%CI: -0.30--0.04, P = 0.009) were significantly lower in the restrictive transfusion group compared to the liberal transfusion group[63].
Therefore, a restrictive transfusion strategy should be employed in managing patients with acute variceal bleeding. The current practice society guidelines do not recommend routine use of plasma products and platelet transfusion in this setting due to inconsistent data on the use of plasma products and reliability of prothrombin time (PT)/international normalized ratio (INR) in patients with cirrhosis[38,46]. However, platelet and plasma transfusion can be done in select patients who are hemodynamically unstable with active variceal bleeding (goal: platelet count > 50000/ μL and INR < 1.5)[47].
Vasoactive agents: Vasoactive agents such as octreotide, terlipressin, somatostatin, and vasopressin cause splanchnic vasoconstriction and thus reduce portal pressure. All patients with confirmed or suspected variceal bleeding should be started on vasoactive agents as early as possible and should be continued for 2-5 d. They can be stopped early if the patient undergoes a TIPS procedure.
Terlipressin is a synthetic analog of vasopressin. The role of terlipressin in acute variceal bleeding was analyzed in a meta-analysis involving 1609 patients from 22 studies. Among those 22 studies, seven studies (443 patients) compared the effect of terlipressin to a placebo group. Terlipressin group was noted to have a statistically significant reduction in all-cause mortality (relative risk = 0.66, 95%CI: 0.49-0.88). Remaining studies compared terlipressin to somatostatin, octreotide, vasopressin or balloon tamponade. There was no significant difference in mortality or adverse events between the groups[64,65].
Use of vasoactive agents has been shown to reduce acute bleeding, need for transfusion, and seven-day all-cause mortality[66]. There was no significant difference in their efficacy or benefits noted between these agents[67].
Antibiotics: Short-term antibiotics should be started in all patients with suspected or confirmed variceal bleeding to reduce bacterial infection, recurrent bleeding, and mortality[38,48,68,69]. Bacterial infection is also considered to be an independent risk factor for variceal rupture. Choice of antibiotics should be based on local resistance pattern. However, third-generation cephalosporins with gram-negative coverage are commonly used. Intravenous ceftriaxone 1 g, every 24 h for a maximum of 7 d is preferred over oral fluroquinolones[38,46].
Other considerations: Most patients with variceal bleeding have loss of intravascular volume, and it is paramount to prevent hypotension. Due to the risk of hypotension and hemodynamic deterioration, nonselective beta-blockers should not be started during acute variceal bleeding and should be discontinued if the patient is already taking. However, nonselective beta-blockers should be restarted after the acute event, once hemostasis is achieved and vasoactive agents are discontinued.
