Published online Oct 6, 2010. doi: 10.4292/wjgpt.v1.i5.112
Revised: September 21, 2010
Accepted: September 28, 2010
Published online: October 6, 2010
AIM: To assess the relative bioavailability and pharmacokinetic properties of two formulations (test and reference) of Lafutidine 10 mg.
METHODS: The study was performed as an open label, randomized, two-way, two-period, two-treatment, single dose cross-over bioequivalence study, under non-fed condition to compare the pharmacokinetic profiles of the lafutidine formulation manufactured by Emcure Pharmaceuticals Ltd., India using an indigenously developed active pharmaceutical ingredient (API) and the commercially available Stogra® formulation, of UCB Japan Co., Ltd., Japan. The two treatments were separated by a wash-out period of 5 d. After an overnight fasting period of 10 h, the subjects were administered either the test or the reference medication as per the randomization schedule. Blood samples were collected at intervals up to 24 h, as per the approved protocol. Concentrations of lafutidine in plasma were analyzed by a validated liquid chromatography/tandem mass spectrometry (LC/MS/MS) method, and a non-compartmental model was used for pharmacokinetic analysis. The pharmacokinetic parameters were subjected to a 4-way ANOVA accounting for sequence, subjects, period and treatment. Statistical significance was evaluated at 95% confidence level (P ≥ 0.05).
RESULTS: The mean (± SD) values of the pharmacokinetic parameters (test vs reference) were Cmax (265.15 ± 49.84 ng/mL vs 246.79 ± 29.30 ng/mL, P < 0.05), Area under the curve (AUC)(0-t) (1033.13 ± 298.74 ng.h/mL vs 952.93 ± 244.07 ng.h/mL, P < 0.05), AUC(0-∞) (1047.61 ± 301.22 ng.h/mL vs 964.21 ± 246.45 ng.h/mL, P < 0.05), and t½(1.92 ± 0.94 h vs 2.05 ± 1.01 h, P < 0.05). The 90% confidence intervals (CI) for the test/reference ratio of mean Cmax, AUC(0-t), and AUC(0-∞) were within the acceptable range of 80.00 to 125.00. The mean times (± SD) to attain maximal plasma concentration (tmax) of lafutidine were 0.95 ± 0.24 h vs 1.01 ± 0.29 h (P < 0.05) for the test and the reference formulations respectively. Both the formulations were well tolerated.
CONCLUSION: In summary, this study has demonstrated the bioequivalence of the two formulations of lafutidine 10 mg. Hence it can be concluded that the two formulations can be used interchangeably in clinical settings.
- Citation: Dewan B, Chimata R. An open-label, randomized, cross-over bioequivalence study of lafutidine 10 mg under fasting condition. World J Gastrointest Pharmacol Ther 2010; 1(5): 112-118
- URL: https://www.wjgnet.com/2150-5349/full/v1/i5/112.htm
- DOI: https://dx.doi.org/10.4292/wjgpt.v1.i5.112
Lafutidine, (±)-2-(furfuryl sulfinyl)-N-[4-[4-(piperidinomethyl)-2-pyridyl]oxy-(Z)-2-butenyl] acetamide (Figure 1), is a newly developed second generation histamine H2-receptor antagonist[1]. It is absorbed in the small intestine, reaches gastric cells via the systemic circulation, and then directly and rapidly binds to gastric cell histamine H2 receptors, resulting in immediate inhibition of gastric acid secretion[2]. Lafutidine is used in the treatment of gastric ulcers, duodenal ulcers, and gastric mucosal lesions associated with acute gastritis and acute exacerbation of chronic gastritis[3]. It has been shown to have mucosal protective action in the gastrointestinal tract, including the esophagus, stomach, small intestine, and large intestine[3-5].
In clinical studies, lafutidine has been shown to inhibit gastric acid secretion during the daytime (i.e. postprandial) as well as during the night[6]. Lafutidine possesses a potent and long lasting gastric antisecretory effect mediated by H2-receptor blockade in animals. Lafutidine has a receptor-binding affinity which is 2-80 times higher than other representative H2-receptor antagonists (e.g. famotidine, ranitidine, and cimetidine)[7]. In addition, lafutidine exerts gastroprotective effects independent of its antisecretory action[8,9]. Lafutidine has been shown to increase the gastric mucosal blood flow[9] and gastric mucus secretion[10,11] and to accelerate epithelial restitution in rats.
