Copyright
©The Author(s) 2024.
World J Gastrointest Pharmacol Ther. May 28, 2024; 15(3): 90757
Published online May 28, 2024. doi: 10.4292/wjgpt.v15.i3.90757
Published online May 28, 2024. doi: 10.4292/wjgpt.v15.i3.90757
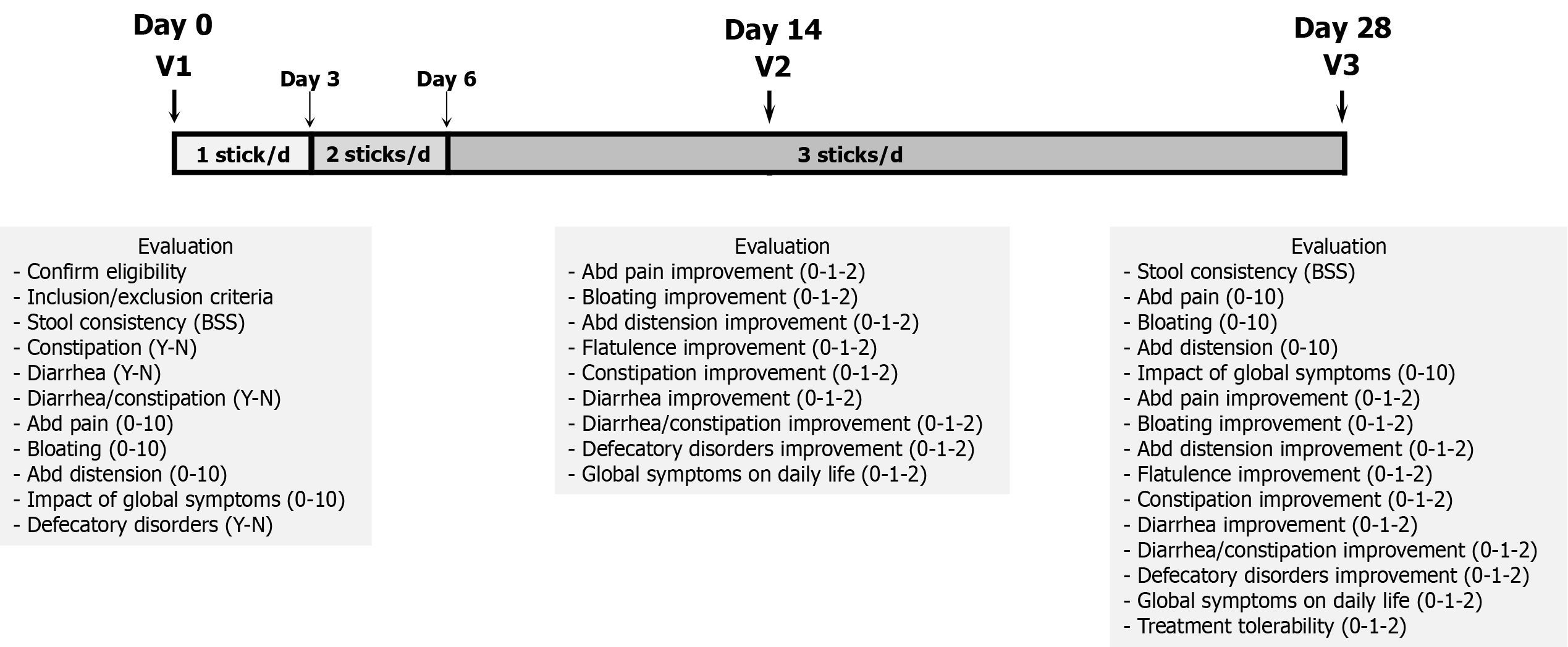
Figure 1 Study design.
D: Day; BSS: British stool scale; d: day; V: Visit; Y: Yes; N: No; abd: Abdominal; SGA: Subjective global assessment.