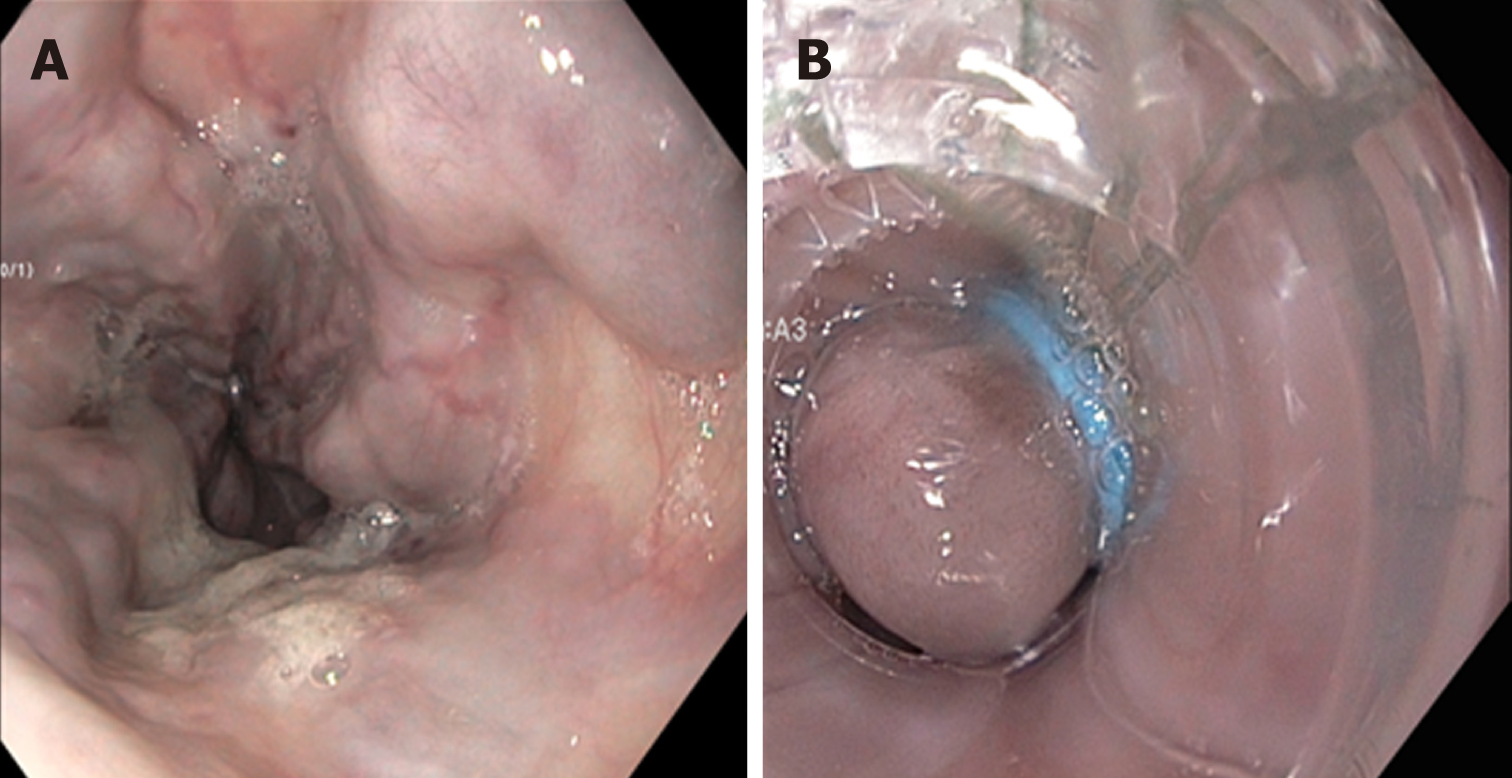
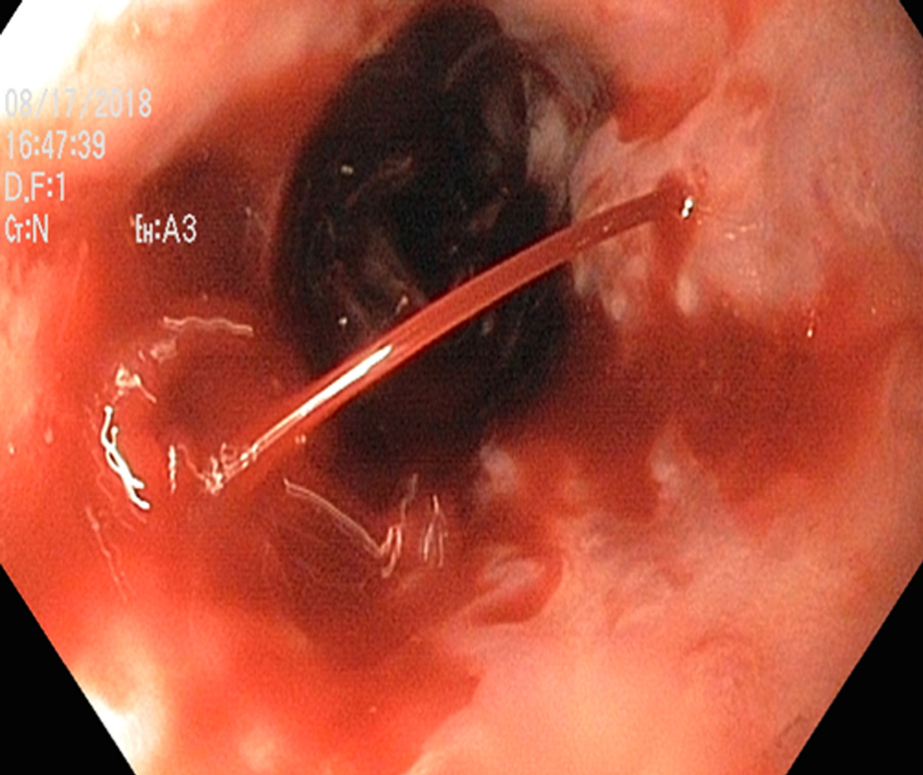
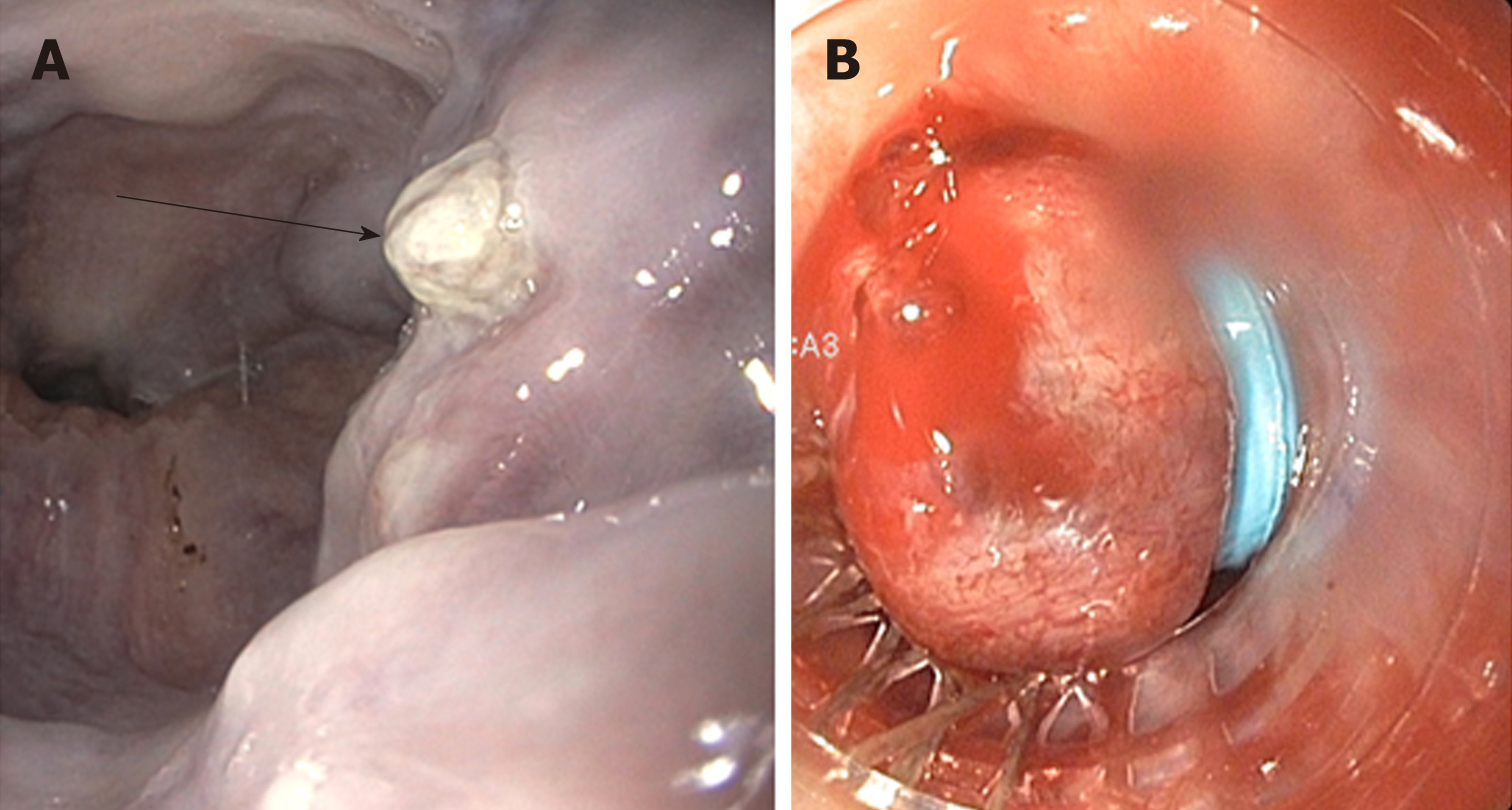
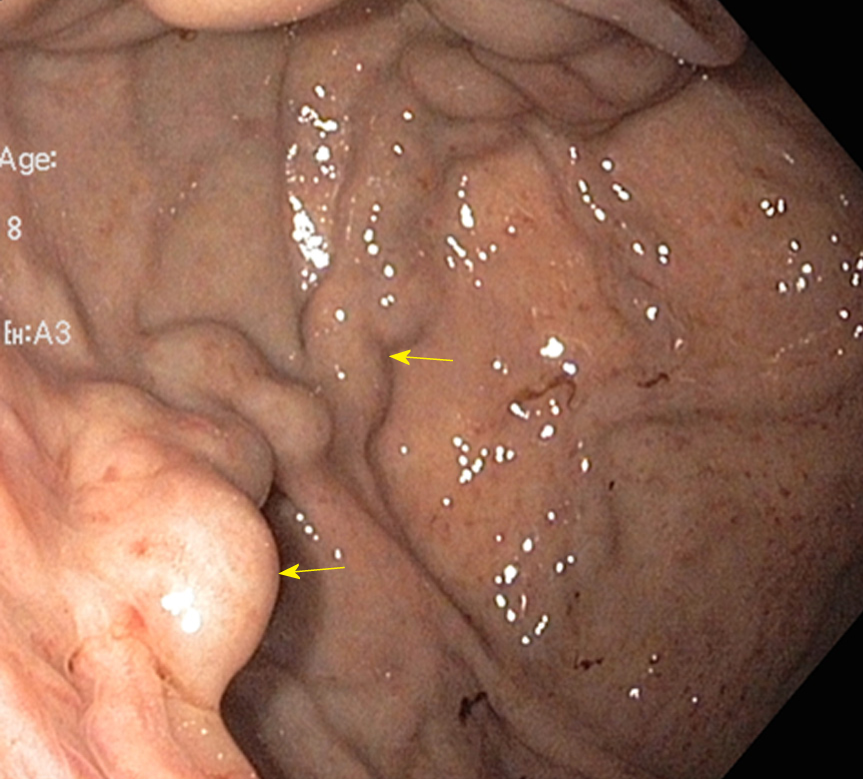
Endoscopic intervention should be performed as early as possible but should be within 12 h from the time of presentation as per practice society guidelines. The diagnosis of variceal bleeding as the etiology of acute upper gastrointestinal bleeding is made when any of the following is noted on upper endoscopy: (1) Actively bleeding varices (Figure 5); (2) Signs of recent bleeding noted on varices or high-risk stigmata; e.g., telangiectasia, red color signs, platelet-fibrin plug (white nipple sign), red wale marking or varices on varices (Figure 6); (3) Presence of varices and blood is noted in the stomach, with no other source of bleeding noted.
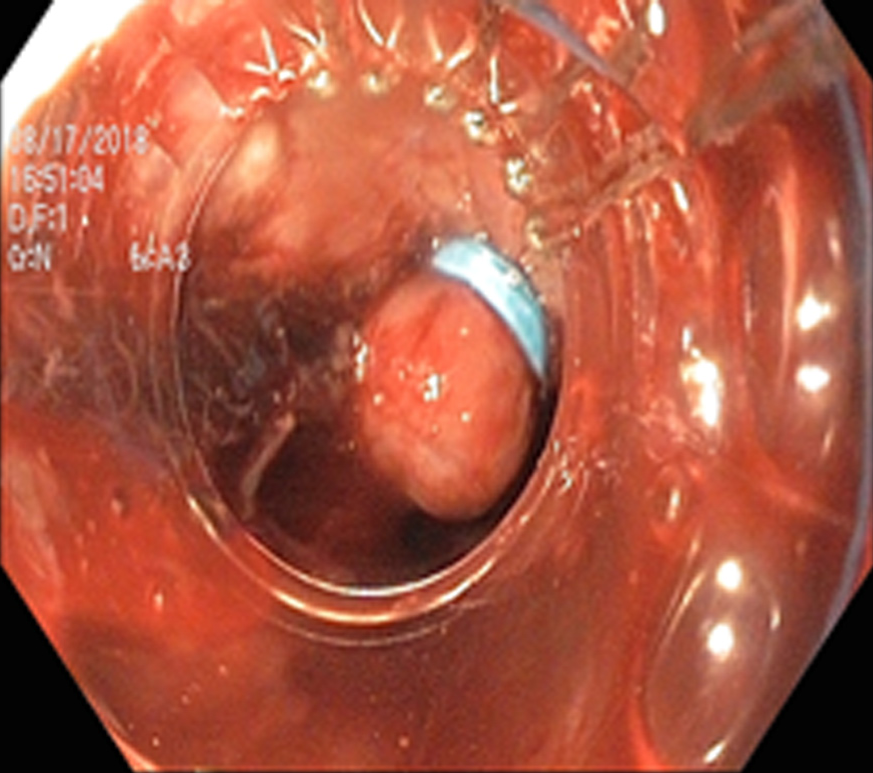
EVL (Figure 7) was first proposed for the treatment of esophageal varices in 1988 by Van Stiegmann et al[70]. Currently, EVL is considered to be the first line of endoscopic treatment for the management of bleeding esophageal varices. EVL has better hemostasis, a lower rate of side effects (ulcer, stricture), a reduced rate of early rebleeding, and a lower rate of early mortality compared to sclerotherapy. Higher rebleeding in sclerotherapy is thought to be due to sustained elevation of HVPG, whereas HVPG returned to baseline with EVL[71-73]. The slightly higher rate of variceal recurrence after EVL, when compared to sclerotherapy is due to its inability to affect the blood flow through perforators and esophageal collateral veins.
Sengstaken-Blakemore tube: Whenever variceal bleeding is not controlled by EVL, temporary hemostatic measures should be used as a bridge to more definitive treatment, such as TIPS or variceal shunt surgery. Sengstaken-Blakemore tube is inserted through the mouth or nose and then distended to achieve hemostasis during active variceal bleeding by tamponading varices. The rate of hemostasis with Sengstaken-Blakemore tube varies (47%-80%). It is associated with a high rate of serious adverse events including aspiration, esophageal ulceration, and rarely esophageal rupture. Sengstaken-Blakemore tubes cannot be left in place for more than 24 h due to an increased risk of adverse events and a high rate of rebleeding (50%)[72,73].
Metal stents: Endoscopically placed self-expanding fully covered metal stents (Figure 8) can achieve hemostasis in most cases (80%-96%). The stents expand inside the esophagus and tamponade the varices to achieve hemostasis. They can be left in place for up to 2 wk and have a lower rate of serious adverse events and transfusion requirements when compared to balloon tamponade[74,75]. Adverse events associated with this modality of treatment include stent migration (28%), rebleeding (16%) and ulcers. However, there was no significant difference in mortality compared to balloon tamponade[76,77].