The gastroprotective effects and intestinal protective effects of lafutidine are due to the activation of capsaicin-sensitive calcitonin gene related peptide (CGRP) containing vasodilator nerves (CGRPergic nerves) via modulation of presynaptic vanilloid-1 receptors[12,13]. Lafutidine causes a sustained increase in intracellular Ca2+ ion concentration in endothelial cells, which induces the release of neurotransmitters including CGRP. Lafutidine induced CGRP release stimulates nitric oxide (NO) production in endothelial cells, where NO participates in the regulation of gastric mucosal blood flow through vasodilation in the gastric microvasculature[14,15].
CGRP released from afferent neurons in the gastric mucosa stimulates D cells in the antral and fundic glands and increases somatostatin secretion from D cells. Somatostatin inhibits gastric acid secretion, acting directly on somatostatin receptors on parietal cells and indirectly by decreasing gastrin from antral G cells[16]. Lafutidine has been shown to significantly increase plasma somatostatin levels 0.3-2 h after a dose has been taken[17].
Lafutidine promptly suppresses gastric acid secretion, hence it is considered to be a useful drug for the on-demand treatment of mild gastroesophageal reflux disease[16]. Studies have shown that a triple therapy with lafutidine, clarithromycin and amoxicillin shows equivalent effect to that of lansoprazole, clarithromycin and amoxicillin in terms of Helicobacter pylori eradication rates, improvements in gastroesophageal reflux and abdominal symptoms after treatment, although the eradication rate with a triple therapy including lafutidine is not influenced by genetic polymorphism of CYP2C19 activity[18,19]. Lafutidine is used as a preanesthetic medication to decrease gastric fluid acidity and volume[20,21].
Lafutidine is presently approved in Japan as a tablet[3]. This product is not available in Europe, USA or India. The objective of the present study was to compare the pharmacokinetic profiles of lafutidine formulation manufactured by Emcure Pharmaceuticals Ltd., India using an indigenously developed active pharmaceutical ingredient (API) and the commercially available Stogra® formulation of UCB Japan Co., Ltd., Japan.
The API along with the test formulation (batch number FD/388/09; manufacturing date March 2009) were indigenously manufactured by Emcure Pharmaceuticals Ltd., India. The reference product Stogra® (batch number 9456, expiry date February’ 2012) was manufactured by UCB Japan Co., Ltd., Japan. Each film coated tablet of both formulations contained lafutidine equivalent to 10 mg. The study was conducted at Therapeutic Drug Monitoring (TDM) Laboratory, Mumbai, India and it was sponsored by Emcure Pharmaceuticals Ltd., India.
Guidelines drawn up by the Institutional Review Board (IRB), which met the requirements of the U.S. code of Federal Regulations, the Canadian MRC guidelines and Declaration of Helsinki, Tokyo 2004 as well as the ethical norms laid down by the Indian Council of Medical Research (ICMR), New Delhi, India, 2006 were followed during the study[22-24]. The protocol was approved by the institutional ethics committee.
Twenty eight healthy male subjects, including 4 subjects as standby to replace dropouts, were included in the study. All participants gave a written informed consent prior to participation, which had the approval of the institutional ethics committee, after they had been informed of the nature and details of the study in a language (both written and verbal) which they understood. Subject inclusion criteria included age between 18-45 years, non-smoker and Asian adult male of Indian origin with no evidence of underlying disease, medical disorders/impairments (hepatic, renal, cardiac, gastrointestinal tract and psychiatric), no vital sign abnormalities, no clinically significant abnormal values during pre-study screening, acceptable ECG, no consumption of drugs for 2 wk prior to the study, and no participation in any bioavailability or bioequivalence study at least 3 mo prior to the present study.
The exclusion criteria included history of hypersensitivity to the study product or related products, significant medical illness or conditions known to interfere with absorption, distribution, metabolism and excretion of the study drugs, significant history of medical illness like asthma, chronic bronchitis or other bronchospastic condition, glaucoma, cardiovascular or hematological disease, diabetes, metabolic acidosis or a known food allergy, significant clinical illness during 4 wk prior to day one of the study or hospitalization during 3 mo prior to the commencement of the study, maintenance therapy with any drug, alcohol abuse, drug dependency, use of enzyme modifying drugs within 30 d prior to day one of the study or use of any systemic medications including over the counter (OTC) drugs within 14 d prior to day one of the study, subjects who had a depot injection or an implant of any drug 3 mo prior to the commencement of the study, HIV or hepatitis positive subjects, and subjects who had donated blood (350 mL) within last 3 mo prior to the study.
The study was performed as an open label, randomized, two-way, two-period, two-treatment, single dose cross-over bioequivalence study, under non fed condition, and the treatments were separated by a wash-out period of 5 d. Each subject was assigned a unique identification number.