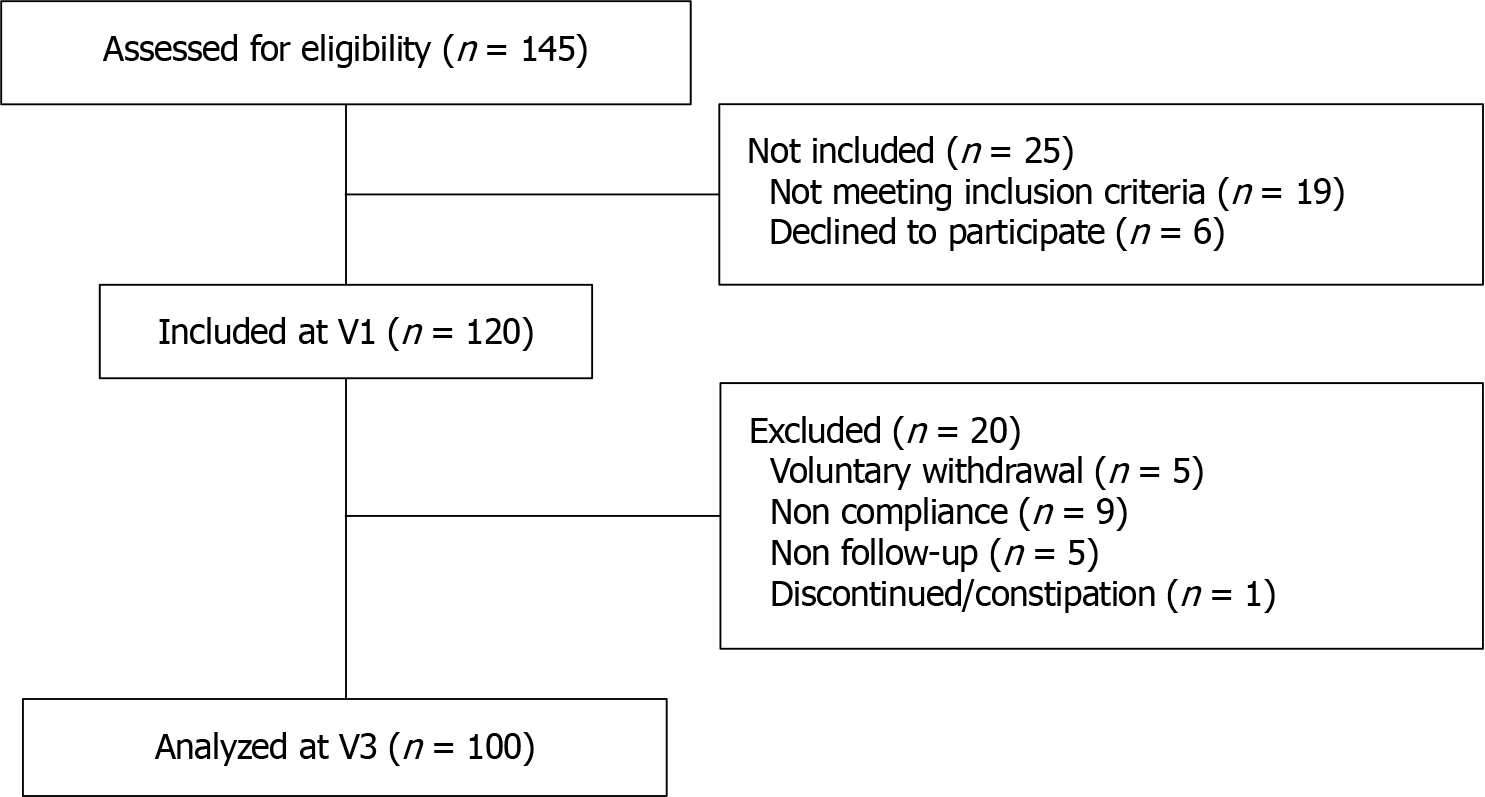
Figure 2 Patient selection flow chart.
N: Number; V: Visit.