In a meta-analysis of 12 studies (n = 155) hemostasis was achieved in 96% (95%CI: 0.90-1.00) of the patients within 24 h with 97% technical success (95%CI: 0.91-1.00). Adverse events (rebleeding, ulceration and stent migration) were noted in 36% (95%CI: 0.23-0.50) of the patients. Pooled susvival rate at 30 d and 60 d were 68% (95%CI: 0.56-0.80) and 64% (95%CI: 0.48-0.78) respectively[78]. Similar results were noted in another meta analsysis of 5 studies (n = 80) with technical success of 96.7% (95%CI: 91.6%-99.5%) and hemostasis of 93.9% (95%CI: 82.2%-99.6%). Rebleeding was observed in 13.2% (95%CI: 1.8%-32.8%) and the overall mortality was 34.5% (95%CI: 24.8%-44.8%)[79].
Based on the above evidence, self-expanding metal stents are a better choice for bridge therapy in uncontrolled esophageal variceal bleeding and should be used whenever available.
Patients with uncontrolled variceal hemorrhage despite the combination of medical and endoscopic treatment should be considered for early TIPS within (24 h) with covered PTFE (polytetrafluoroethylene) stents. TIPS is a shunt created by placing a stent between the portal vein and hepatic vein to reduce the portal pressure and thereby portal hypertension. Also, early rebleeding (within five days of initial bleeding) can be treated with repeat endoscopic intervention or covered TIPS stent[38,46,48].
In a meta-analysis of six comparative studies, TIPS was compared with medical and endoscopic treatment for acute variceal bleeding. In this study, the survival rate (HR = 0.55; 95%CI: 0.38-0.812) was better in TIPS patients, and the incidence of bleeding-related death (OR = 0.19; 95%CI: 0.06-0.59) was lower compared to medical/endoscopic treatment. There was no significant increase in hepatic encephalopathy (OR = 1.37; 95%CI: 0.63-2.99) in TIPS patients. Although there was no significant difference in rebleeding rate between the two groups, it was evident that rebleeding in the high-risk patients was higher on subgroup analysis[80].
Patients with Child-Pugh class B with active bleeding and class C are considered high-risk due to increased risk of treatment failure and rebleeding.
In a 2010 study, early TIPS was compared with pharmacotherapy (vasoactive agents) and EVL in Child-Pugh class C patients and class B patients with a high risk of treatment failure. Sixty-two patients were randomized into the treatment group (early TIPS, n = 32), and control group (pharmacotherapy and EVL, n = 31). Rescue TIPS was used in control group as needed for treatment failure. Rebleeding or failure to control bleeding occurred in one patient in the early TIPS group and 14 patients in the control group (P = 0.001). The one-year actuarial survival rate was 61% in the control group vs 86% in the early-TIPS group (P < 0.001)[81].
In another international multicenter observational study (671 patients from 34 centers) patients who were admitted for acute variceal bleeding with Child-Pugh class C, and Child-Pugh class B with active bleeding were included in the study. Patients were treated with either pharmacotherapy and endoscopic interventions or preemptive TIPS. Preemptive TIPS was associated with significantly lower one-year mortality (22% vs 47%, P = 0.002), treatment failure and rebleeding (92% vs 74%, P = 0.017) when compared to patients treated with pharmacotherapy and endoscopic interventions. TIPS also prevented the development of new ascites or worsening of pre-existing ascites[82]. Even though these results are encouraging, it was an observational study, and patients were not randomized. Each center chose to treat the patient with either preemptive TIPS or medications and endoscopy at its discretion. Therefore, the results may not be generalized. However, large RCTs can determine the use of preemptive TIPS in this high-risk population[82].
Complications from TIPS include hepatic encephalopathy, heart failure, and stent stenosis. The incidence of hepatic encephalopathy is close to 50% without a significant difference in mortality[83]. Absolute contraindications for TIPS include heart failure, severe pulmonary hypertension, severe tricuspid valve regurgitation, sepsis, and unrelieved biliary obstruction. Relative contraindications are portal vein thrombosis, hepatoma, uncorrected coagulopathy, and severe thrombocytopenia (platelet count < 20000/μL).
Patients who failed TIPS, those who have altered anatomy due to previous surgery or congenital anomaly, or are otherwise not candidates for TIPS, can be treated with direct ultrasound-guided direct intrahepatic porto-caval shunt (DIPS)[84]. The DIPS is a modified TIPS procedure, and it involves percutaneous ultrasound-guided puncture from the inferior vena cava to the portal vein through the caudate lobe of the liver.
Surgical shunts are considered when all other treatment modalities fail. Portocaval surgery has a very high rate of encephalopathy but does have good bleeding control. Most patients who undergo portocaval shunt surgery already have high morbidity and surgery adds to it further[85]. In a recent RCT, emergency TIPS procedure was compared with emergency portocaval shunt surgery, and shunt surgery was noted to have superior bleeding control, long-term survival (10 years vs 1.99 years) and low rate of encephalopathy. However, this has not been replicated, and more evidence is required before using portocaval shunt surgery as a salvage procedure after failure of first-line treatment with medical therapy and EVL[86].
Patients who were treated with EVL and medical therapy without TIPS are at high risk for rebleeding. Approximately 60% of patients will experience rebleeding during the first year and have a high mortality rate (up to 33%) with no further intervention. Combination therapy with nonselective beta blockers (propranolol and nadolol) and EVL is the first line of treatment for secondary prophylaxis with a goal to eradicate varices and prevent recurrent bleeding[87]. TIPS should be considered if patients do not tolerate or fail the combination of nonselective beta-blockers and EVL.
A multicenter RCT compared TIPS with the combination of EVL or glue injection and nonselective beta-blockers. Patients in the TIPS group had a significantly lower rebleeding rate (0%) compared to the EVL or glue injection and nonselective beta blockers group (29%) without a significant difference in survival benefit[88].
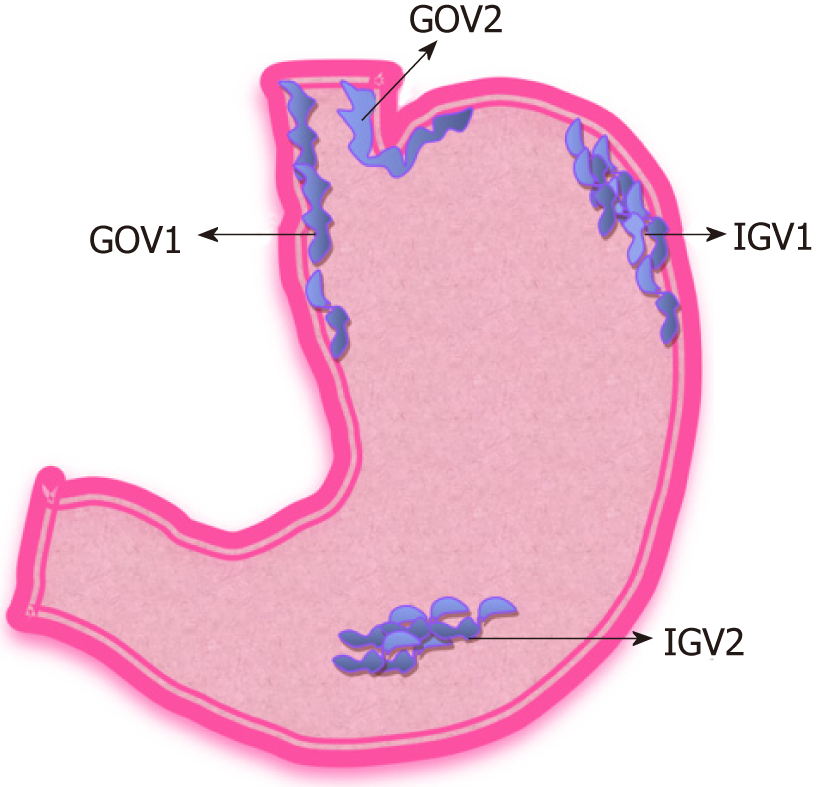
GVs are less frequent compared to esophageal varices and are reported to be seen in 20% of the patients with portal hypertension[38,89]. Bleeding from GVs account for 20% of all variceal bleeding[48]. The annual bleeding rate in GVs, which have never bled before is reported to be as low as 16% per year. Sarin et al[90] classified GVs based on their location.
Shown in Figure 9. Gastroesophageal varix type 1 (GOV1): Extension of esophageal varices along lesser curvature (most common 75% of GVs); GOV2: Extension of esophageal varices along the greater curvature; Isolated gastric varix type 1 (IGV1): Isolated varices seen in the fundus of the stomach; IGV2: Isolated varices in the stomach (body, pylorus, antrum).
Location (IGV1 > GOV2 > GOV1); The severity of liver disease; Stigmata of high-risk bleeding such as ‘red wale’ sign.
GVs bleed less frequently but have high mortality due to the severity of bleeding. Bleeding from IGV is associated with the highest risk of death[38,48,91].
GVs have complex anatomy and understanding the anatomy assists in the endoscopic management of GVs. The most common type of GVs are GOV1 and are usually associated with portal hypertension due to cirrhosis. They are a continuation of esophageal varices along the lesser curvature of the stomach. These are supplied by the esophageal collateral veins and are also treated similarly to esophageal varices.