All the subjects arrived at the study center at least 13 h prior to the start of the study. They were housed in an air-conditioned facility and were given a standard dinner, which was finished at least 10 h before dosing in each period of the study. After an overnight fasting period of 10 h, the subjects were administered the medications as per the randomization schedule, for the test or the reference products, with 240 mL of plain drinking water. The intake of the study formulations was closely monitored by a physician and the oral cavity was checked properly to ensure completion of the administration process. Subjects were instructed to remain inclined on the bed for the first 2.0 h after dosing.
No meal was allowed until 4 h after dosing. Drinking water was restricted from 1 h before dosing till 2 h after dosing and ad libitum thereafter. Excess fluid intake (> 120 mL/h) was not allowed. Lunch, snacks and dinner were served as per the scheduled time.
All the subjects were abstained from any xanthine-containing food or beverages or alcoholic products for 72 h prior to formulation administration and throughout the sampling schedule during each period.
Subjects were informed not to take any drug at least 14 d prior to the study, especially cold preparations, aspirin, vitamins and antacid preparations. No concomitant medication was permitted during the study period.
Blood samples (5 mL) were collected from an antecubital vein by an indwelling venous cannula using coded, sterile vacutainers containing ethylenediamine tetraacetic acid (EDTA) as an anticoagulant. Blood samples were obtained immediately prior to dosing (predose sampling, 0.00 h) and at 0.25, 0.50, 0.75, 1.00, 1.25, 1.50, 1.75, 2.00, 2.50, 3.00, 3.50, 4.00, 5.00, 6.00, 8.00, 10.00, 12.00, 16.00, 18.00, and at 24.00 h after dosing. Blood samples were centrifuged at 4000 r/min (at 0-4°C) for 10 min, within 10 min of the sample collection, to separate the plasma. The plasma was separated and stored frozen at -20°C ± 5°C until assayed.
During the study periods, all the subjects were under medical supervision. Vital signs were examined at scheduled time as per the protocol.
A validated liquid chromatography/tandem mass spectrometry (LC/MS/MS) method was used for determination of lafutidine concentration in human plasma. Equipment used was a Perkin Elmer Series 200 pump fitted with Perkin Elmer series 200 autosampler and the software used was Analyst Software Integrator. Column type used was Cosmosil C18 (150 mm × 4.6 mm, i.d.) 5 μ and the mobile phase used was 0.01% Formic acid: Acetonitrile (20:80). Procedures of validation and acceptance criteria were based on “FDA Bio-analytical Method validation guidelines”[24].
Aliquots of 480.00 μL of drug free human plasma were taken in tubes and standard solutions were spiked to obtain concentrations of 5.00, 25.00, 50.00, 100.00, 200.00 and 400.00 ng/mL. The tubes were vortexed for 30 s. 100 μL of 0.2 mol/L sodium hydroxide was added and vortexed for 30 s. 5 mL of ethyl acetate was added to the tubes and the tubes were shaken for 10 min at 10 r/min in a shaker. The tubes were then centrifuged for 10 min at 3000 r/min. 4 mL of organic layer was collected and evaporated at 80°C until dryness under a stream of nitrogen for 10 min in a low volume evaporator. The residue was then reconstituted in 200 μL of the mobile phase, and 10 μL of the reconstituted residue was injected onto the LC/MS/MS system.
All pharmacokinetic parameters were determined by non-compartmental methods. Values below the quantification limit (< 5.00 ng/mL) were set to zero for calculation purposes.
The maximum plasma concentration (Cmax) and the time to reach Cmax (tmax) were taken directly from observed concentration vs time data. The elimination rate constant (Kel) was estimated by a non-linear least square regression analysis of the individual concentrations observed as a function of time during the elimination phase. The apparent elimination half life (t½) was obtained by dividing 0.693 by Kel. The area under the curve (AUC) of lafutidine in plasma from time zero to last quantifiable time point (t), AUC(0-t), was calculated using the linear trapezoidal rule. The AUC from time zero to infinity, AUC(0-∞), was calculated from the sum of AUC(0-t) and Clast/Kel, where Clast is the last measurable concentration of lafutidine in plasma.
The test and the reference formulations were considered to be bioequivalent if the calculated 90% confidence intervals (CI) for the log transformed ratios (test/reference) of the Cmax, AUC(0-t), and AUC(0-∞) were within the bioequivalence criteria range of 80.00-125.00 as established by the Central Drug Standard Control Organization (CDSCO), India; US Food and Drug Administration (US FDA) and European Medicines Evaluation Agency (EMEA).
