INTRODUCTION
Acute pancreatitis (AP) is an inflammatory process of the pancreatic gland that exhibits a broad clinical spectrum and its severity may vary from a mild, edematous to a severe, necrotizing disease with high morbidity and mortality. In the most severe forms, the process involves remote organ systems. In fact, systemic inflammatory response syndrome (SIRS) is one of the major pathobiological processes underlying severe acute pancreatitis. This is of major importance because half of deaths in the first week of the process are attributed to organ failure and, in particular, the acute respiratory distress syndrome associated with SIRS[1]. Despite advances in diagnosis and treatment of inflammatory pancreatic disease, to date, supportive care remains the only treatment for patients with pulmonary complications.
It is widely accepted that the premature activation of digestive enzymes (trypsin, elastase and lipase) within the pancreatic acinar cells is a critical initiating event that leads to autodigestion of the pancreas[2]. However, acute pancreatitis is also an inflammatory disorder which develops a complex cascade of immunological events which not only affect the pathogenesis but also the course of the disease. Although intra-acinar or interstitial activation of trypsinogen is most probably the trigger of acute pancreatitis[3], in recent years much emphasis has been put on the role of leukocytes[4]. In addition, a number of proinflammatory mediators have been identified to play a role in the progression of local pancreatic damage to systemic inflammation. This includes tumor necrosis factor α (TNFα), interleukin (IL)-1β, IL-6, MCP-1 and Platelet activating factor[5]. Some of these mediators are initially released by pancreatic acinar cells and results in the recruitment of neutrophils and monocytes. Numerous experimental and clinical data indicate that more pro-inflammatory mediators including cytokines, arachidonic acid derivatives, activated oxygen species and proteases are released locally by over activated neutrophils and monocytes/macrophages among other cells[6]. When released, these mediators gain access to the systemic circulation and play a central role in the progression of multisystem organ failure[7].
MACROPHAGES
In addition to pancreatic cells, other cell populations contribute to the systemic generation of inflammatory mediators. In particular, it has been reported that peritoneal macrophages, alveolar macrophages and Kupffer cells become activated in different stages of severe acute pancreatitis[8-10].
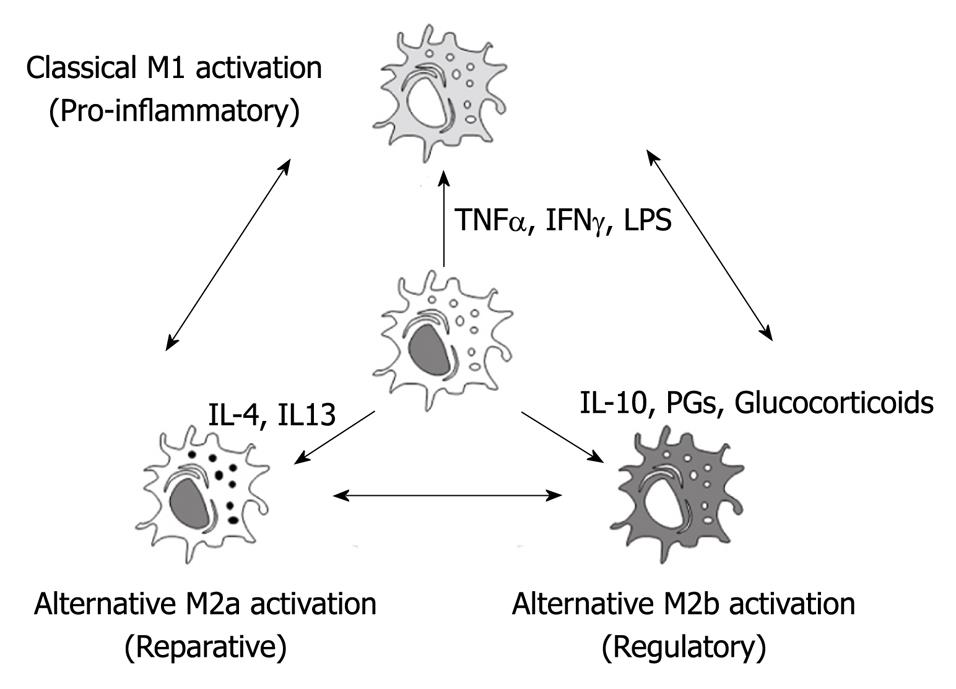
Macrophages display remarkable plasticity and can change their physiology in response to environmental cues[11]. Depending on their microenvironmental stimulation, macrophages could follow different activation pathways resulting in marked phenotypic heterogeneity (Figure 1)[12]. These changes can give rise to different populations of cells with distinct functions.