On the other hand, GOV2 and IGV1 are supplied by the posterior and left gastric vein, which later drains into left renal vein due to porto-systemic shunting. Therefore GOV2 and IGV1 are together called cardiofundal varices[91]. Isolated IGV1 can be associated with splenic vein thrombosis in the setting of pancreatitis or malignancy.
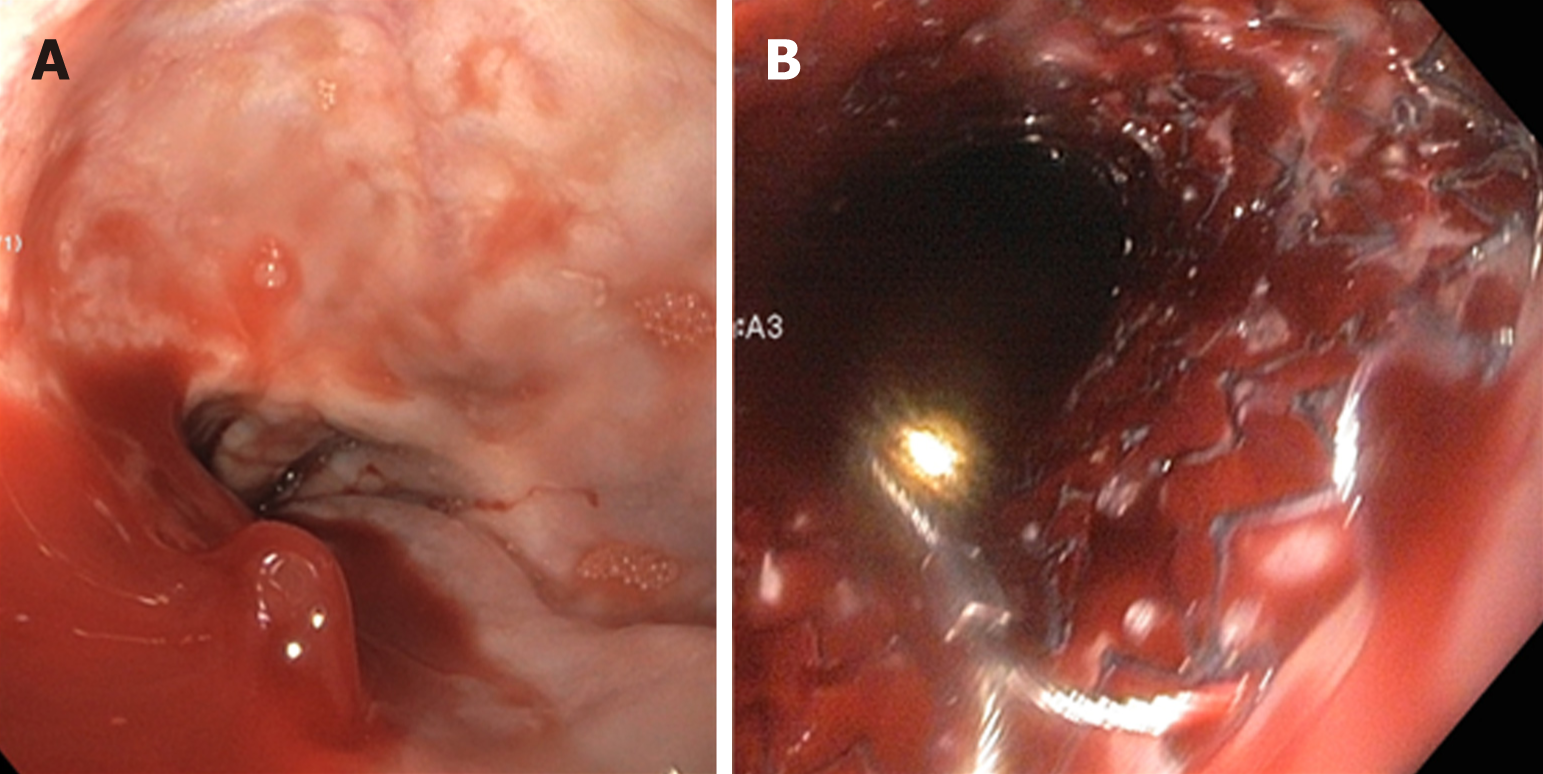
Diagnosis of GVs (Figure 10) is commonly done with endoscopy. However, the recent use of EUS has increased the sensitivity of detecting GVs. No guidelines are currently available regarding the use of endoscopy or EUS specifically to diagnose GVs.
Primary prophylaxis for GVs is not well established. Currently, nonselective beta-blockers are the first line of treatment as per practice society guidelines, in large part due to their ability to prevent other complications of cirrhosis. The role of endoscopic glue (N-butyl-2-cyanoacrylate) injection and EVL in primary prophylaxis are not clear. One study has shown glue injection was associated with lower bleeding and mortality due to GVs when compared to nonselective beta blockers[92]. Prophylactic EUS guided injection has also shown to be equally beneficial, and further studies are required to evaluate its role in primary prophylaxis for GVs.
Medical management of suspected gastric variceal bleeding is similar to esophageal variceal bleeding as described above, including airway protection, admission to the intensive care unit, blood transfusion, vasoactive agents, and antibiotics (Figure 11).
Diagnosis of gastric variceal bleeding can be made based on endoscopic findings. Most practice guidelines recommend endoscopic glue injection as the first line of treatment in the management of acute gastric variceal bleeding. However, glue injection comes with the risk of several complications including venous and systemic thromboembolism (pulmonary embolism, stroke), ulcers, protracted bleeding, splenic and portal vein thrombosis[93]. Portal vein thrombosis due to embolized glue can render a future plan for TIPS and liver transplantation ineffective. Embolized glue can also act as a nidus of infection and cause recurrent bacteremia[94]. Successful glue injection requires experience due to gastric anatomy. Because of the drawbacks mentioned above, many centers use TIPS as the first line of treatment in managing acute gastric variceal bleeding.
A RCT compared efficacy and complication of TIPS and glue injection in treating GVs. Rebleeding (11% vs 38%, P = 0.014; OR = 3.6, 95%CI: 1.2-11.1) and transfusion requirements were lower (P < 0.01) in TIPS compared to endoscopic glue injection with similar initial hemostasis, side effects, and mortality[95].
Even though initial hemostasis in both glue injection and EVL is similar for GOV1 GVs, rebleeding is high in EVL. So EVL should be avoided[96-98]. Combination of sclerotherapy and EVL is currently not recommended due to a higher rate of complications, and adverse events without mortality benefit[99]. In a recent RCT, scleroligation (variceal ligation + sclerotherapy) compared to EVL alone, in the management of GOVs, the scleroligation group required a lower number of endoscopic procedures, transfusion, and bands used, without a significant difference in recurrence rate, major side effects, and mortality[100]. Further research is needed to prove the benefits of scleroligation.
The recent emergence of EUS guided glue and coil injection in treating GVs has shown a lower bleeding rate, transfusion requirements, and mortality when compared to glue injection. When EUS guided coil embolization alone was compared with EUS guided glue injection, both had similar hemostasis rates, but coil embolization had fewer adverse events and required a fewer number of endoscopies[101]. When these two techniques were combined (glue + coil), the mean number of coils used, mean volume of glue used, and the recurrence rate was lower compared to either of them alone[102].
Patients with uncontrolled gastric variceal bleeding despite endoscopic intervention should be managed with balloon tamponade with Sengstaken-Blakemore tube or Linton-Nachlas tube as a bridge to definitive treatment. In a controlled trial Sengstaken-Blakemore tube failed to control gastric variceal bleeding in all the cases, and 50% hemostasis was achieved by Linton-Nachlas tube. Types of GVs and their frequency between the two groups was not available[73]. This difference could be attributed to a larger gastric balloon (500 mL) when compared to smaller gastric balloon in the Sengstaken-Blakemore tube. Therefore, Linton-Nachlas tube should be used whenever possible.
Hemostatic powder (TC 325 - hemospray) and similar products have been used as bridging therapy in controlling acute peptic ulcer bleeding in the past. The hemostatic powder when sprayed at the bleeding site, it absorbs water and creates a mechanical barrier to achieve hemostasis. Recently one study assessed its role in acute variceal bleeding. Hemostasis in the study group was better than the control group, with fewer study group patients requiring rescue endoscopy (12%). Rescue endoscopy was performed if initial hemostasis was not achieved within the first 12 h with hemospray. All patients were later treated with definitive endoscopic intervention after 24 h. Larger RCTs are required to evaluate the role of hemostasis powder, and currently not approved by Food and Drug Administration[103,104].
Patients with GVs who fail to respond to the endoscopic treatment will require TIPS or shunt surgery to control acute variceal bleeding. Recurrent bleeding is noted in 11%-30% of the patients who undergo TIPS.
Patients with GVs and gastro-renal collaterals can be treated with balloon-occluded retrograde transvenous obliteration (BRTO). This procedure involves retrograde cannulation of the outflow channels which drain the GVs through the femoral or jugular vein, and obliteration of the varices and collaterals assisted by balloon occlusion and followed by coil and sclerosant. Various studies have evaluated its efficacy in treating GVs. A recent meta-analysis showed a success rate for obliteration was 97.3%, and 33.3% recurrence. BRTO can be considered as an alternative to TIPS in managing GVs. A retrospective review of 142 consecutive patients treated for acute gastric variceal bleeding comparing the efficacy of BRTO (n = 95) and TIPS (n = 47) showed significantly lower rebleeding rate in BRTO (8.6%) group compared to TIPS (19.8%)[105] at the end of the first year. There was no significant difference in mortality. BRTO is mostly done in Asian countries, but recently it is gaining popularity in the United States[38,48,106].