Certified and validated WinNonlin version 3.0 (Pharsight Corp., USA) and Statistical Analysis System 9.1 (SAS 9.1) (SAS Institute Inc., USA) programs were used for statistical evaluations of the pharmacokinetic parameters.
The pharmacokinetic parameters were statistically analyzed by analysis of variance (ANOVA) test, and Schuermann’s two one sided t-test. Standard descriptive analysis including mean, standard deviation (SD) and standard error (SE) were used for variables such as height, weight and age. These statistical parameters including coefficient of variance were used to describe plasma concentrations at each individual time point as well as pharmacokinetic parameters. AUC(0-t), AUC(0-∞) and Cmax were subjected to a four-way ANOVA accounting for sequence, subjects, period and treatment and the statistical significance was evaluated at 95% confidence level (P≥ 0.05). The statistical method for testing bioequivalence was based upon the 90% CI for the ratio of the calculated means (test/reference) for the parameter under consideration. The statistical analysis (e.g. ANOVA) took into account sources of variation that can be reasonably assumed to have an effective response.
Twenty eight healthy male subjects, including 4 subjects as standby to replace dropouts, were included in the study. Subject number 21 did not report for the second period of the study, and hence was considered as a dropout. Therefore subject number 26, carrying similar sequence of drug administration as that of subject number 21, was included for analysis. Thus twenty seven adult males completed the study. However twenty four subjects were considered for evaluation of pharmacokinetic parameters.
The two formulations were well tolerated by the subjects. No adverse event was observed during both the periods of the study in any of the subjects. Both clinical and laboratory parameters of all subjects showed no clinically significant changes.
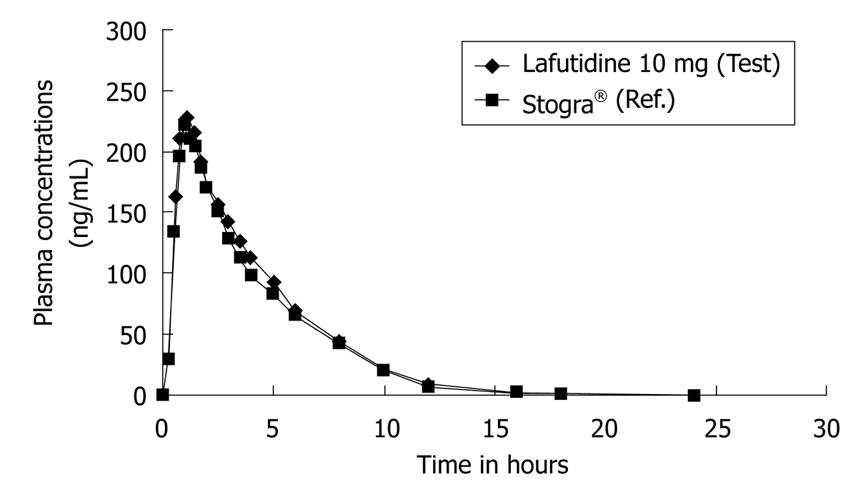
The mean age, mean weight and mean height of twenty four subjects were (± SD) of 27.9 ± 5.22 years (range 19-37 years), 63.3 ± 7.74 kg (range 51-76 kg) and 167.2 ± 6.62 cm (range 157-184 cm) respectively. Figure 2 shows the plots of mean serum concentrations of lafutidine vs time. Both the formulations were rapidly absorbed and detected from 0.25 h in plasma.
Mean pharmacokinetic parameters of lafutidine for the test and the reference formulation, in 24 healthy Indian subjects are presented in Table 1.
| Parameters | Test (mean ± SD) | Reference (mean ± SD) |
| Cmax (ng/mL) | 265.15 ± 49.84 | 246.79 ± 29.30 |
| AUC(0-t) (ng.h /mL) | 1033.13 ± 298.74 | 952.94 ± 244.07 |
| AUC(0-∞) (ng.h/mL) | 1047.61 ± 301.22 | 964.22 ± 246.45 |
| tmax (h) | 0.95 ± 0.24 | 1.01 ± 0.29 |
| Kel (h-1) | 0.44 ± 0.19 | 0.42 ± 0.22 |
| t½ (h) | 1.92 ± 0.94 | 2.05 ± 1.05 |
The results of ANOVA revealed that sequence and period had no statistically significant effects on Cmax (P > 0.05). However there was a significant effect of subject and treatment on Cmax (P < 0.05). Similarly, there was a significant effect of sequence, subject and treatment on AUC(0-t) and AUC(0-∞) (P > 0.05). The period had no significant effect on either AUC(0-t) or AUC(0-∞) (P < 0.05). The intra-subject variation, calculated using mean square error obtained from the logarithmically transformed Cmax, AUC(0-t) and AUC(0-∞) values, were 9.36%, 10.88% and 10.73% respectively. Additionally the 90% CI for the ratios of mean Cmax, AUC(0-t), and AUC(0-∞) were within the range of 80.00 to 125.00 (using log transformed data), meeting the regulatory criterion for bioequivalence as mentioned above. Table 2 represents the ratio (test/reference), 90% CI and the intra-subject variations of the Cmax, AUC(0-t) and AUC(0-∞).
