Copyright
©The Author(s) 2016.
World J Gastrointest Pharmacol Ther. May 6, 2016; 7(2): 274-282
Published online May 6, 2016. doi: 10.4292/wjgpt.v7.i2.274
Published online May 6, 2016. doi: 10.4292/wjgpt.v7.i2.274
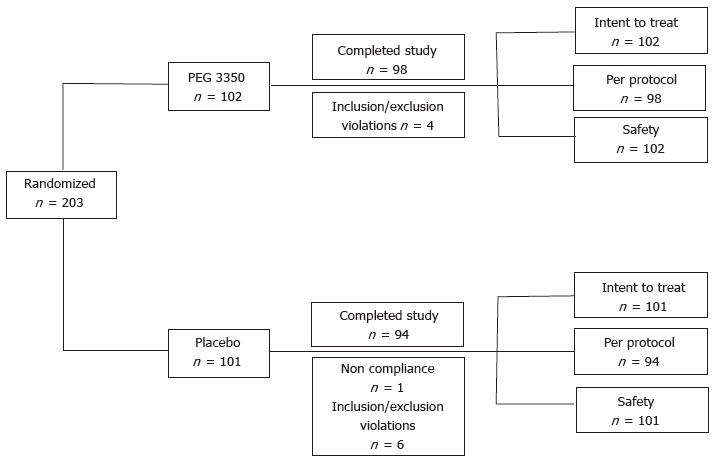
Figure 1 Disposition of subjects.
The ITT population included all subjects randomized to a study treatment and receiving at least one dose of the assigned drug. The per-protocol population included all ITT subjects who additionally exhibited no major protocol violations or other events considered biasing the study outcome. The safety population included all subjects who received one or more dose of the study medication. ITT: Intent-to-treat; PEG: Polyethylene glycol.
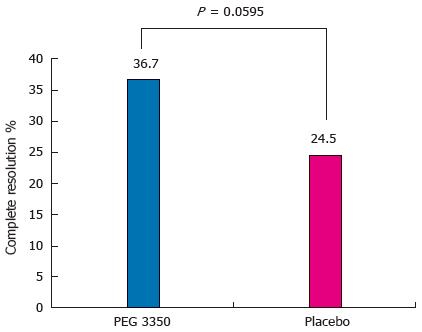
Figure 2 Assessment of primary outcome between polyethylene glycol 3350 and placebo (per protocol population).
Resolution was recorded if the subject reported no occurrence of two or more consecutive unsuccessful bowel movements (BMs) for the rest of the study following the first successful BM. PEG: Polyethylene glycol.
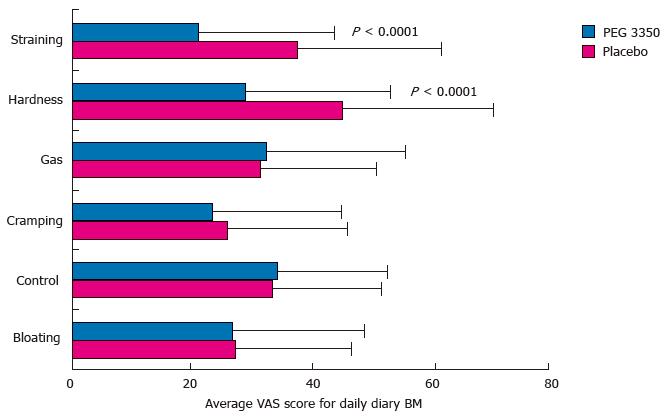
Figure 3 Average visual analog scale scores for daily diary entries of bowel movements of subjects receiving polyethylene glycol 3350 or placebo (PP population).
Error bars represent standard deviations of mean. BM: Bowel movement; PEG: Polyethylene glycol; VAS: Visual analog scale.
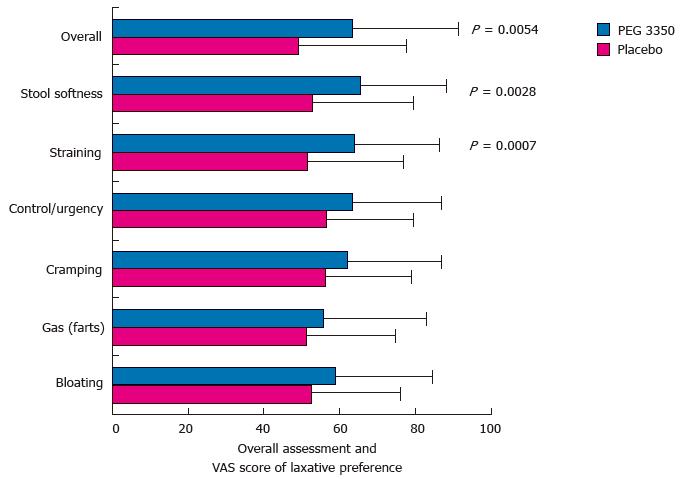
Figure 4 Visual analog scale scores for laxative preference of subjects receiving polyethylene glycol 3350 or placebo (per protocol population).
Error bars represent standard deviations of mean. PEG: Polyethylene glycol; VAS: Visual analog scale.
- Citation: McGraw T. Polyethylene glycol 3350 in occasional constipation: A one-week, randomized, placebo-controlled, double-blind trial. World J Gastrointest Pharmacol Ther 2016; 7(2): 274-282
- URL: https://www.wjgnet.com/2150-5349/full/v7/i2/274.htm
- DOI: https://dx.doi.org/10.4292/wjgpt.v7.i2.274