Risk of rebleeding among patients who are treated with glue injection for gastric variceal bleeding was noted to vary from 15%-72%[98,107,108]. TIPS is considered to be superior to endoscopic glue injection for secondary prophylaxis of GVs[38]. However, there is no significant mortality benefit when compared to glue injection. The role of nonselective beta-blockers is not evident in secondary prophylaxis of GVs. Data on EUS guided glue injection and coiling for primary and secondary prophylaxis is lacking. Larger multicenter RCTs will help in understanding the role of EUS in the management of GVs.
Gastrointestinal varices can develop in the duodenum, rectum, colon, small bowel, gallbladder and the retroperitoneal areas. The prevalence of ectopic gastrointestinal varices is unknown. According to one estimate, among patients with cirrhosis and portal hypertension who underwent angiography, 40% of patients had duodenal varices. Ectopic varices are responsible for up to 1%-5% of all variceal bleeding. Understanding the complex anatomy of ectopic varices, and their anastomosis with mesenteric veins is essential in managing ectopic varices[91,109].
Duodenal varices are more commonly seen in noncirrhotic, extrahepatic portal hypertension (e.g., portal vein thrombosis, splenic vein thrombosis) and their prevalence is around 0.4%[109]. Duodenal varices form due to Porto-mesenteric and Porto-portal anastomosis. Duodenal varices are noted on endoscopy as submucosal dilated veins, usually arising from anastomosis between tributaries of the superior mesenteric vein and portal vein draining into inferior vena cava. EUS is notably superior in diagnosing duodenal varices compared to EGD[110]. Acute duodenal variceal bleeding is usually treated with endoscopic glue injection. There have been no RCTs evaluating the treatment strategies for duodenal varices owing to their rarity. In the largest case series involving ten patients with duodenal variceal glue injection, 4 out of the five patients who presented with acute bleeding were treated with endoscopic glue injection and had 100% hemostasis rate without recurrence[110]. Duodenal varices bleed at a lower hepatic venous pressure gradient, and therefore TIPS may not be sufficient to treat duodenal varices and need further definitive treatment with intravascular obliteration with glue injection, or embolization through BRTO. BRTO can also be used for patients who fail endoscopic therapy and are not candidates for TIPS[111].
Rectal varices usually arise from portosystemic anastomosis between superior hemorrhoidal veins (a tributary of the inferior mesenteric vein) and the middle or inferior hemorrhoidal veins (tributaries of iliac or pudendal veins). Prevalence of rectal varices patients with portal hypertension varies from 28%-56% in cirrhotic patients[112], and are more common among patients with extrahepatic portal vein obstruction(up to 90%)[113]. EUS has a higher sensitivity to detect rectal varices compared to endoscopy. Risk of bleeding from rectal varices is 8%-38%[112]. Rectal varices bleed at the lower hepatic venous pressure gradient and may not disappear with TIPS. Endoscopic variceal band ligation is the preferred method of treatment for rectal varices compared to endoscopic sclerotherapy or glue injection, but the recurrence rate of rebleeding is high with Endoscopic variceal band ligation. Recurrent bleeding in endoscopic sclerotherapy (33%) was much lower compared to EVL (55.6%)[114] but not commonly used due to the occurrence of severe ulcers. Endoscopic glue injection can be useful in managing rectal varices, but nearly 0.5%-4.3% of these patients develop embolization. EUS guided coil and glue embolization is also considered useful in large rectal varices that are not amenable to variceal ligation[115]. Role of BRTO has been evaluated in small case series; no RCTs are available to compare its efficacy. Optimal management of rectal varices is not yet established.
Stomal varices usually occur at the mucocutaneous junction of the stoma, due to portosystemic shunt between the portal circulation of the bowel and systemic circulation of the abdominal wall. Diagnosis of stomal varices is difficult, on physical exam, they appear as bluish discoloration of the skin. Visibly dilated veins and characteristic raspberry appearance of the stoma should prompt further evaluation for the cause of bleeding. Patients with stomal varices can be treated with a glue injection. Percutaneous sclerotherapy is not recommended due to increased risk of damaging the stoma. Gastrointestinal varices can also form in other parts of the gastrointestinal tract including jejunum, ileum, and colon as well. The actual prevalence of these varices is unknown but considered to be low.
In summary, development, and utilization of newer treatment modalities such as therapeutic EUS, BRTO, and hemospray in managing gastrointestinal varices will help to reduce further- the morbidity and mortality related to variceal bleeding. Further research in understanding the risk factors, mechanism of liver injury, and evaluation of antifibrotic agents to prevent architectural changes to the liver can revolutionize the management of portal hypertension and its complications.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Lo GH, Qi XS S- Editor: Ma RY L- Editor: A E- Editor: Bian YN
| 1. | Scaglione S, Kliethermes S, Cao G, Shoham D, Durazo R, Luke A, Volk ML. The Epidemiology of Cirrhosis in the United States: A Population-based Study. J Clin Gastroenterol. 2015;49:690-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 502] [Article Influence: 50.2] [Reference Citation Analysis (0)] |
| 2. | Kovalak M, Lake J, Mattek N, Eisen G, Lieberman D, Zaman A. Endoscopic screening for varices in cirrhotic patients: data from a national endoscopic database. Gastrointest Endosc. 2007;65:82-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 130] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 3. | Mann RE, Smart RG, Govoni R. The epidemiology of alcoholic liver disease. Alcohol Res Health. 2003;27:209-219. [PubMed] |
| 4. | Liou IW. Screening for Varices and Prevention of Bleeding. Hepat C Online. 2013;1-14. |
| 5. | Davis GL, Alter MJ, El-Serag H, Poynard T, Jennings LW. Aging of hepatitis C virus (HCV)-infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology. 2010;138:513-521, 521.e1-521.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 671] [Cited by in RCA: 665] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 6. | Abrutyn S, Mueller AS. Are Suicidal Behaviors Contagious in Adolescence?: Using Longitudinal Data to Examine Suicide Suggestion. Am Sociol Rev. 2014;79:211-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 88] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 7. | Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol. 2013;10:330-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1066] [Cited by in RCA: 1317] [Article Influence: 109.8] [Reference Citation Analysis (0)] |
| 8. | Wong RJ, Aguilar M, Cheung R, Perumpail RB, Harrison SA, Younossi ZM, Ahmed A. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148:547-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1211] [Cited by in RCA: 1379] [Article Influence: 137.9] [Reference Citation Analysis (1)] |
| 9. | Masuoka HC, Chalasani N. Nonalcoholic fatty liver disease: an emerging threat to obese and diabetic individuals. Ann N Y Acad Sci. 2013;1281:106-122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 203] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 10. | D’Amico G, Pagliaro L, Bosch J. The treatment of portal hypertension: a meta-analytic review. Hepatology. 1995;22:332-354. [PubMed] |
| 11. | Merli M, Nicolini G, Angeloni S, Rinaldi V, De Santis A, Merkel C, Attili AF, Riggio O. Incidence and natural history of small esophageal varices in cirrhotic patients. J Hepatol. 2003;38:266-272. [PubMed] |
| 12. | North Italian Endoscopic Club for the Study and Treatment of Esophageal Varices. Prediction of the first variceal hemorrhage in patients with cirrhosis of the liver and esophageal varices. A prospective multicenter study. N Engl J Med. 1988;319:983-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 996] [Cited by in RCA: 840] [Article Influence: 22.7] [Reference Citation Analysis (1)] |
| 13. | Amitrano L, Guardascione MA, Manguso F, Bennato R, Bove A, DeNucci C, Lombardi G, Martino R, Menchise A, Orsini L, Picascia S, Riccio E. The effectiveness of current acute variceal bleed treatments in unselected cirrhotic patients: refining short-term prognosis and risk factors. Am J Gastroenterol. 2012;107:1872-1878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 133] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 14. | Bosch J, García-Pagán JC. Prevention of variceal rebleeding. Lancet. 2003;361:952-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 288] [Article Influence: 13.1] [Reference Citation Analysis (2)] |
| 15. | Khanna R, Sarin SK. Non-cirrhotic portal hypertension - diagnosis and management. J Hepatol. 2014;60:421-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 261] [Article Influence: 23.7] [Reference Citation Analysis (2)] |
| 16. | Baker LA, Smith C, Lieberman G. The natural history of esophageal varices; a study of 115 cirrhotic patients in whom varices were diagnosed prior to bleeding. Am J Med. 1959;26:228-237. [PubMed] |
| 17. | Dagradi AE. The natural history of esophageal varices in patients with alcoholic liver cirrhosis. An endoscopic and clinical study. Am J Gastroenterol. 1972;57:520-540. [PubMed] |