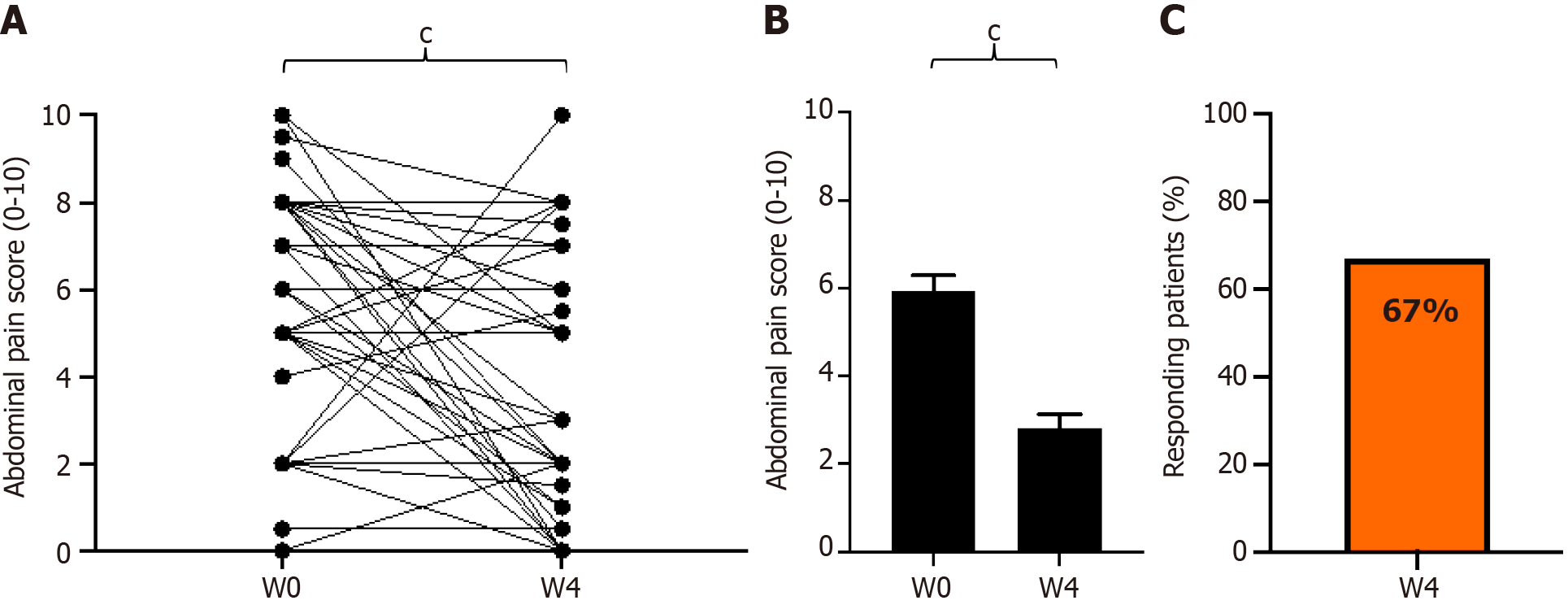
Figure 3 Change in paired abdominal pain scores from baseline to week 4 of GASTRAP® DIRECT treatment (visit 1 to visit 3).
A: Paired abdominal pain scores; B: Abdominal pain scores (0–10); C: Patient responders (delta > 30%) with respect to abdominal pain. cP < 0.001. W: Weeks.
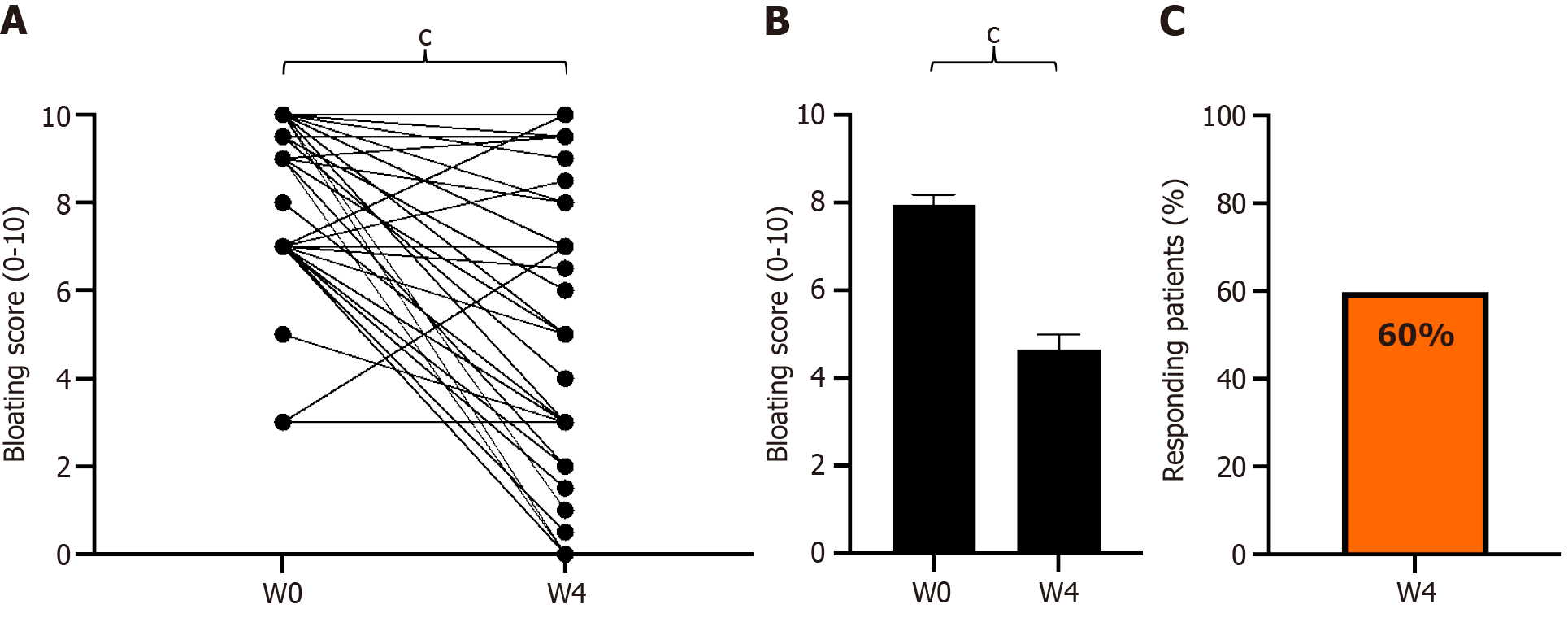
Figure 4 Change in paired bloating scores from baseline to week 4 of GASTRAP® DIRECT treatment (visit 1 to visit 3).
A: Change in paired bloating scores; B: Abdominal bloating scores (0–10); C: Patient responders (delta > 30%) with respect to bloating. cP < 0.001. W: Weeks.
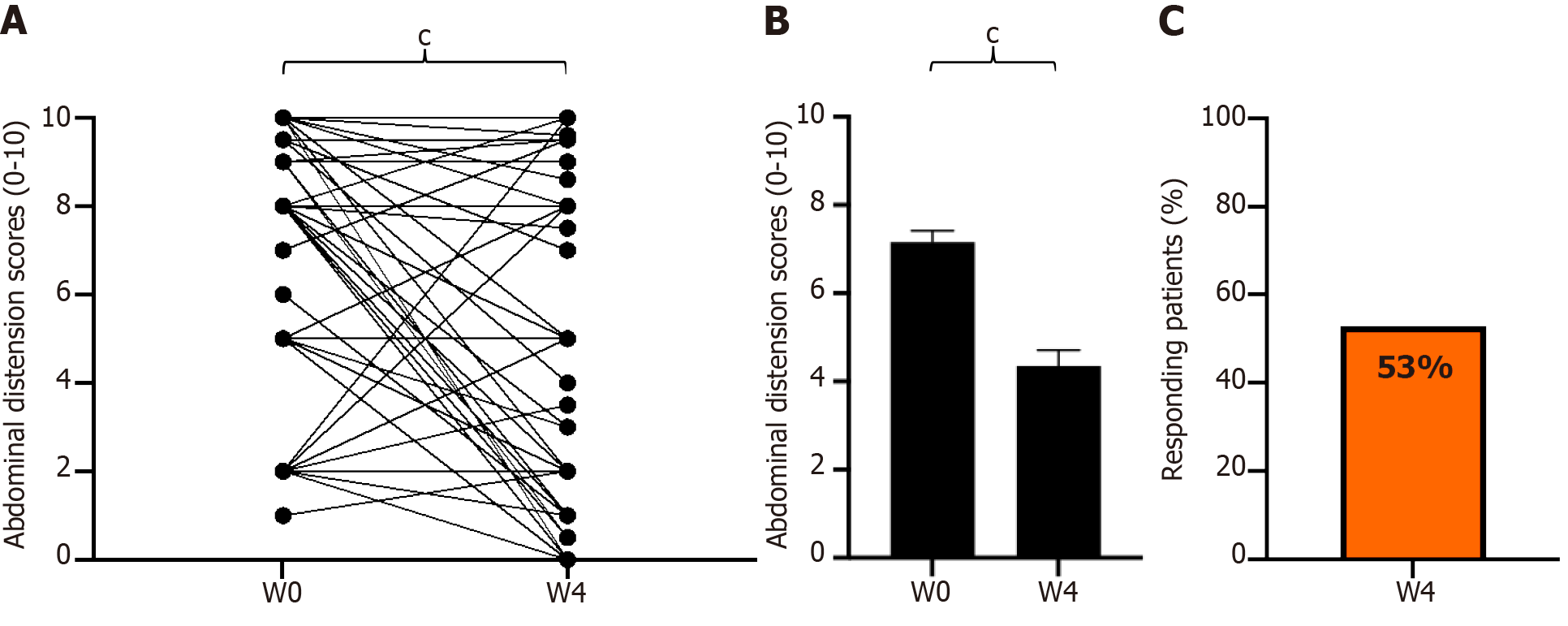
Figure 5 Change in abdominal distension scores from baseline to week 4 of GASTRAP® DIRECT treatment (visit 1 to visit 3).
A: Change in paired abdominal distension scores; B: Abdominal distension scores (0–10); C: Patient responders (delta > 30%) with respect to abdominal distension. cP < 0.001. W: Weeks.
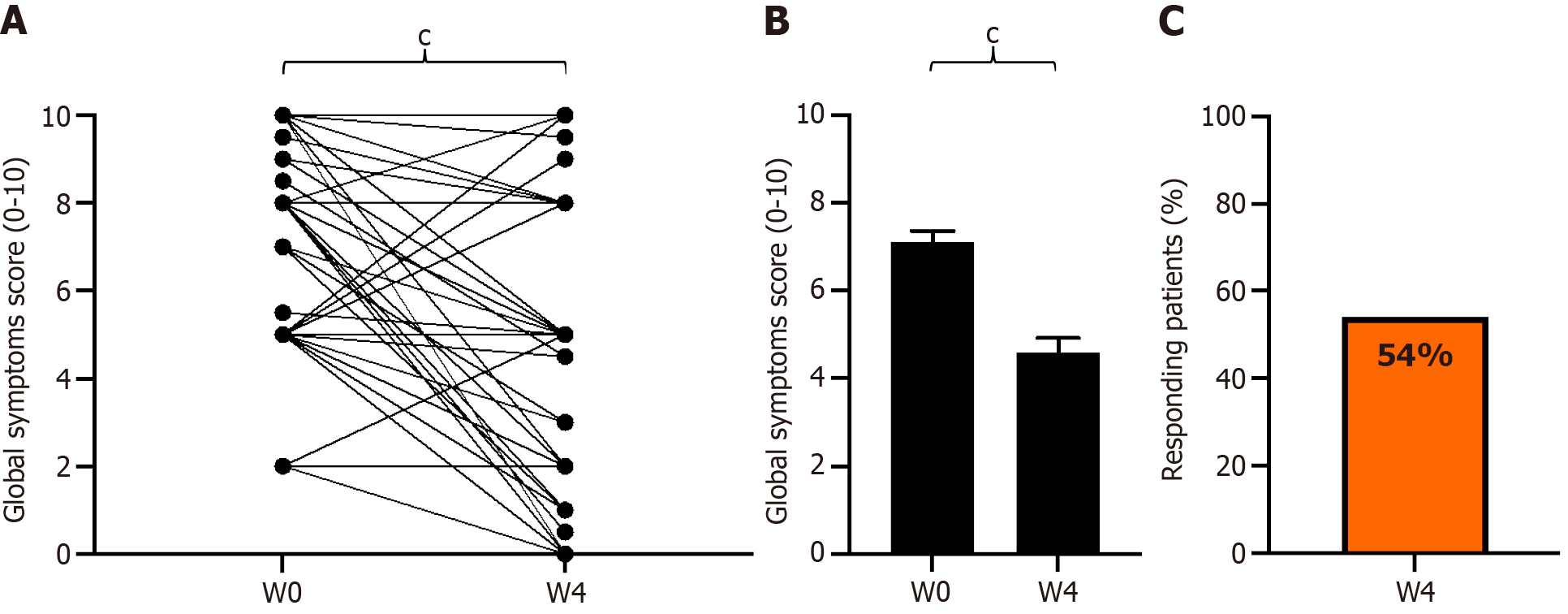
Figure 6 Change in impact of global symptoms on daily life scores from baseline to week 4 of GASTRAP® DIRECT treatment (visit 1 to visit 3).
A: Paired global symptom scores; B: Global symptom scores (0–10); C: Patient responders (delta > 30%) with respect to global symptoms. cP < 0.001. W: Weeks.
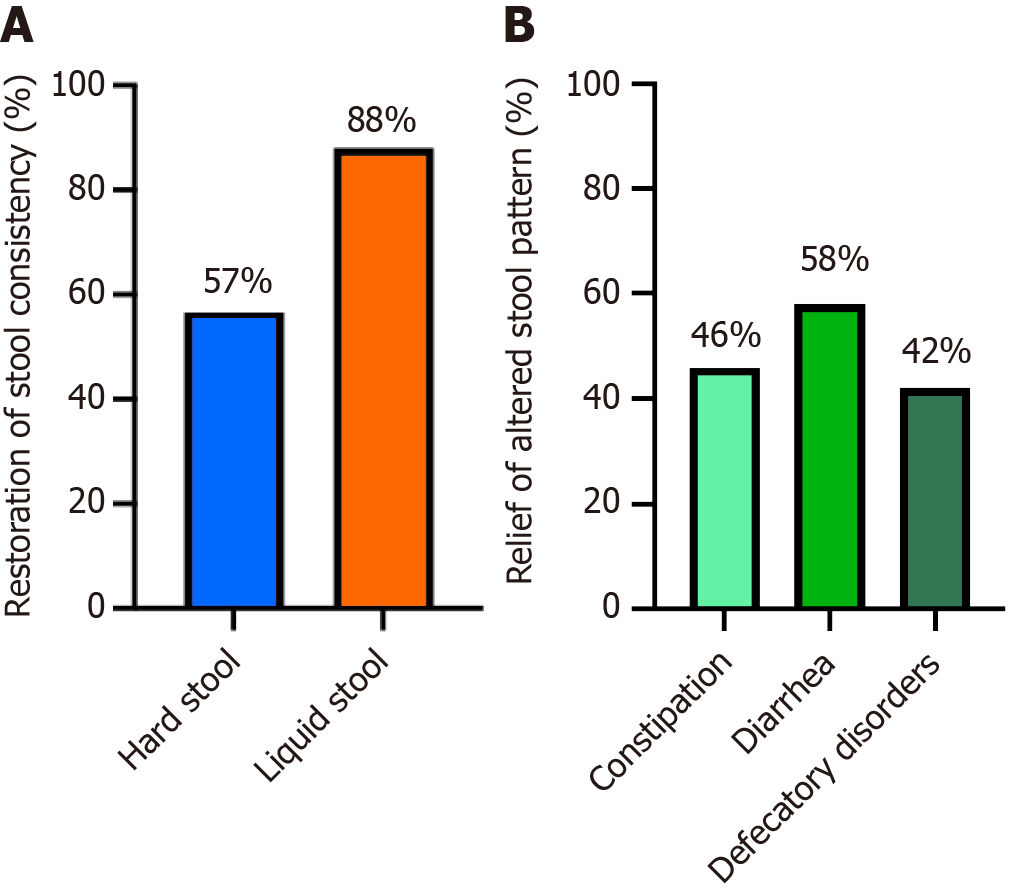
Figure 7 Changes in stool from baseline to week 4 of GASTRAP® DIRECT treatment (visit 1 to visit 3).
A: Restoration of stool consistency {hard stool [Bristol stool scale (BSS) score 1–2] to normal; liquid stool (BSS 6–7) to normal}; B: Relief of altered stool pattern.
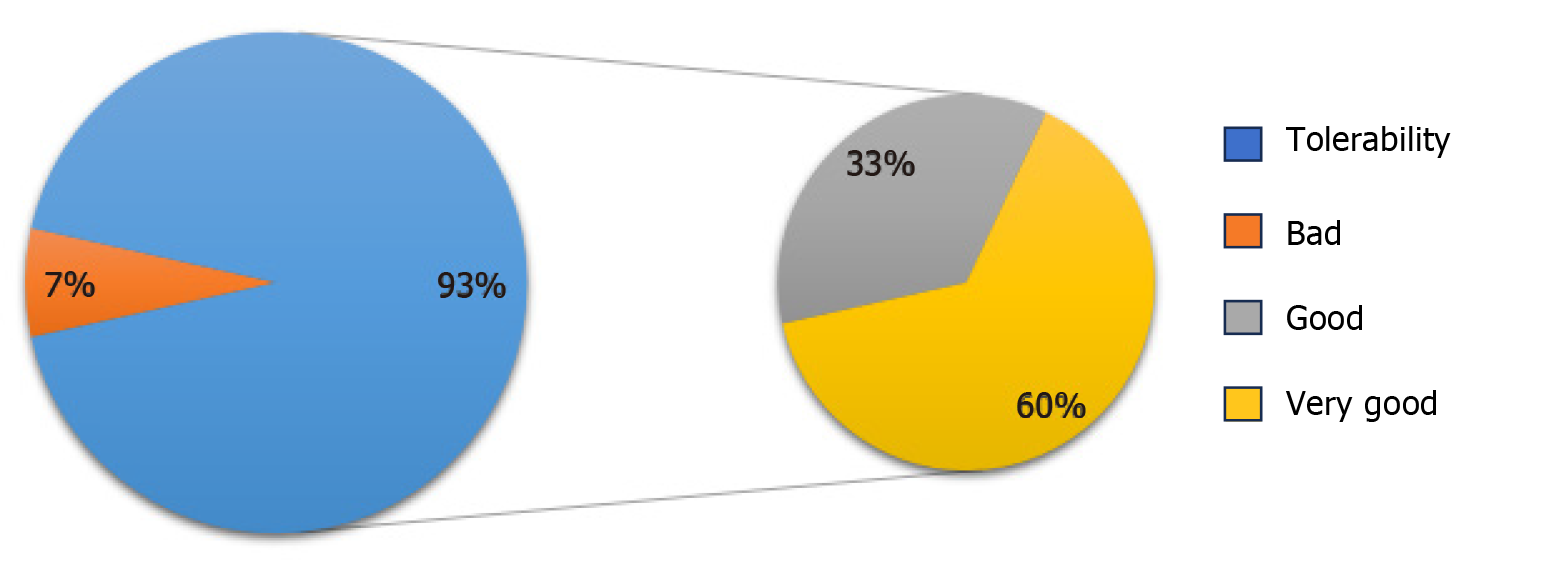
Figure 8
Tolerability of GASTRAP® DIRECT evaluated at week 4.
- Citation: Talbodec N, Le Roy P, Fournier P, Lesage B, Lepoutre E, Castex F, Godchaux JM, Vandeville L, Bismuth B, Lesage X, Bayart P, Genin M, Rousseaux C, Maquet V, Modica S, Desreumaux P, Valibouze C. Efficacy and tolerability of chitin-glucan combined with simethicone (GASTRAP® DIRECT) in irritable bowel syndrome: A prospective, open-label, multicenter study. World J Gastrointest Pharmacol Ther 2024; 15(3): 90757
- URL: https://www.wjgnet.com/2150-5349/full/v15/i3/90757.htm
- DOI: https://dx.doi.org/10.4292/wjgpt.v15.i3.90757