Figure 1 Depending on the microenvironment, macrophages could follow different activation processes.
Classical M1 activation is induced by bacterial products or pro-inflammatory cytokines as tumor necrosis factor α or IFNγ. Regulatory M2b activation is induced by glucocorticoids, prostaglandins or interleukin (IL)-10. Finally, reparative M2a activation depends mainly on IL-4 and IL-13. Since these phenotypes could be redirected, the modulation of macrophage phenotype could be a promising therapeutical approach for the treatment of the systemic effects of acute pancreatitis. TNFα: Tumor necrosis factor α.
During the initial stages of the inflammatory response, the presence of pro-inflammatory stimulus induces classically activated macrophages (M1 macrophages). Under this activation, M1 macrophages are characterized by the secretion of proinflammatory cytokines including TNFα, IL1β and IL6 and the induction of enzymes, such as iNOS or COX2, involved in the generation of other pro-inflammatory mediators as nitric oxide or arachidonic acid metabolites[11].
In addition to classical M1 activation, a second population of macrophages was identified in the presence of the Th2 cytokines IL4 and IL13. These macrophages are termed alternative, wound healing or M2a. Under this activation, these macrophages fail to produce NO and to present antigens to T cells but they up-regulate mannose receptor expression and arginase II[13,14]. These cells also contribute to the production of the extra-cellular matrix.
Finally, a third population of activated macrophages termed regulatory or M2b have been described in the later stages of immune responses. The primary role of these macrophages seems to be to limit the inflammatory response. The production of the regulatory cytokine TGFβ and IL10 can inhibit the production of proinflammatory mediators by the classical M1 macrophages[15]. These regulatory macrophages do not contribute to the production of the extracellular matrix but express high levels of co-stimulatory molecules (CD80 and CD86) and can present antigens to T cells[11].
Macrophages seem to retain their plasticity and respond to environmental signals[16]. Several in vitro studies indicate that the phenotype of a macrophage population can change in response to different stimuli[17]. On the other hand, in vivo there are some cases in which a phenotypic switch in the macrophage population occurs over time and is associated with pathology[18]. However, it is less clear whether these changes in the phenotype are the result of differentiation of the original macrophages or of the migration of a new population of macrophages into the tissue site where they replace the original cells[11].
Since macrophages orchestrate both the initiation and the resolution of inflammation, it is clear that the degree of macrophage activation could be one of the factors that finally determine the severity of the inflammatory process.
MACROPHAGE ACTIVATION DURING ACUTE PANCREATITIS
The fact that macrophages could generate both pro and anti-inflammatory mediators confers a pivotal role to these cells in the progression of acute pancreatitis. Several reports demonstrate that macrophages could be activated by mediators released during pancreatitis by a damaged pancreas. In particular, pancreatic enzymes such as trypsin, elastase, carboxypeptidase A and lipase induce the generation of TNFα in cultured peritoneal macrophages or in macrophage cell lines[19,20]. The fact that these effects are mediated by IκB degradation and NFκB activation indicates that these enzymes trigger macrophage activation through specific membrane-bound receptors[21].
In vitro activation of macrophages could also be observed by treating these cells with supernatants of pancreatic acinar cells cultures incubated with cerulein[22]. Similarly, ascitic fluid collected from rats after the induction of experimental models of pancreatitis activates macrophages in vitro[23,24]. This activation could also be blocked by treating the cells with NFκB inhibitors as pyrrolidine dithiocarbamate. Interestingly, lipids present in ascitic fluid also have an effect on the activation of macrophages. It has been observed that lipid fraction of ascitic fluid does not activate macrophages but interferes in the inhibitory activity of PPARγ nuclear receptor, thus resulting in an increased activation of these inflammatory cells[24].
Direct measurement of circulating monocytes also revealed a significant degree of activation. Increased expression of iNOS[25] and TLR4[26] was reported in blood monocytes obtained from human patients with severe acute pancreatitis. In an experimental model of taurocholate-induced pancreatitis, the activation of NFκB and p38 MAPK signalling pathways in circulating monocytes has also been observed[27].
These results indicate that during acute pancreatitis there are mediators released with the capability to activate these inflammatory cells. When activated, macrophages could act to amplify the inflammatory response triggered in the pancreas through the generation of more cytokines and inflammatory mediators in systemic organs[28]. The importance of this activation on the progression of the disease was shown by the use of different inhibitors.
In this line, the administration of drugs as the macrophage-pacifying compound CNI-1493 prior to the induction of severe acute pancreatitis in rats results in an increased survival and a reduction in the severity of the process characterized by lower levels of circulating enzymes, cytokines as well as transaminases[29,30]. A protective effect was also observed by depleting macrophages with the injection of liposome-encapsulated dichloromethylene-diphosphonate, a macrophage-depleting agent, in a mice model of virus -induced pancreatitis[31]. However, in this case, a reduction in the levels of potentially protective cytokines as IL-10 was also observed.
MACROPHAGE POPULATIONS IN PANCREATITIS: PERITONEAL MACROPHAGES, KUPFFER CELLS AND ALVEOLAR MACROPHAGES
During the progression of acute pancreatitis from local pancreatic damage to systemic organ inflammation, several macrophage populations are of particular importance. Peritoneal macrophages are in direct contact with ascitic fluid secreted by the pancreas. This fluid generated in severe acute pancreatitis contains pancreatic enzymes and cytokines in a concentration that exceeds that observed in plasma in an order of magnitude[32]. Consequently, peritoneal macrophages are exposed to an intense pro-inflammatory environment and strong activation could be expected. The importance of this activation is the fact that mediators released by these macrophages to the peritoneal cavity could easily achieve the bloodstream through mesenteric absorption, thus contributing to the inflammatory response associated with acute pancreatitis[32].
A number of studies using different models of acute pancreatitis confirmed an early and intense M1 activation of peritoneal macrophages, reflected in the high expression of proinflammatory cytokines such as TNFα, IL1β, IL6 and enzymes such as iNOS[33]. The contribution of peritoneal macrophage-derived mediators to the toxicity of ascitic fluid was shown when a peritoneal lavage was carried out before the induction of pancreatitis in order to remove the macrophages[34]. In these conditions, ascitic fluid was generated by the effect of acute pancreatitis but the cytotoxic effects of this fluid, and in particular its apoptosis-inducing activity, was significantly reduced.
Another population of macrophages that has been involved in the pathogenesis of acute pancreatitis are Kupffer cells. They are the resident macrophages in the liver and participate in the acute response of this organ to toxic compounds. Since mediators released by a damaged pancreas or present in ascitic fluid are carried to the systemic circulation via the portal vein, the Kupffer cells could interact with all these products before they become diluted into the systemic circulation[26]. In vitro analysis of Kupffer cell activity revealed that these macrophages could also be activated by pancreatic enzymes[35,36].
Several works reported on the effect of gadolinium chloride administration to inhibit Kupffer cells activity before the induction of acute pancreatitis[37-41]. This inhibition results in lower levels of circulating cytokines and the pathological injury of the lung was ameliorated. By contrast, pancreatic damage was not affected by Kupffer cell blockage. These results indicate that the liver acts to amplify the inflammatory signal triggered by the pancreas in a process that is mediated by the activation of hepatic macrophages. Interestingly, the liver itself is not affected by this process and hepatic damage is not evident in early stages of pancreatitis.
The third family of macrophages involved in the progression of acute pancreatitis is alveolar macrophages. The capacity of alveolar macrophages to mobilize a large amount of leukocytes and release secretory products such as cytokines, arachidonic acid metabolites and nitric oxide (NO) after their activation in the course of different pulmonary inflammatory diseases suggests that these cells can be involved in the lung damage associated with AP. These macrophages exhibit particular characteristics, probably as a consequence of their anatomical situation, in direct contact with the environmental pollutants present in the breathing air.
A number of works reported on the changes presented by these cells during the acute lung injury secondary to pancreatitis. In particular, increased NO synthesis related to the induction of iNOS has been shown[42,43]. The use of phospholipase A2 inhibitors indicates that this enzyme could be involved in the activation of alveolar macrophages to generate nitric oxide[44]. However, the role of alveolar macrophages in the progression of acute lung injury during pancreatitis remains controversial. The use of inhibitors could affect other pulmonary cells involved in the generation of cytokines and the level of activation observed in alveolar macrophages seems to be lower than that observed in peritoneal or hepatic macrophages.
MACROPHAGES: A THERAPEUTIC TARGET?
The role of macrophages in the progression from local inflammation of the pancreas to a systemic inflammation and multiple-organ dysfunction made these cells interesting therapeutical targets. In particular, the capacity of macrophages to sequentially exhibit pro- and anti- inflammatory properties is of interest. This capacity suggests that macrophages not only are mediators in the inflammatory injury but can be induced to modify the sequence of events that occurs during acute pancreatitis.
Initial strategies were focused on the inhibition of macrophages. In experimental models, the use of gadolinium chloride to inhibit Kupffer cells[28,35], liposome-encapsulated dichloromethylene-diphosphonate to act on peritoneal macrophages[26] or PAF antagonists to block the activation of alveolar macrophages[45] resulted in the modulation of the systemic inflammatory response. Unfortunately, in these studies macrophage inhibitors were administered before the induction of pancreatitis and the application of this approach in clinical practice is difficult. Another problem is the long time needed to effectively inhibit macrophage activity or to deplete the macrophage population. Consequently, other approaches have been assayed based on the capability of macrophages to modify its phenotype.
The administration of IL-4 and IL-13 has been evaluated in order to derivate the pancreatitis-activated M1 peritoneal macrophages to a reparative M2a phenotype. However, despite this treatment effectively reverting the M1 pro-inflammatory macrophages to M2b reparative cells in vitro, it failed when assayed in vivo[33]. The reason for this failure seems to be the related to the ascitic fluid present in the peritoneal cavity. The high concentration of hydrolytic enzymes in this fluid result in the degradation of cytokines and its activity was lost before any effect on the macrophages could be observed.
Another interesting strategy was to programme macrophages ex vivo with anti-inflammatory or protective characteristics and to transfer these cells into pancreatitis-induced animals. This has been carried out using an experimental model of diet- induced pancreatitis in mice and transferring heme-activated macrophages before starting the diet[46]. Heme activated macrophages express high amount of heme-oxygenase-1 that unveil several potential protective mechanisms mediated by IL-10 and p38 MAPK activity. This approach results in a reduction of histological score and in the levels of circulating amylase. However, despite these data confirming the role of inflammatory cells on the progression of local pancreatic damage during pancreatitis, the long time needed to obtain heme-activated macrophages is a challenge to apply this approach as a therapeutical strategy.
CONCLUSION
Different populations of resident macrophages are involved in the progression of acute pancreatitis from local pancreatic damage to a multiple organ failure response. The plasticity of these cells makes them an attractive target to manipulate the systemic inflammatory response associated with acute pancreatitis. Therefore, future studies are needed to improve the manipulation or selective depletion of macrophages.
Peer reviewers: Ilker Tasci, MD, Associate Professor, Department of Internal Medicine, Gulhane School of Medicine, Etlik 06018, Ankara, Turkey; Maxim Petrov, MD, MPH, Department of Surgery, University of Auckland, Private Bag 921019, Auckland 1142, New Zealand
S- Editor Wang JL L- Editor Roemmele A E- Editor Yang C