| 18. | Taura K, De Minicis S, Seki E, Hatano E, Iwaisako K, Osterreicher CH, Kodama Y, Miura K, Ikai I, Uemoto S, Brenner DA. Hepatic stellate cells secrete angiopoietin 1 that induces angiogenesis in liver fibrosis. Gastroenterology. 2008;135:1729-1738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 217] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 19. | Thabut D, Shah V. Intrahepatic angiogenesis and sinusoidal remodeling in chronic liver disease: new targets for the treatment of portal hypertension? J Hepatol. 2010;53:976-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 218] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 20. | Bauer M, Bauer I, Sonin NV, Kresge N, Baveja R, Yokoyama Y, Harding D, Zhang JX, Clemens MG. Functional significance of endothelin B receptors in mediating sinusoidal and extrasinusoidal effects of endothelins in the intact rat liver. Hepatology. 2000;31:937-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 77] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 21. | Kawada N, Tran-Thi TA, Klein H, Decker K. The contraction of hepatic stellate (Ito) cells stimulated with vasoactive substances. Possible involvement of endothelin 1 and nitric oxide in the regulation of the sinusoidal tonus. Eur J Biochem. 1993;213:815-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 268] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 22. | Gracia-Sancho J, Laviña B, Rodríguez-Vilarrupla A, García-Calderó H, Bosch J, García-Pagán JC. Enhanced vasoconstrictor prostanoid production by sinusoidal endothelial cells increases portal perfusion pressure in cirrhotic rat livers. J Hepatol. 2007;47:220-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 94] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 23. | Graupera M, March S, Engel P, Rodés J, Bosch J, García-Pagán JC. Sinusoidal endothelial COX-1-derived prostanoids modulate the hepatic vascular tone of cirrhotic rat livers. Am J Physiol Gastrointest Liver Physiol. 2005;288:G763-G770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 24. | Shah V, Haddad FG, Garcia-Cardena G, Frangos JA, Mennone A, Groszmann RJ, Sessa WC. Liver sinusoidal endothelial cells are responsible for nitric oxide modulation of resistance in the hepatic sinusoids. J Clin Invest. 1997;100:2923-2930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 232] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 25. | Iwakiri Y. Pathophysiology of portal hypertension. Clin Liver Dis. 2014;18:281-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 192] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 26. | Iwakiri Y. Endothelial dysfunction in the regulation of cirrhosis and portal hypertension. Liver Int. 2012;32:199-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 132] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 27. | Abraldes JG, Iwakiri Y, Loureiro-Silva M, Haq O, Sessa WC, Groszmann RJ. Mild increases in portal pressure upregulate vascular endothelial growth factor and endothelial nitric oxide synthase in the intestinal microcirculatory bed, leading to a hyperdynamic state. Am J Physiol Gastrointest Liver Physiol. 2006;290:G980-G987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 139] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 28. | Tsai MH, Iwakiri Y, Cadelina G, Sessa WC, Groszmann RJ. Mesenteric vasoconstriction triggers nitric oxide overproduction in the superior mesenteric artery of portal hypertensive rats. Gastroenterology. 2003;125:1452-1461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 49] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 29. | Iwakiri Y, Tsai MH, McCabe TJ, Gratton JP, Fulton D, Groszmann RJ, Sessa WC. Phosphorylation of eNOS initiates excessive NO production in early phases of portal hypertension. Am J Physiol Heart Circ Physiol. 2002;282:H2084-H2090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 53] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 30. | Huang HC, Haq O, Utsumi T, Sethasine S, Abraldes JG, Groszmann RJ, Iwakiri Y. Intestinal and plasma VEGF levels in cirrhosis: the role of portal pressure. J Cell Mol Med. 2012;16:1125-1133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | Bosch J, Abraldes JG, Fernández M, García-Pagán JC. Hepatic endothelial dysfunction and abnormal angiogenesis: new targets in the treatment of portal hypertension. J Hepatol. 2010;53:558-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 152] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 32. | Rigau J, Bosch J, Bordas JM, Navasa M, Mastai R, Kravetz D, Bruix J, Feu F, Rodés J. Endoscopic measurement of variceal pressure in cirrhosis: correlation with portal pressure and variceal hemorrhage. Gastroenterology. 1989;96:873-880. [PubMed] |
| 33. | Groszmann RJ, Bosch J, Grace ND, Conn HO, Garcia-Tsao G, Navasa M, Alberts J, Rodes J, Fischer R, Bermann M. Hemodynamic events in a prospective randomized trial of propranolol versus placebo in the prevention of a first variceal hemorrhage. Gastroenterology. 1990;99:1401-1407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 533] [Cited by in RCA: 452] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 34. | Abraldes JG, Villanueva C, Bañares R, Aracil C, Catalina MV, Garci A-Pagán JC, Bosch J; Spanish Cooperative Group for Portal Hypertension and Variceal Bleeding. Hepatic venous pressure gradient and prognosis in patients with acute variceal bleeding treated with pharmacologic and endoscopic therapy. J Hepatol. 2008;48:229-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 222] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 35. | Feu F, García-Pagán JC, Bosch J, Luca A, Terés J, Escorsell A, Rodés J. Relation between portal pressure response to pharmacotherapy and risk of recurrent variceal haemorrhage in patients with cirrhosis. Lancet. 1995;346:1056-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 457] [Cited by in RCA: 391] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 36. | D’Amico G, Garcia-Pagan JC, Luca A, Bosch J. Hepatic vein pressure gradient reduction and prevention of variceal bleeding in cirrhosis: a systematic review. Gastroenterology. 2006;131:1611-1624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 349] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 37. | Abraldes JG, Tarantino I, Turnes J, Garcia-Pagan JC, Rodés J, Bosch J. Hemodynamic response to pharmacological treatment of portal hypertension and long-term prognosis of cirrhosis. Hepatology. 2003;37:902-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 366] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 38. | Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology. 2017;65:310-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1108] [Cited by in RCA: 1431] [Article Influence: 178.9] [Reference Citation Analysis (3)] |
| 39. | Konishi Y, Nakamura T, Kida H, Seno H, Okazaki K, Chiba T. Catheter US probe EUS evaluation of gastric cardia and perigastric vascular structures to predict esophageal variceal recurrence. Gastrointest Endosc. 2002;55:197-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 40. | Lee YT, Chan FK, Ching JY, Lai CW, Leung VK, Chung SC, Sung JJ. Diagnosis of gastroesophageal varices and portal collateral venous abnormalities by endosonography in cirrhotic patients. Endoscopy. 2002;34:391-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 41. | Hammoud GM, Ibdah JA. Utility of endoscopic ultrasound in patients with portal hypertension. World J Gastroenterol. 2014;20:14230-14236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 42. | McCarty TR, Afinogenova Y, Njei B. Use of Wireless Capsule Endoscopy for the Diagnosis and Grading of Esophageal Varices in Patients With Portal Hypertension: A Systematic Review and Meta-Analysis. J Clin Gastroenterol. 2017;51:174-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 43. | Annicchiarico BE, Riccioni ME, Siciliano M, Urgesi R, Spada C, Caracciolo G, Gasbarrini A, Costamagna G. A pilot study of capsule endoscopy after a standard meal for the detection and grading of oesophageal varices in cirrhotic patients. Dig Liver Dis. 2014;46:997-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 44. | Laurain A, de Leusse A, Gincul R, Vanbiervliet G, Bramli S, Heyries L, Martane G, Amrani N, Serraj I, Saurin JC, Borentain P, Filoche B, Duburque C, Gaudric M, Sogni P, Dumortier J. Oesophageal capsule endoscopy versus oesophago-gastroduodenoscopy for the diagnosis of recurrent varices: a prospective multicentre study. Dig Liver Dis. 2014;46:535-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 45. | Ding NS, Nguyen T, Iser DM, Hong T, Flanagan E, Wong A, Luiz L, Tan JY, Fulforth J, Holmes J, Ryan M, Bell SJ, Desmond PV, Roberts SK, Lubel J, Kemp W, Thompson AJ. Liver stiffness plus platelet count can be used to exclude high-risk oesophageal varices. Liver Int. 2016;36:240-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 46. | de Franchis R; Baveno VI Faculty. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2011] [Cited by in RCA: 2282] [Article Influence: 228.2] [Reference Citation Analysis (3)] |
| 47. | Reverter E, Tandon P, Augustin S, Turon F, Casu S, Bastiampillai R, Keough A, Llop E, González A, Seijo S, Berzigotti A, Ma M, Genescà J, Bosch J, García-Pagán JC, Abraldes JG. A MELD-based model to determine risk of mortality among patients with acute variceal bleeding. Gastroenterology. 2014;146:412-419.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 265] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 48. | Jalan R, Hayes PC. UK guidelines on the management of variceal haemorrhage in cirrhotic patients. British Society of Gastroenterology. Gut. 2000;46 Suppl 3-4:III1-III15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 454] [Cited by in RCA: 412] [Article Influence: 41.2] [Reference Citation Analysis (2)] |
| 49. | Tripathi D, Ferguson JW, Kochar N, Leithead JA, Therapondos G, McAvoy NC, Stanley AJ, Forrest EH, Hislop WS, Mills PR, Hayes PC. Randomized controlled trial of carvedilol versus variceal band ligation for the prevention of the first variceal bleed. Hepatology. 2009;50:825-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 184] [Article Influence: 11.5] [Reference Citation Analysis (1)] |