| Parameters | Ratio (Test/Reference, %) | 90% Confidence interval (Log transformed data) | Intrasubject variability (Log transformed data, %) |
| Cmax | 106.69 | 101.86-111.74 | 9.36 |
| AUC(0-t) | 107.90 | 102.25-113.86 | 10.88 |
| AUC(0-∞) | 108.14 | 102.56-114.03 | 10.73 |
For overall extent of absorption, both the formulations were equivalent, with test/reference formulation ratios of both AUC(0-t) and AUC(0-∞) very close to 100%. Based on the plasma levels of the 24 completed subjects, the mean relative bioavailability of lafutidine was 107.90% as compared with the reference.
The pharmacokinetic data for each subject are illustrated in Table 3.
| Subject | Cmax (ng/mL) | tmax (h) | AUC(0-∞) (ng.h/mL) | t½(h) | ||||
| No. | T | R | T | R | T | R | T | R |
| 1 | 251.39 | 206.84 | 0.75 | 2.00 | 832.06 | 739.16 | 2.10 | 1.86 |
| 2 | 262.45 | 177.45 | 0.75 | 0.75 | 710.95 | 488.76 | 0.76 | 1.41 |
| 3 | 296.14 | 261.31 | 1.25 | 1.25 | 1194.42 | 1216.98 | 2.15 | 2.38 |
| 4 | 437.81 | 329.52 | 1.00 | 1.25 | 1794.64 | 1616.96 | 2.66 | 2.04 |
| 5 | 272.06 | 266.29 | 0.50 | 1.00 | 1514.49 | 1362.13 | 3.88 | 5.33 |
| 6 | 226.16 | 211.52 | 0.75 | 1.50 | 817.78 | 826.44 | 4.66 | 3.49 |
| 7 | 333.21 | 262.45 | 1.50 | 1.00 | 1670.60 | 919.94 | 2.74 | 2.38 |
| 8 | 367.03 | 284.66 | 1.00 | 0.75 | 1351.55 | 1029.20 | 2.83 | 2.60 |
| 9 | 217.84 | 264.98 | 0.75 | 0.75 | 915.96 | 946.37 | 1.47 | 1.17 |
| 10 | 239.84 | 263.04 | 0.75 | 1.00 | 908.95 | 955.31 | 1.68 | 2.17 |
| 11 | 261.86 | 230.72 | 1.00 | 0.75 | 934.60 | 1025.74 | 1.19 | 2.00 |
| 12 | 261.43 | 239.22 | 0.75 | 1.00 | 711.94 | 724.40 | 2.09 | 1.28 |
| 13 | 272.29 | 274.25 | 1.00 | 0.75 | 1079.63 | 1054.03 | 1.11 | 3.49 |
| 14 | 242.47 | 239.57 | 1.25 | 0.75 | 1338.51 | 1297.85 | 1.34 | 1.66 |
| 15 | 252.28 | 248.71 | 1.00 | 1.25 | 1132.98 | 1158.95 | 1.64 | 2.66 |
| 16 | 255.77 | 258.87 | 1.25 | 1.00 | 927.31 | 953.39 | 1.13 | 0.63 |
| 17 | 230.14 | 228.79 | 0.75 | 1.00 | 775.57 | 701.88 | 1.82 | 1.96 |
| 18 | 244.41 | 241.83 | 1.00 | 0.75 | 893.76 | 803.78 | 2.01 | 2.16 |
| 19 | 224.93 | 225.22 | 0.75 | 1.00 | 774.27 | 704.02 | 0.92 | 2.14 |
| 20 | 238.08 | 232.65 | 1.25 | 1.00 | 992.35 | 884.79 | 2.41 | 1.19 |
| 22 | 246.95 | 242.79 | 0.75 | 1.00 | 1109.70 | 1100.98 | 1.71 | 0.86 |
| 23 | 239.34 | 237.60 | 1.25 | 1.00 | 1163.50 | 984.41 | 0.95 | 1.31 |
| 24 | 240.45 | 245.86 | 0.75 | 1.00 | 727.85 | 825.47 | 1.32 | 1.01 |
| 26 | 249.33 | 248.84 | 1.00 | 0.75 | 869.33 | 820.28 | 1.42 | 1.97 |
This is the first bioequivalence study of lafutidine conducted on an Indian population. This study assessed the bioequivalence of a 10 mg lafutidine tablet formulation with the Stogra® 10 mg tablet manufactured by Japanese company UCB Japan Co. Ltd. API and the test formulation (batch number FD/388/09; manufacturing date March’ 2009) were indigenously manufactured by Emcure Pharmaceuticals Ltd., India.
Lafutidine has great potential for use in the treatment of gastric ulcers, duodenal ulcers, and gastric mucosal lesions associated with acute gastritis and acute exacerbation of chronic gastritis. Many published comparative clinical studies have established the superiority of lafutidine over proton pump inhibitors[2,6,16] and other H2-receptor antagonists[1,25]. The normal dose of lafutidine is 10 mg once a day for gastric mucosal lesions associated with acute gastritis and acute exacerbation of chronic gastritis; 10 mg once a day as a preanesthetic medication; and 10 mg twice a day for gastric ulcers, duodenal ulcers and stomal ulcers[20]. As the dosage of 10 mg remains the mainstay, the pharmacokinetics of single dose of lafutidine 10 mg was evaluated in healthy male volunteers.
An internationally published pharmacokinetic study of lafutidine performed on healthy Japanese male volunteers showed a Cmax of 133.90 ng/mL and tmax of 1.84 h[1]. A similar study performed on healthy Chinese volunteers showed a Cmax of 151.55 ± 54.49 ng/mL and tmax of 1.60 ± 0.40 h[26]. This particular study performed on an Indian subpopulation showed a Cmax of 265.15 ± 49.84 ng/mL and tmax of 0.95 ± 0.24 h. The estimated pharmacokinetic parameters of the test and the reference formulations in this study have higher levels than Japanese and Chinese clinical studies probably due to racial and genetic differences in the population studied.
The measured AUC and Cmax values following oral administration of both formulations (test and reference) maintained 90% CI within 80.00-125.00 for the log transformed values, suggesting that the two formulations were bioequivalent.
Lafutidine was found to be well tolerated in the present study. No adverse effects were reported or observed in any of the subjects. This finding is consistent with previously published clinical study by Ohya et al[27] where in no adverse event was observed in subjects given lafutidine.
In summary this study has demonstrated the bioequivalence of the 10 mg lafutidine tablet manufactured by Emcure Pharmaceuticals Ltd., India and the reference product, Stogra® manufactured by UCB Japan Co., Ltd., Japan. Hence it can be concluded that the two formulations can be used interchangeably in clinical settings.
Lafutidine is a newly developed second generation histamine H2-receptor antagonist used in the treatment of gastric ulcers, duodenal ulcers, and gastric mucosal lesions associated with acute gastritis and acute exacerbation of chronic gastritis. The mechanism of lafutidine encompasses a multimodal action that not only reduces the gastric acid output but also exhibits mucosal anti-inflammatory and mucosal protective activity. That is why it is categorized as a second-generation H2-receptor antagonist. The objective of the present study was to compare the pharmacokinetic profiles of a lafutidine formulation manufactured by Emcure Pharmaceuticals Ltd., India using indigenously developed active pharmaceutical ingredient and the commercially available formulation Stogra®, of UCB Japan Co., Ltd., Japan.
The control of acid-peptic disease represents a major triumph for modern pharmacology. Second generation H2-receptor antagonists, owing to their faster and multimodal mechanisms of action, can be used for on-demand treatment of mild to moderate acid-peptic disorders. Among the various second generation H2-receptor antagonists available, Lafutidine seems to hold its own niche. Lafutidine boasts of a multi-modal and potent armamentarium of mechanisms of action thus giving it an edge over the other representative H2-receptor antagonists. Lafutidine is presently approved in Japan as a tablet. This particular research aims at investigating the relative bioavailability and pharmacokinetic properties of two formulations of Lafutidine, and monitoring the safety and tolerability of a single dose of lafutidine 10 mg tablet in healthy adult Indian male volunteers.
Administration of the two lafutidine formulations (test and reference) to healthy adult Indian male volunteers did not significantly alter the pharmacokinetic profiles of either drug, demonstrating bioequivalence of the two formulations.
The study results suggest that lafutidine is safe and well tolerated. The formulations can be used interchangeably in clinical setting.
Bioavailability: Bioavailability refers to the relative amount of drug from an administered dosage form which enters the systemic circulation and the rate at which the drug appears in the systemic circulation. Bioequivalence: Bioequivalence of a drug is achieved if its extent and rate of absorption are not statistically significantly different from those of the reference product when administered at the same molar dose. Pharmacokinetics: Pharmacokinetics describes the movement of the drug into, within, and out of the body, and its time-course. Tmax: Time taken to achieve maximum plasma concentration. Cmax: Maximum plasma concentration. AUC: Area under the plasma concentration time curve. Kel: Mean elimination rate constant. t1/2: Mean elimination half-life. ANOVA: Analysis of variance.
The study was well performed and the results are well discussed. The statistical tests are adequate.
Peer reviewers: Elham Rahme, PhD, Associate Professor, Department of Medicine, McGill University Health Centre, 687 Pine Avenue West, V Building, Room V2.11 Montreal, Quebec, H3A1A1, Canada; Dr. Andreas Marc Palmer, PhD, Department of Medicinal Chemistry, Nycomed GmbH, Byk Gulden Str. 2, Konstanz 78467, Germany; Tomohiko Shimatani, MD, PhD, Professor, Department of General Medicine, Hiroshima UniversityHospital, 5-1-1 Hirokoshingai, Kure-shi, Hiroshima 737-0112, Japan
S- Editor Wang JL L- Editor Hughes D E- Editor Yang C
| 1. | Ikawa K, Shimatani T, Hayato S, Morikawa N, Tazuma S. Pharmacokinetic and pharmacodynamic properties of lafutidine after postprandial oral administration in healthy subjects: comparison with famotidine. Biol Pharm Bull. 2007;30:1003-1006. |
| 2. | Yamagishi H, Koike T, Ohara S, Horii T, Kikuchi R, Kobayashi S, Abe Y, Iijima K, Imatani A, Suzuki K. Stronger inhibition of gastric acid secretion by lafutidine, a novel H2 receptor antagonist, than by the proton pump inhibitor lansoprazole. World J Gastroenterol. 2008;14:2406-2410. |
| 3. | Onodera S, Shibata M, Tanaka M, Inaba N, Yamaura T, Ohnishi H. Gastroprotective activity of FRG-8813, a novel histamine H2-receptor antagonist, in rats. Jpn J Pharmacol. 1995;68:161-173. |
| 4. | Shibata M, Yamaura T, Inaba N, Onodera S, Chida Y, Ohnishi H. Gastric antisecretory effect of FRG-8813, a new histamine H2 receptor antagonist, in rats and dogs. Eur J Pharmacol. 1993;235:245-253. |
| 5. | Kato S, Tanaka A, Kunikata T, Umeda M, Takeuchi K. Protective effect of lafutidine against indomethacin-induced intestinal ulceration in rats: relation to capsaicin-sensitive sensory neurons. Digestion. 2000;61:39-46. |
| 6. | Shimatani T, Inoue M, Kuroiwa T, Horikawa Y, Mieno H, Nakamura M. Effect of omeprazole 10 mg on intragastric pH in three different CYP2C19 genotypes, compared with omeprazole 20 mg and lafutidine 20 mg, a new H2-receptor antagonist. Aliment Pharmacol Ther. 2003;18:1149-1157. |
| 7. | Inaba N, Shibata M, Onodera S, Tanaka M, Suzuki T, Yamaura T, Ohnishi H. [Studies on histamine H2-receptor antagonistic property of FRG-8813, a novel anti-ulcer drug]. Nippon Yakurigaku Zasshi. 1995;105:231-241. |
| 8. | Onodera S, Tanaka M, Aoyama M, Arai Y, Inaba N, Suzuki T, Nishizawa A, Shibata M, Sekine Y. Antiulcer effect of lafutidine on indomethacin-induced gastric antral ulcers in refed rats. Jpn J Pharmacol. 1999;80:229-235. |
| 9. | Onodera S, Shibata M, Tanaka M, Inaba N, Arai Y, Aoyama M, Lee B, Yamaura T. Gastroprotective mechanism of lafutidine, a novel anti-ulcer drug with histamine H2-receptor antagonistic activity. Arzneimittelforschung. 1999;49:519-526. |
| 10. | Ichikawa T, Ishihara K, Saigenji K, Hotta K. Effects of acid-inhibitory antiulcer drugs on mucin biosynthesis in the rat stomach. Eur J Pharmacol. 1994;251:107-111. |
| 11. | Ichikawa T, Ishihara K, Saigenji K, Hotta K. Lafutidine-induced stimulation of mucin biosynthesis mediated by nitric oxide is limited to the surface mucous cells of rat gastric oxyntic mucosa. Life Sci. 1998;62:PL259-PL264. |
| 12. | Kunieda K, Someya A, Horie S, Ajioka H, Murayama T. Lafutidine-induced increase in intracellular ca(2+) concentrations in PC12 and endothelial cells. J Pharmacol Sci. 2005;97:67-74. |
| 13. | Sugiyama T, Hatanaka Y, Iwatani Y, Jin X, Kawasaki H. Lafutidine facilitates calcitonin gene-related peptide (CGRP) nerve-mediated vasodilation via vanilloid-1 receptors in rat mesenteric resistance arteries. J Pharmacol Sci. 2008;106:505-511. |
| 14. | Chen RY, Guth PH. Interaction of endogenous nitric oxide and CGRP in sensory neuron-induced gastric vasodilation. Am J Physiol. 1995;268:G791-G796. |
| 15. | Holzer P. Neural emergency system in the stomach. Gastroenterology. 1998;114:823-839. |
| 16. | Inamori M, Togawa J, Iwasaki T, Ozawa Y, Kikuchi T, Muramatsu K, Chiguchi G, Matsumoto S, Kawamura H, Abe Y. Early effects of lafutidine or rabeprazole on intragastric acidity: which drug is more suitable for on-demand use? J Gastroenterol. 2005;40:453-458. |
| 17. | Itoh H, Naito T, Takeyama M. Lafutidine changes levels of somatostatin, calcitonin gene-related peptide, and secretin in human plasma. Biol Pharm Bull. 2002;25:379-382. |
| 18. | Hagiwara T, Kato M, Anbo T, Imamura A, Suga T, Uchida T, Fujinaga A, Nakagawa M, Nakagawa S, Shimizu Y. Improvement in symptoms after H2-receptor antagonist-based therapy for eradication of H pylori infection. World J Gastroenterol. 2007;13:3836-3840. |
| 19. | Isomoto H, Inoue K, Furusu H, Nishiyama H, Shikuwa S, Omagari K, Mizuta Y, Murase K, Murata I, Kohno S. Lafutidine, a novel histamine H2-receptor antagonist, vs lansoprazole in combination with amoxicillin and clarithromycin for eradication of Helicobacter pylori. Helicobacter. 2003;8:111-119. |
| 20. | Available from: http://www.ucb.com/_up/ucb_com_products/documents/STOGAR-April2005(050414)_tcm62-3741_tcm81-8593.pdf. |
| 21. | Uesugi T, Mikawa K, Nishina K, Morikawa O, Takao Y, Obara H. The efficacy of lafutidine in improving preoperative gastric fluid property: a comparison with ranitidine and rabeprazole. Anesth Analg. 2002;95:144-147, table of contents. |
| 22. | Uesugi T; WMA. Ethical principles for medical research involving human subjects. Declaration of Helsinki. 2004; Available from: http://www.laakariliitto.fi/e/ethics/helsinki.html. |
| 23. | Ethical Guidelines For Biomedical Research On Human Participants, Indian Council of Medical Research. ICMR Guidelines 2006; New Delhi 1-111;. Available from: http://www.icmr.nic.in/ethical_guidelines.pdf. |
| 24. | Uesugi T; FDA. Bioanalytical method validation. Food and drug administration. Guidance for Industry. 2001;1-22; Available from: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM070107.pdf. |
| 25. | Mikawa K, Nishina K, Uesugi T, Shiga M, Obara H. Lafutidine vs cimetidine to decrease gastric fluid acidity and volume in children. Can J Anaesth. 2003;50:425-426. |
| 26. | Chen WD, Liang Y, Li H, Xiong Y, Liu XD, Wang GJ, Xie L. Simple, sensitive and rapid LC-ESI-MS method for the quantitation of lafutidine in human plasma--application to pharmacokinetic studies. J Pharm Biomed Anal. 2006;41:256-260. |
| 27. | Ohya TR, Endo H, Kawagoe K, Yanagawa T, Hanawa K, Ohata K, Asayama M, Hisatomi K, Teratani T, Gunji T. A prospective randomized trial of lafutidine vs rabeprazole on post-ESD gastric ulcers. World J Gastrointest Endosc. 2010;16:36-40. |
| 28. | Aihara T, Nakamura E, Amagase K, Tomita K, Fujishita T, Furutani K, Okabe S. Pharmacological control of gastric acid secretion for the treatment of acid-related peptic disease: past, present, and future. Pharmacol Ther. 2003;98:109-127. |