| 50. | Li L, Yu C, Li Y. Endoscopic band ligation versus pharmacological therapy for variceal bleeding in cirrhosis: a meta-analysis. Can J Gastroenterol. 2011;25:147-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 51. | Gluud LL, Krag A. Banding ligation versus beta-blockers for primary prevention in oesophageal varices in adults. Cochrane Database Syst Rev. 2012;CD004544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 52. | Shah HA, Azam Z, Rauf J, Abid S, Hamid S, Jafri W, Khalid A, Ismail FW, Parkash O, Subhan A, Munir SM. Carvedilol vs. esophageal variceal band ligation in the primary prophylaxis of variceal hemorrhage: a multicentre randomized controlled trial. J Hepatol. 2014;60:757-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 53. | Merkel C, Marin R, Angeli P, Zanella P, Felder M, Bernardinello E, Cavallarin G, Bolognesi M, Donada C, Bellini B, Torboli P, Gatta A; Gruppo Triveneto per l’Ipertensione Portale. A placebo-controlled clinical trial of nadolol in the prophylaxis of growth of small esophageal varices in cirrhosis. Gastroenterology. 2004;127:476-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 167] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 54. | Qi XS, Bao YX, Bai M, Xu WD, Dai JN, Guo XZ. Nonselective beta-blockers in cirrhotic patients with no or small varices: A meta-analysis. World J Gastroenterol. 2015;21:3100-3108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 46] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 55. | Sarin SK, Wadhawan M, Agarwal SR, Tyagi P, Sharma BC. Endoscopic variceal ligation plus propranolol versus endoscopic variceal ligation alone in primary prophylaxis of variceal bleeding. Am J Gastroenterol. 2005;100:797-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 107] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 56. | Sersté T, Melot C, Francoz C, Durand F, Rautou PE, Valla D, Moreau R, Lebrec D. Deleterious effects of beta-blockers on survival in patients with cirrhosis and refractory ascites. Hepatology. 2010;52:1017-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 376] [Article Influence: 25.1] [Reference Citation Analysis (1)] |
| 57. | Mandorfer M, Bota S, Schwabl P, Bucsics T, Pfisterer N, Kruzik M, Hagmann M, Blacky A, Ferlitsch A, Sieghart W, Trauner M, Peck-Radosavljevic M, Reiberger T. Nonselective β blockers increase risk for hepatorenal syndrome and death in patients with cirrhosis and spontaneous bacterial peritonitis. Gastroenterology. 2014;146:1680-1690.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 280] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 58. | Facciorusso A, Roy S, Livadas S, Fevrier-Paul A, Wekesa C, Kilic ID, Chaurasia AK, Sadeq M, Muscatiello N. Nonselective Beta-Blockers Do Not Affect Survival in Cirrhotic Patients with Ascites. Dig Dis Sci. 2018;63:1737-1746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 59. | Al-Freah MA, Gera A, Martini S, McPhail MJ, Devlin J, Harrison PM, Shawcross D, Abeles RD, Taylor NJ, Auzinger G, Bernal W, Heneghan MA, Wendon JA. Comparison of scoring systems and outcome of patients admitted to a liver intensive care unit of a tertiary referral centre with severe variceal bleeding. Aliment Pharmacol Ther. 2014;39:1286-1300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 60. | Moitinho E, Escorsell A, Bandi JC, Salmerón JM, García-Pagán JC, Rodés J, Bosch J. Prognostic value of early measurements of portal pressure in acute variceal bleeding. Gastroenterology. 1999;117:626-631. [PubMed] |
| 61. | Fortune BE, Garcia-Tsao G, Ciarleglio M, Deng Y, Fallon MB, Sigal S, Chalasani NP, Lim JK, Reuben A, Vargas HE, Abrams G, Lewis MD, Hassanein T, Trotter JF, Sanyal AJ, Beavers KL, Ganger D, Thuluvath PJ, Grace ND, Groszmann RJ; Vapreotide Study Group. Child-Turcotte-Pugh Class is Best at Stratifying Risk in Variceal Hemorrhage: Analysis of a US Multicenter Prospective Study. J Clin Gastroenterol. 2017;51:446-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 62. | Villanueva C, Colomo A, Bosch A, Concepción M, Hernandez-Gea V, Aracil C, Graupera I, Poca M, Alvarez-Urturi C, Gordillo J, Guarner-Argente C, Santaló M, Muñiz E, Guarner C. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368:11-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1186] [Cited by in RCA: 1068] [Article Influence: 89.0] [Reference Citation Analysis (0)] |
| 63. | Wang J, Bao YX, Bai M, Zhang YG, Xu WD, Qi XS. Restrictive vs liberal transfusion for upper gastrointestinal bleeding: a meta-analysis of randomized controlled trials. World J Gastroenterol. 2013;19:6919-6927. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 64. | Ioannou G, Doust J, Rockey DC. Terlipressin for acute esophageal variceal hemorrhage. Cochrane Database Syst Rev. 2003;CD002147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 87] [Article Influence: 3.6] [Reference Citation Analysis (1)] |
| 65. | Wang C, Han J, Xiao L, Jin CE, Li DJ, Yang Z. Efficacy of vasopressin/terlipressin and somatostatin/octreotide for the prevention of early variceal rebleeding after the initial control of bleeding: a systematic review and meta-analysis. Hepatol Int. 2015;9:120-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 66. | Wells M, Chande N, Adams P, Beaton M, Levstik M, Boyce E, Mrkobrada M. Meta-analysis: vasoactive medications for the management of acute variceal bleeds. Aliment Pharmacol Ther. 2012;35:1267-1278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 147] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 67. | Seo YS, Park SY, Kim MY, Kim JH, Park JY, Yim HJ, Jang BK, Kim HS, Hahn T, Kim BI, Heo J, An H, Tak WY, Baik SK, Han KH, Hwang JS, Park SH, Cho M, Um SH. Lack of difference among terlipressin, somatostatin, and octreotide in the control of acute gastroesophageal variceal hemorrhage. Hepatology. 2014;60:954-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 142] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 68. | Bernard B, Grangé JD, Khac EN, Amiot X, Opolon P, Poynard T. Antibiotic prophylaxis for the prevention of bacterial infections in cirrhotic patients with gastrointestinal bleeding: a meta-analysis. Hepatology. 1999;29:1655-1661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 594] [Cited by in RCA: 461] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 69. | Chavez-Tapia NC, Barrientos-Gutierrez T, Tellez-Avila F, Soares-Weiser K, Mendez-Sanchez N, Gluud C, Uribe M. Meta-analysis: antibiotic prophylaxis for cirrhotic patients with upper gastrointestinal bleeding - an updated Cochrane review. Aliment Pharmacol Ther. 2011;34:509-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 183] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 70. | Van Stiegmann G, Goff JS. Endoscopic esophageal varix ligation: preliminary clinical experience. Gastrointest Endosc. 1988;34:113-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 100] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 71. | Villanueva C, Piqueras M, Aracil C, Gómez C, López-Balaguer JM, Gonzalez B, Gallego A, Torras X, Soriano G, Sáinz S, Benito S, Balanzó J. A randomized controlled trial comparing ligation and sclerotherapy as emergency endoscopic treatment added to somatostatin in acute variceal bleeding. J Hepatol. 2006;45:560-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 137] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 72. | Panés J, Terés J, Bosch J, Rodés J. Efficacy of balloon tamponade in treatment of bleeding gastric and esophageal varices. Results in 151 consecutive episodes. Dig Dis Sci. 1988;33:454-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 130] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 73. | Terés J, Cecilia A, Bordas JM, Rimola A, Bru C, Rodés J. Esophageal tamponade for bleeding varices. Controlled trial between the Sengstaken-Blakemore tube and the Linton-Nachlas tube. Gastroenterology. 1978;75:566-569. [PubMed] |
| 74. | Dechêne A, El Fouly AH, Bechmann LP, Jochum C, Saner FH, Gerken G, Canbay A. Acute management of refractory variceal bleeding in liver cirrhosis by self-expanding metal stents. Digestion. 2012;85:185-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 75. | Escorsell À, Pavel O, Cárdenas A, Morillas R, Llop E, Villanueva C, Garcia-Pagán JC, Bosch J; Variceal Bleeding Study Group. Esophageal balloon tamponade versus esophageal stent in controlling acute refractory variceal bleeding: A multicenter randomized, controlled trial. Hepatology. 2016;63:1957-1967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 126] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 76. | Wright G, Lewis H, Hogan B, Burroughs A, Patch D, O’Beirne J. A self-expanding metal stent for complicated variceal hemorrhage: experience at a single center. Gastrointest Endosc. 2010;71:71-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 77. | Marot A, Trépo E, Doerig C, Moreno C, Moradpour D, Deltenre P. Systematic review with meta-analysis: self-expanding metal stents in patients with cirrhosis and severe or refractory oesophageal variceal bleeding. Aliment Pharmacol Ther. 2015;42:1250-1260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 78. | McCarty TR, Njei B. Self-expanding metal stents for acute refractory esophageal variceal bleeding: A systematic review and meta-analysis. Dig Endosc. 2016;28:539-547. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 79. | Shao XD, Qi XS, Guo XZ. Esophageal Stent for Refractory Variceal Bleeding: A Systemic Review and Meta-Analysis. Biomed Res Int. 2016;2016:4054513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 80. | Qi X, Jia J, Bai M, Guo X, Su C, García-Pagán JC, Han G, Fan D. Transjugular Intrahepatic Portosystemic Shunt for Acute Variceal Bleeding: A Meta-analysis. J Clin Gastroenterol. 2015;49:495-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 81. | García-Pagán JC, Caca K, Bureau C, Laleman W, Appenrodt B, Luca A, Abraldes JG, Nevens F, Vinel JP, Mössner J, Bosch J; Early TIPS (Transjugular Intrahepatic Portosystemic Shunt) Cooperative Study Group. Early use of TIPS in patients with cirrhosis and variceal bleeding. N Engl J Med. 2010;362:2370-2379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 826] [Cited by in RCA: 838] [Article Influence: 55.9] [Reference Citation Analysis (0)] |
| 82. | Hernández-Gea V, Procopet B, Giráldez Á, Amitrano L, Villanueva C, Thabut D, Ibañez-Samaniego L, Silva-Junior G, Martinez J, Genescà J, Bureau C, Trebicka J, Llop E, Laleman W, Palazon JM, Castellote J, Rodrigues S, Gluud LL, Noronha Ferreira C, Barcelo R, Cañete N, Rodríguez M, Ferlitsch A, Mundi JL, Gronbaek H, Hernández-Guerra M, Sassatelli R, Dell’Era A, Senzolo M, Abraldes JG, Romero-Gómez M, Zipprich A, Casas M, Masnou H, Primignani M, Krag A, Nevens F, Calleja JL, Jansen C, Robic MA, Conejo I, Catalina MV, Albillos A, Rudler M, Alvarado E, Guardascione MA, Tantau M, Bosch J, Torres F, Garcia-Pagán JC; International Variceal Bleeding Observational Study Group and Baveno Cooperation. Preemptive-TIPS improves outcome in high-risk variceal bleeding: An observational study. Hepatology. 2018; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 95] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 83. | Mamiya Y, Kanazawa H, Kimura Y, Narahara Y, Yamate Y, Nakatsuka K, Sakamoto C. Hepatic encephalopathy after transjugular intrahepatic portosystemic shunt. Hepatol Res. 2004;30:162-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 84. | Ward TJ, Techasith T, Louie JD, Hwang GL, Hofmann LV, Sze DY. Emergent salvage direct intrahepatic portocaval shunt procedure for acute variceal hemorrhage. J Vasc Interv Radiol. 2015;26:829-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 85. | Haydon GH, Isaac J, Buckels JAC, Olliff SP. Portal hypertension. ClinicalKey. 2018;1-17. |
| 86. | Orloff MJ, Hye RJ, Wheeler HO, Isenberg JI, Haynes KS, Vaida F, Girard B, Orloff KJ. Randomized trials of endoscopic therapy and transjugular intrahepatic portosystemic shunt versus portacaval shunt for emergency and elective treatment of bleeding gastric varices in cirrhosis. Surgery. 2015;157:1028-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 87. | Puente A, Hernández-Gea V, Graupera I, Roque M, Colomo A, Poca M, Aracil C, Gich I, Guarner C, Villanueva C. Drugs plus ligation to prevent rebleeding in cirrhosis: an updated systematic review. Liver Int. 2014;34:823-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 88] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 88. | Holster IL, Tjwa ET, Moelker A, Wils A, Hansen BE, Vermeijden JR, Scholten P, van Hoek B, Nicolai JJ, Kuipers EJ, Pattynama PM, van Buuren HR. Covered transjugular intrahepatic portosystemic shunt versus endoscopic therapy + β-blocker for prevention of variceal rebleeding. Hepatology. 2016;63:581-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 163] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 89. | Ryan BM, Stockbrugger RW, Ryan JM. A pathophysiologic, gastroenterologic, and radiologic approach to the management of gastric varices. Gastroenterology. 2004;126:1175-1189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 223] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 90. | Sarin SK, Lahoti D, Saxena SP, Murthy NS, Makwana UK. Prevalence, classification and natural history of gastric varices: a long-term follow-up study in 568 portal hypertension patients. Hepatology. 1992;16:1343-1349. [PubMed] |
| 91. | Gulamhusein AF, Kamath PS. The epidemiology and pathogenesis of gastrointestinal varices. Tech Gastrointest Endosc. 2017;19:62-68. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 92. | Mishra SR, Sharma BC, Kumar A, Sarin SK. Primary prophylaxis of gastric variceal bleeding comparing cyanoacrylate injection and beta-blockers: a randomized controlled trial. J Hepatol. 2011;54:1161-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 142] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 93. | Cheng LF, Wang ZQ, Li CZ, Lin W, Yeo AE, Jin B. Low incidence of complications from endoscopic gastric variceal obturation with butyl cyanoacrylate. Clin Gastroenterol Hepatol. 2010;8:760-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 122] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 94. | Wright G, Matull WR, Zambreanu L, O’Neill S, Smith R, O’Beirne J, Morgan MY. Recurrent bacteremia due to retained embolized glue following variceal obliteration. Endoscopy. 2009;41 Suppl 2:E56-E57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 95. | Lo GH, Liang HL, Chen WC, Chen MH, Lai KH, Hsu PI, Lin CK, Chan HH, Pan HB. A prospective, randomized controlled trial of transjugular intrahepatic portosystemic shunt versus cyanoacrylate injection in the prevention of gastric variceal rebleeding. Endoscopy. 2007;39:679-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 222] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 96. | Tan PC, Hou MC, Lin HC, Liu TT, Lee FY, Chang FY, Lee SD. A randomized trial of endoscopic treatment of acute gastric variceal hemorrhage: N-butyl-2-cyanoacrylate injection versus band ligation. Hepatology. 2006;43:690-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 226] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 97. | Tantau M, Crisan D, Popa D, Vesa S, Tantau A. Band ligation vs. N-Butyl-2-cyanoacrylate injection in acute gastric variceal bleeding: a prospective follow-up study. Ann Hepatol. 2013;13:75-83. [PubMed] |
| 98. | Lo GH, Lai KH, Cheng JS, Chen MH, Chiang HT. A prospective, randomized trial of butyl cyanoacrylate injection versus band ligation in the management of bleeding gastric varices. Hepatology. 2001;33:1060-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 302] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 99. | Singh P, Pooran N, Indaram A, Bank S. Combined ligation and sclerotherapy versus ligation alone for secondary prophylaxis of esophageal variceal bleeding: a meta-analysis. Am J Gastroenterol. 2002;97:623-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 100. | Mansour L, El-Kalla F, El-Bassat H, Abd-Elsalam S, El-Bedewy M, Kobtan A, Badawi R, Elhendawy M. Randomized controlled trial of scleroligation versus band ligation alone for eradication of gastroesophageal varices. Gastrointest Endosc. 2017;86:307-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 101. | Romero-Castro R, Ellrichmann M, Ortiz-Moyano C, Subtil-Inigo JC, Junquera-Florez F, Gornals JB, Repiso-Ortega A, Vila-Costas J, Marcos-Sanchez F, Muñoz-Navas M, Romero-Gomez M, Brullet-Benedi E, Romero-Vazquez J, Caunedo-Alvarez A, Pellicer-Bautista F, Herrerias-Gutierrez JM, Fritscher-Ravens A. EUS-guided coil versus cyanoacrylate therapy for the treatment of gastric varices: a multicenter study (with videos). Gastrointest Endosc. 2013;78:711-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 156] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 102. | Bhat YM, Weilert F, Fredrick RT, Kane SD, Shah JN, Hamerski CM, Binmoeller KF. EUS-guided treatment of gastric fundal varices with combined injection of coils and cyanoacrylate glue: a large U.S. experience over 6 years (with video). Gastrointest Endosc. 2016;83:1164-1172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 161] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 103. | Ibrahim M, El-Mikkawy A, Abdel Hamid M, Abdalla H, Lemmers A, Mostafa I, Devière J. Early application of haemostatic powder added to standard management for oesophagogastric variceal bleeding: a randomised trial. Gut. 2018; Epub ahead of print. [PubMed] |
| 104. | Ibrahim M, Mostafa I, Devière J. New Developments in Managing Variceal Bleeding. Gastroenterology. 2018;154:1964-1969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 105. | Lee SJ, Kim SU, Kim MD, Kim YH, Kim GM, Park SI, Won JY, Lee DY, Lee KH. Comparison of treatment outcomes between balloon-occluded retrograde transvenous obliteration and transjugular intrahepatic portosystemic shunt for gastric variceal bleeding hemostasis. J Gastroenterol Hepatol. 2017;32:1487-1494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 106. | Park JK, Saab S, Kee ST, Busuttil RW, Kim HJ, Durazo F, Cho SK, Lee EW. Balloon-Occluded Retrograde Transvenous Obliteration (BRTO) for Treatment of Gastric Varices: Review and Meta-Analysis. Dig Dis Sci. 2015;60:1543-1553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 104] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 107. | Mishra SR, Chander Sharma B, Kumar A, Sarin SK. Endoscopic cyanoacrylate injection versus beta-blocker for secondary prophylaxis of gastric variceal bleed: a randomised controlled trial. Gut. 2010;59:729-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 117] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 108. | Soehendra N, Grimm H, Nam VC, Berger B. N-butyl-2-cyanoacrylate: a supplement to endoscopic sclerotherapy. Endoscopy. 1987;19:221-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 131] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 109. | Henry ZH, Caldwell SH. Management of bleeding ectopic varices. Tech Gastrointest Endosc. 2017;19:101-107. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 110. | Rana SS, Bhasin DK, Sharma V, Chaudhary V, Sharma R, Singh K. Clinical, endoscopic and endoscopic ultrasound features of duodenal varices: A report of 10 cases. Endosc Ultrasound. 2014;3:54-57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 111. | Copelan A, Chehab M, Dixit P, Cappell MS. Safety and efficacy of angiographic occlusion of duodenal varices as an alternative to TIPS: review of 32 cases. Ann Hepatol. 2015;14:369-379. [PubMed] |
| 112. | Ganguly S, Sarin SK, Bhatia V, Lahoti D. The prevalence and spectrum of colonic lesions in patients with cirrhotic and noncirrhotic portal hypertension. Hepatology. 1995;21:1226-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (1)] |
| 113. | Chawla Y, Dilawari JB. Anorectal varices--their frequency in cirrhotic and non-cirrhotic portal hypertension. Gut. 1991;32:309-311. [PubMed] |
| 114. | Sato T, Yamazaki K, Akaike J, Toyota J, Karino Y, Ohmura T. Retrospective analysis of endoscopic injection sclerotherapy for rectal varices compared with band ligation. Clin Exp Gastroenterol. 2010;3:159-163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 115. | Weilert F, Shah JN, Marson FP, Binmoeller KF. EUS-guided coil and glue for bleeding rectal varix. Gastrointest Endosc. 2012;76:915-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |