Published online Nov 15, 2017. doi: 10.4291/wjgp.v8.i4.176
Peer-review started: March 20, 2017
First decision: May 3, 2017
Revised: May 20, 2017
Accepted: June 30, 2017
Article in press: July 3, 2017
Published online: November 15, 2017
Processing time: 240 Days and 11.6 Hours
To determine if almond extracts reduce the severity of chemotherapy-induced mucositis as determined through biochemical, histological and behavioural markers.
Intestinal mucositis is a debilitating condition characterized by inflammation and ulceration of the gastrointestinal mucosa experienced by cancer patients undergoing chemotherapy. Certain bioactive plant products have shown promise in accelerating mucosal repair and alleviating clinical symptoms. This study evaluated almond extracts for their potential to reduce the severity of chemotherapy-induced mucositis in Dark Agouti rats. Female Dark Agouti rats were gavaged (days 3-11) with either PBS, almond hull or almond blanched water extract at two doses, and were injected intraperitoneally with 5-fluorouracil (5-FU-150 mg/kg) or saline on day 9 to induce mucositis. Burrowing behavior, histological parameters and myeloperoxidase activity were assessed.
Bodyweight was significantly reduced in rats that received 5-FU compared to saline-treated controls (P < 0.05). Rats administered 5-FU significantly increased jejunal and ileal MPO levels (1048%; P < 0.001 and 409%; P < 0.001), compared to healthy controls. Almond hull extract caused a pro-inflammatory response in rats with mucositis as evidenced by increased myeloperoxidase activity in the jejunum when compared to 5-FU alone (rise 50%, 1088 ± 96 U/g vs 723 ± 135 U/g, P = 0.02). Other extract-related effects on inflammatory activity were minimal. 5-FU significantly increased histological severity score compared to healthy controls confirming the presence of mucositis (median of 9.75 vs 0; P < 0.001). The extracts had no ameliorating effect on histological severity score in the jejunum or ileum. Burrowing behavior was significantly reduced in all chemotherapy-treated groups (P = 0.001). The extracts failed to normalize burrowing activity to baseline levels.
Almond extracts at these dosages offer little beneficial effect on mucositis severity. Burrowing provides a novel measure of affective state in studies of chemotherapy-induced mucositis.
Core tip: In spite of their procyanadin content and anti-oxidant capacity the almond extracts tested failed to provide any ameliorating effect on chemotherapy-induced mucositis in rats as determined through biochemical and histological evaluation. Currently studies utilising animal models of gastrointestinal disease largely fail to assess measures of patient affect. In order to achieve successful translational outcomes assessment of patient experience is important since these disease conditions are often self-limiting. Burrowing behaviour shows promise as a novel measure of affective state in studies of chemotherapy-induced mucositis.
- Citation: Whittaker AL, Zhu Y, Howarth GS, Loung CS, Bastian SEP, Wirthensohn MG. Effects of commercially produced almond by-products on chemotherapy-induced mucositis in rats. World J Gastrointest Pathophysiol 2017; 8(4): 176-187
- URL: https://www.wjgnet.com/2150-5330/full/v8/i4/176.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v8.i4.176
Patients undergoing chemotherapy treatment for cancer frequently experience severe side-effects which can cause significant deterioration to quality of life[1]. A common side-effect, affecting approximately 60% of patients is mucositis[2]. The term mucositis refers to inflammation and ulceration of the gastrointestinal tract. Anatomical sites commonly affected are the buccal mucosa (oral mucositis), and the small intestinal mucosa (intestinal mucositis)[3]. Histologically, intestinal mucositis is characterized by crypt cell apoptosis and hypo-proliferation, villous atrophy, and ulcerative lesions[4]. These pathophysiological processes result in a range of clinical symptoms which may include pain, diarrhea, nausea and inappetance[5]. The severe nature of the symptoms can lead to patient-requested reduction of chemotherapy dose. Additionally, intestinal mucositis can increase the frequency of peripheral parenteral nutrition prescription, which can predispose to high morbidity. Any reduction in therapeutic level has the potential to impact on tumor kill rates; hence the need to discover new treatments or preventative agents which will reduce mucositis side-effects[6]. These treatments should not just provide symptomatic relief but target the pathogenesis of the condition[7].
Almonds belong to the genus Prunus or Amygdalus. A number of fruit extracts from these genera have been investigated for their anti-cancer properties. Many have had moderate success in suppressing growth and reducing proliferation in in-vitro studies using various cancer cell lines[8-11]. Almonds have also been determined to play a chemo-preventative role[12,13], and to exhibit anti-inflammatory properties[14]. It should be noted that cultivated (sweet) almonds differ in composition to bitter almonds[15]. Bitter almonds contain amygdalin which releases hydrocyanic acid[16]. Amygdalin has been widely proposed as a potential anti-cancer treatment. However, amygdalin use has been controversial due to the risk of cyanide poisoning, and its effectiveness has not been substantiated[17].
The various health related benefits conferred through sweet almond consumption are presumed to be due to their content of arginine, fiber, mono- and polyunsaturated fat, vitamin E, and other nutrients. Presence of phenolic acids and polyphenols may also contribute to their health profile[18] . Procyanidins are a class of polyphenolic compounds that have bioactive properties[19]. Grape-seed extract has recently shown promise in the alleviation of intestinal injury in chemotherapy-induced mucositis, and at enhancing chemotherapeutic effectiveness in colon cancer cell lines[19]. This beneficial effect is thought to be brought about through the action of the procyanidins. Almonds also contain procyanidins[12], and thus they may similarly ameliorate the gut inflammation caused by chemotherapy administration.
This study was designed to assess whether two types of sweet almond extracts were safe and efficacious in the prevention of chemotherapy-induced mucositis damage in a rat model. Furthermore, we incorporated a behavioral measure of affective state (burrowing) into the study in order to determine validity of the technique, and to provide a further measure of therapeutic outcome.
This study utilized two different by-products of the commercial almond production process; almond hull extract (AHE) and almond blanched water (ABW). Almond hulls (50 g; Riverland, South Australia) were extracted in 1250 mL of reverse osmosis treated water in a slow cooker. Temperature within the cooker increased from 21 °C to 80 °C over 5 h. Following this, the dry matter was maintained in water overnight until the solution was completely cooled. Extract was then stored at 4 °C until use. Almond blanched water was sourced from a different processing plant (Almond Co., Riverland, South Australia) and was taken directly from the process line and stored at 4 °C until use. The total polyphenol levels and antioxidant capacity of both sources were measured using the total polyphenol gallic acid equivalent test and L-ascorbic acid equivalent test described below.
Both extracts were prepared similarly by drying 1 mL of the stored extracts in a centrifuge dryer (45 °C, 3-4 h). Using the gravimetric method, the resultant dry matter was measured and re-dissolved in 1.5 mL Dulbecco’s Modified Eagle’s Minimum Essential Medium (DMEM) (Gibco BRL, Life Technologies Pty Ltd, Australia). This process generated concentrations of 24.6 mg/mL and 7.08 mg/mL of AHE and ABW, respectively.
Total phenolic content (TPC) was measured by the reaction between gallic acid and Folin-Ciocalteu reagent and described as gallic acid equivalent (TPC-GAE). Samples were added in triplicate to non-sterile 96 well plates and incubated with 100 μL Folin-Ciocalteu reagent for 5 min. Sodium carbonate solution (300 μL, 20% w/v) was added and further incubated for 4 h in the dark. The calibration curve was prepared as follows: 20 μL of gallic acid ethanol solution at concentrations of 0.23 mmol/L, 0.46 mmol/L, 0.92 mmol/L, 1.84 mmol/L, 3.68 mmol/L, 7.35 mmol/L. The plate was read at 765 nm by a spectrometer (BioRad UV, NSW, Australia). Measurement of TPC in the extracts was performed using 20 μL of the extract in place of the gallic acid. Data were expressed as gallic acid equivalents (TPC-GAE).
Antioxidant capacity was measured by the reaction between L-ascorbic acid and 1,1 diphenyl-2-picrylhydrazyl (DPPH). Samples (0.1 mL) were added in triplicate to non-sterile 96 well plates and incubated with 1.5 mL of DPPH ethanol solution (25.36 mmol/L) for 30 min in the dark. The calibration curve was prepared as follows: 0.1 mL of L-ascorbic acid ethanol solution as concentrations of 110.9 μmol/L, 221.8 μmol/L, 443.5 μmol/L, 887 μmmol/L, 1774 μmol/L. The plate was read at 515 nm by a spectrometer (BioRad UV, NSW, Australia). Data were expressed as ascorbic acid equivalents (AC-AAE).
The animal protocol was designed to minimize pain or discomfort to the animals. Female DA/Arc rats (100-140 g, n = 80) were sourced from a barrier-maintained Specific-Pathogen Free production facility (Laboratory Animal Services, the University of Adelaide, Adelaide, SA, Australia). Following facility acclimatization they were housed individually in metabolic cages (Tecniplast, Exton, PA, United States). Room temperature was maintained at a constant 22 °C with a 12 h reversed light-dark cycle (lights off at 0800). Rats were provided ad libitum access to food (18% casein-based diet)[20] and water purified by reverse osmosis. This study was approved by the Animal Ethics Committee of the University of Adelaide and conducted in accordance with the provisions of the Australian Code for the Care and Use of Animals for Scientific Purposes[21].
Rats were stratified into 10 treatment groups (n = 8) on the basis of bodyweight and burrowing ability (see later) such that both covariates demonstrated similar mean values between groups. Treatments were: Phosphate-buffered saline (PBS) + saline injection, AHE 31.25 μg/mL + saline injection, AHE 1000 μg/mL + saline injection, ABW 31.25 μg/mL + saline injection, ABW 1000 μg/mL + saline injection, PBS + 5-fluorouracil (5-FU), AHE 31.25 μg/mL + 5-FU injection, AHE 1000 μg/mL + 5-FU injection, ABW 31.25 μg/mL + 5-FU injection, ABW 1000 μg/mL + 5-FU injection. The low and high dose extracts were referred to as (1) and (2) respectively. Dose selection was based on previous in vitro data (unpublished). Rats were acclimatized to metabolic cages on experimental days 0-2. On experimental days 3-11 rats were gavaged daily with 1 mL of PBS or almond extract dependent on group allocation. On day 9 rats were injected intraperitoneally with either saline or 5-FU (150 mg/kg, Mayne Pharma Pty Ltd. Mulgrave, Vic, Australia).
Daily measurements of bodyweight, food and water intake and urine and fecal output were performed. On day 12, rats were humanely killed by CO2 asphyxiation followed by cervical dislocation. Visceral organs were weighed. Lengths and weights of the small intestine, duodenum and colon were recorded. All lengths were measured un-stretched prior to emptying of content. Weights were subsequently measured after gastrointestinal content had been removed. Midsection samples (2 cm) of the gastrointestinal organs were collected into 10% buffered formalin for histological analysis. Further 4 cm samples were snap frozen in liquid nitrogen and stored at -80 °C for later biochemical analysis.
The burrowing apparatus was adapted from Andrews et al[22] 2012. Burrows were hollow plastic tubes (200 mm length × 90 mm diameter) sealed at one end with a plastic cap. The open end was raised 60 mm above the cage floor by the use of a plastic stand in order to minimize gravel loss when placed in the test cage. Each burrow was filled with 1 kg of gravel (7 mm pea-shingle decorative gravel; Tuscan Path, Hawthorn East, Victoria, Australia). The test cage was a standard open-top rat cage (590 mm × 385 mm × 200 mm; Tecniplast, Rydalmere, New South Wales, Australia) with the burrow placed to the rear of the cage. These cages were devoid of bedding substrate. In order to reduce the effects of cage novelty on propensity to perform burrowing behaviour, animals were always placed in the same cage for testing.
Rats were habituated to the test cages and burrowing apparatus in pairs on two consecutive days prior to experimental treatments (experimental days -2 and -1). To perform the test, rats were removed from their home cages and placed into the test cage for one hour. Following the test hour the remaining gravel in the burrow was weighed and the volume of gravel displaced calculated. Rats were assessed for level of baseline burrowing on experimental days 1 and 2. The mean of these two values was used to determine baseline burrowing level and to select animals into their treatment groups. Since, it had been previously shown that there was wide inter-individual variability in propensity to burrow[23], burrowing ability was categorised as good or poor using the method described by Deacon 2006[24]. Animals were then allocated into their treatment groups to include an equivalent number of good and poor burrowers in each group. Post-treatment burrowing was performed on experimental day 12. On test days burrowing was conducted between 1000 and 1200 h.
Small intestinal tissue samples (4 cm) of jejunum and ileum were thawed on ice and homogenized with 1.5 mL of phosphate buffer (10 mmol/L, pH 6.1) for 60 s until the solution was homogenous. Homogenates were stored at -80 °C until required for assay.
Level of myeloperoxidase (MPO) in the intestinal tissue was determined by a slight modification of the assay described by Krawisz et al[25]. Tissue homogenates were thawed on ice and centrifuged at 13000 g for 13 min. The supernatant was discarded and cell pellets were re-suspended in hexadecyltrimethyl ammonium bromide (0.5%, pH 6.0). Samples were then vortexed for 2 min and centrifuged at 13000 g for 3 min. Supernatants were reacted with o-dianisidine and absorbance measured at 450 nm at 1 min intervals for a period of 15 min using a microplate reader (Sunrise Microplate Reader, Tecan Austria GmbH, Grodig, Austria). Absorbance readings were used to calculate MPO activity using a pre-designed macro. Activity was expressed as units MPO per gram of tissue.
The collected tissue samples were formalin-fixed for 24 h and then transferred to 70% ethanol. Transverse tissue sections (4 μm) were embedded in paraffin wax, H&E stained and viewed using a light microscope. The histological disease severity score was assessed semi-quantitatively based on scoring of eight independent histological criteria using a slight modification to the method described by Howarth et al[26] 1996. The criteria scored were: villus blunting, crypt distortion, reduction in goblet cell number, dilation of lymphatics, thickening of the submucosa, thickening of the muscularis externa, enterocyte disruption and lymphocytic and polymorphonuclear cell infiltration. Each criterion was scored from zero (normal) to three (maximal damage) and expressed as a median score. Saline control rat intestinal tissue was used as a baseline reference to grade the criteria. Villus heights and crypt depths (40 villi and 40 crypts per section) were determined in the jejunal and ileal sections as described in Howarth et al[26]. All microscope-based analyses were performed in a blinded fashion using a light microscope (Nikon, ProgRes® CS, Tokyo, Japan) and image ProPlus software version 5.1 (Media Cybernetics, Silver Spring MD, United States).
Statistical analyses were conducted using PASW 21 (SPSS, Inc., Chicago, IL, United States) and Megastat Excel Add-In (version 10.2, McGraw-Hill Higher Education, New York, NY, United States). Data were tested for normality using the Shapiro-Wilk test. All parametric data including bodyweight, daily metabolic data, histological parameters, MPO and villus height and crypt depth, were compared using one-way analysis of variance (ANOVA) with a Tukey’s post-hoc test. Burrowing behavior was analyzed using a 3-way ANOVA with treatment, injection type and time as fixed factors, both adjusted and unadjusted for baseline burrowing values. The histological disease severity score was analyzed by a Kruskal-Wallis test with a Mann Whitney U-test to determine between-group significance. Significance was determined at P < 0.05.
The phenolic content of AHE and ABW as measured by the polyphenol gallic acid equivalent test was 1.06 g/L and 0.48 g/L Respectively. Anti-oxidant capacity measured by the L-ascorbic acid equivalent test yielded values of 0.27 mg/mL for AHE, and 0.25 mg/mL for ABW.
Administration of 5-FU significantly increased urine output and reduced bodyweight and food intake compared to rats that received a saline injection (Table 1). Despite the increase in urine output a concomitant increase in water intake was not observed except in the group receiving 5-FU + ABW1. The treatments provided no advantage in terms of normalizing these clinical parameters in the chemotherapy-treated groups.
| Sal+PBS | Sal+ AHE1 | Sal+ AHE2 | Sal+ ABW1 | Sal+ ABW2 | 5-FU+PBS | 5-FU+AHE1 | 5-FU+AHE2 | 5-FU+ ABW1 | 5-FU+ ABW2 | |
| Body weight change with respect to day 9(%) | 3.7 ± 0.6 | 4.0 ± 1.0 | 1.6 ± 0.9 | 2.3 ± 1.1 | 2.6 ± 0.9 | -7.2 ± 1.5b | -9.5 ± 1.9b | -7.3 ± 1.3b | -7.0 ± 1.1b | -7.2 ± 1.2b |
| Water intake (mL/kgbw) | 256 ± 49 | 247 ± 28 | 184 ± 18 | 236 ± 22 | 200± 28 | 275 ± 27 | 244 ± 18 | 296 ± 22 | 348 ± 38a | 284 ± 28 |
| Food intake (%bw) | 6.7 ± 0.5 | 6.9 ± 0.5 | 6.9 ± 0.4 | 6.7 ± 0.6 | 6.4 ± 0.5 | 5.1 ± 0.3b | 5.2 ± 0.4a | 5.4 ± 0.3a | 4.9 ± 0.3b | 4.8 ± 0.3b |
| Urine output (mL/kgbw) | 103 ± 11 | 99 ± 12 | 98 ± 8 | 103 ± 9 | 89 ± 6 | 175 ± 19b | 202 ± 20b | 180 ± 25b | 215 ± 19b | 164 ± 18b |
| Faeces output (g/kgbw) | 39 ± 9 | 31 ± 5 | 40 ± 10 | 34 ± 6 | 24 ± 3 | 31 ± 4 | 35 ± 5 | 29 ± 6 | 31 ± 4 | 28 ± 5 |
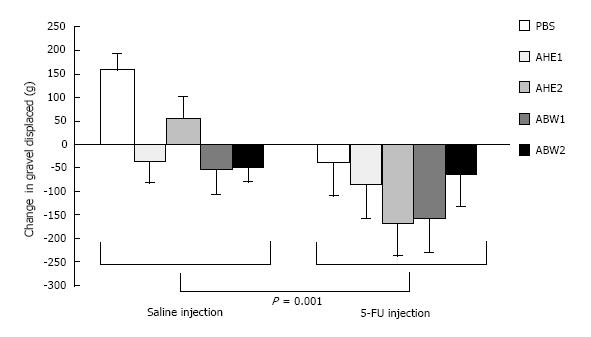
Simple main effects analysis demonstrated a change in mass of gravel displaced between baseline values and 72 h following chemotherapy injection between groups administered chemotherapy versus those receiving saline. Chemotherapy treated groups having a significantly greater, and more negative, change in mass of gravel displaced in comparison to all saline-treated groups [F (2, 68) = 7.514, P = 0.001; Figure 1]. However, there was no interaction between type of injection (5-FU or saline) and almond extract administered F (4, 68) = 1.41, P = 0.24.
Administration of 5-FU significantly reduced thymus weight by 50% (P < 0.001), spleen weight by 22% (P = 0.01) and increased caecum weight by 38% (P = 0.002) compared to saline-injected rats. Whilst almond extracts did not prevent the occurrence of these 5-FU induced changes, in general they failed to produce any negative effects on organ weights (Table 2). However, ABW2 caused an increase in liver weight in advance of that seen in chemotherapy-treated control animals by ABW2 (15%, P = 0.005).
| Sal+PBS | Sal+ AHE1 | Sal+ AHE2 | Sal+ABW1 | Sal+ ABW2 | 5-FU+PBS | 5-FU+AHE1 | 5-FU+AHE2 | 5-FU+ ABW1 | 5-FU+ ABW2 | 5-FU+AHE2 | |
| Thymus | 20 ± 1 | 21 ± 1 | 19 ± 1 | 20 ± 1 | 21 ± 1 | 10 ± 2b | 10 ± 3b | 8 ± 1b | 8 ± 1b | 10 ± 3b | 8 ± 1b |
| Heart | 40 ± 1 | 40 ± 1 | 42 ± 1 | 41 ± 1 | 42 ± 1 | 40 ± 2 | 43 ± 3 | 42 ± 1 | 40 ± 1 | 40 ± 3 | 42 ± 1 |
| Lung | 64 ± 3 | 74 ± 8 | 67 ± 3 | 69 ± 2 | 71 ± 4 | 77 ± 4 | 71 ± 6 | 72 ± 5 | 78 ± 1 | 77 ± 5 | 72 ± 5 |
| Liver | 374 ± 16 | 361 ± 13 | 372 ± 10 | 358 ± 11 | 380 ± 8 | 360 ± 14 | 383 ± 21 | 384 ± 9 | 380 ± 12 | 416 ± 16ad | 384 ± 9 |
| Stomach | 58 ± 4 | 62 ± 2 | 64 ± 2 | 52 ± 5 | 57 ± 4 | 58 ± 5 | 63 ± 5 | 60 ± 4 | 55 ± 5 | 52 ± 5 | 60 ± 4 |
| Caecum | 40 ± 2 | 35 ± 4 | 38 ± 2 | 37 ± 1 | 38 ± 2 | 55 ± 5b | 48 ± 4a | 46 ± 4a | 57 ± 2b | 51 ± 5a | 46 ± 4a |
| Spleen | 23 ± 1 | 27 ± 2 | 24 ± 1 | 26 ± 2 | 25 ± 2 | 18 ± 1a | 18 ± 1a | 17 ± 1b | 17 ± 1b | 18 ± 1a | 17 ± 1c |
| Left kidney | 41 ± 2 | 43 ± 2 | 42 ± 1 | 40 ± 1 | 40 ± 1 | 40 ± 1 | 43 ± 2 | 41 ± 1 | 42 ± 1 | 43 ± 1 | 41 ± 1 |
| Right kidney | 42 ± 2 | 44 ± 2 | 43 ± 1 | 41 ± 1 | 42 ± 1 | 44 ± 1 | 43 ± 1 | 44 ± 1 | 42 ± 1 | 43 ± 1 | 44 ± 1 |
| Adrenal glands | 3 ± 1 | 3 ± 1 | 3 ± 1 | 2 ± 1 | 3 ± 1 | 3 ± 1 | 8 ± 1 | 3 ± 5 | 7 ± 4 | 3 ± 1 | 3 ± 5 |
Similarly, 5-FU significantly reduced the length of the jejunum + ileum (5% reduction, P = 0.03) and colon (12% reduction, P = 0.02) (Table 3). A corresponding decrease in weight of the jejunum + ileum occurred (9% reduction, P = 0.02) although a similar weight reduction in the colon was not evidenced, and in fact the contrary occurred with a general increase in weight in 5-FU treated animals. This weight increase showed significance in the groups treated with 5-FU and AHE2, ABW1 and ABW2 (respective P values 0.03, 0.001 and 0.009). This increase in colon weight showed a trend towards significance (P = 0.06) in the 5-FU + PBS treated animals in comparison with saline control animals (Table 3).
| Sal +PBS | Sal + AHE1 | Sal + AHE2 | Sal + ABW1 | Sal + ABW2 | 5-FU +PBS | 5-FU +AHE1 | 5-U +AHE2 | 5-FU + ABW1 | 5-FU + ABW2 | |
| Duodenum | ||||||||||
| Weight (10-2) | 31 ± 1 | 31 ± 2 | 33 ± 3 | 31 ± 2 | 30 ± 1 | 31 ± 3 | 28 ± 1 | 31 ± 2 | 32 ± 1 | 30 ± 2 |
| Length | 6.2 ± 0.2 | 6.0 ± 0.2 | 6.1 ± 0.2 | 6.3 ± 0.1 | 6.1 ± 0.1 | 5.9 ± 0.3 | 5.9 ± 0.2 | 5.9 ± 0.1 | 5.7 ± 0.1 | 6.0 ± 0.3 |
| Jejunum + ileum | ||||||||||
| Weight (10-2) | 230 ± 5 | 226 ± 8 | 221 ± 6 | 222 ± 7 | 229 ± 7 | 210 ± 11a | 208 ± 10b | 203 ± 6b | 212 ± 11a | 209 ± 9a |
| Length | 75.1 ± 1.3 | 74.8 ± 1.4 | 75.8 ± 1.2 | 74.5 ± 0.9 | 75.4 ± 1.0 | 71.3 ± 0.9a | 70 ± 1.5b | 70.1 ± 1.9b | 71.5 ± 0.7a | 71.6 ± 1.0a |
| Colon | ||||||||||
| Weight | 58 ± 1 | 59 ± 2 | 58 ± 2 | 58 ± 2 | 60 ± 2 | 66 ± 2 | 64 ± 3 | 68 ± 4a | 73 ± 4b | 69 ± 4b |
| Length | 13.0 ± 0.6 | 13.1 ± 0.5 | 13.0 ± 0.4 | 12.8 ± 0.2 | 13.0 ± 0.2 | 11.5 ± 0.3a | 11.3 ± 0.6a | 11.5 ± 0.4a | 11.0 ± 0.4b | 10.9 ± 0.8b |
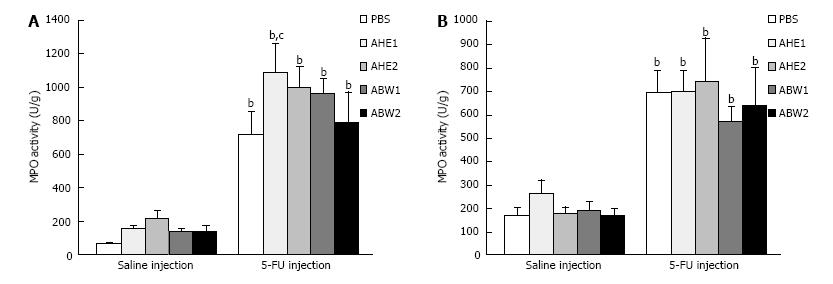
Injection of 5-FU caused a significant (P < 0.001) increase in MPO activity in the proximal jejunum and ileum of 5-FU + PBS treated rats (1048% and 409% respectively) compared to saline + PBS control animals (Figure 2).
In the jejunum AHE1 caused a statistically significant elevation in MPO activity in comparison with chemotherapy-treated control animals (50%, P = 0.02). This effect was not observed in the ileum. All other almond extracts had no ameliorating effect on 5-FU induced MPO activity, but did not worsen the effect. There was no significant difference in MPO activity between almond extract-treated animals and PBS-treated animals determining that almond extracts caused no adverse effects on MPO activity in healthy animals.
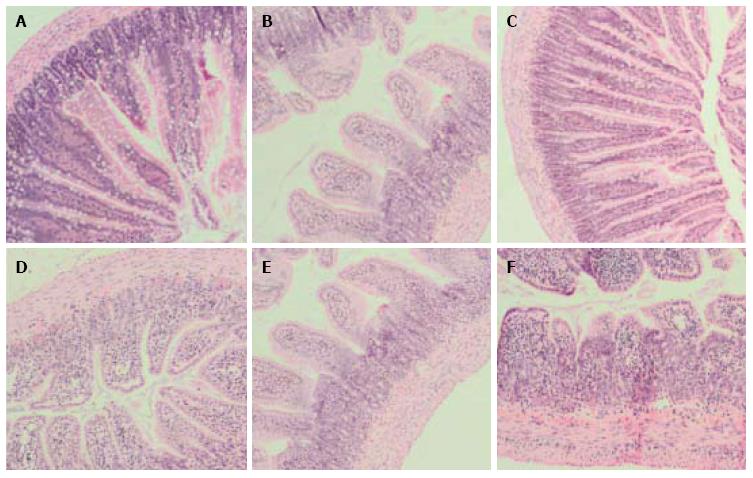
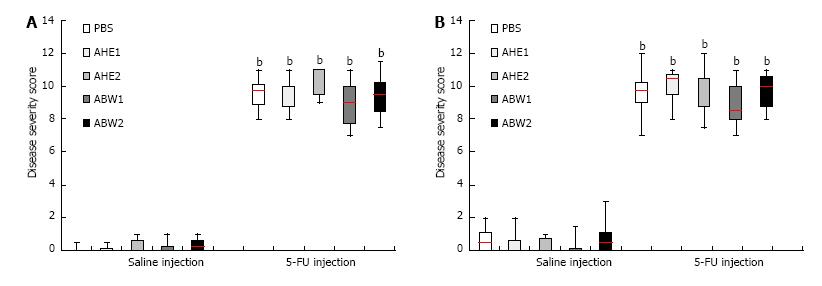
Mucositis caused characteristic histological changes in the mucosal architecture of the jejunum and ileum (Figure 3). Chemotherapy administration significantly increased disease severity score in the proximal jejunum and ileum in all treated animals (Figure 4) in comparison with PBS- treated saline control animals (P < 0.001 for all). Almond extract treatments failed to normalize histological severity score (Figure 3). Presence of mucositis with or without almond extracts led to increases in all individual severity scoring parameters (Table 4). Scores for these parameters were not significantly different between almond extract-treated and 5-FU control groups.
| Villus blunting | Crypt distortion | Reduction in goblet cell numbers | Dilation of lymphatics | Thickening of submucosa | Thickening of muscularis externa | Enterocyte disruption | Lymphocytic and PMN infiltration | ||
| Sal + PBS | J | 0 | 0 | 0 | 0 | 0.06 ± 0.06 | 0 | 0 | 0 |
| I | 0.19 ± 0.12 | 0 | 0 | 0 | 0.31 ± 0.15 | 0.19 ± 0.12 | 0 | 0 | |
| Sal + AHE1 | J | 0 | 0 | 0 | 0 | 0.13 ± 0.08 | 0 | 0 | 0 |
| I | 0 | 0 | 0 | 0 | 0.31 ± 0.15 | 0.13 ± 0.12 | 0 | 0 | |
| Sal + AHE2 | J | 0 | 0 | 0 | 0 | 0.21 ± 0.14 | 0 | 0 | 0 |
| I | 0.07 ± 0.07 | 0 | 0.14 ± 0.13 | 0 | 0.07 ± 0.07 | 0.07 ± 0.07 | 0 | 0 | |
| Sal + ABW1 | J | 0.13 ± 0.12 | 0 | 0 | 0 | 0.13 ± 0.12 | 0 | 0 | 0 |
| I | 0 | 0.06 ± 0.06 | 0 | 0 | 0.13 ± 0.12 | 0.06 ± 0.06 | 0 | 0 | |
| Sal + ABW2 | J | 0 | 0 | 0 | 0 | 0.25 ± 0.13 | 0.13 ± 0.08 | 0 | 0 |
| I | 0.13 ± 0.12 | 0 | 0.12 ± 0.12 | 0 | 0.38 ± 0.15 | 0.19 ± 0.12 | 0 | 0 | |
| 5-FU +PBS | J | 1.63 ± 0.15b | 1.38 ± 0.17b | 1.81 ± 0.12b | 1 ± 0b | 0.88 ± 0.12b | 0.88 ± 0.12b | 1 ± 0b | 1 ± 0b |
| I | 1.56 ± 0.24b | 1.62 ± 0.17b | 1.89 ± 0.12b | 1 ± 0b | 0.88 ± 0.21b | 0.63 ± 0.17a | 1 ± 0.18b | 1 ± 0b | |
| 5-FU +AHE1 | J | 1.71 ± 0.17b | 1.43 ± 0.19b | 1.86 ± 0.13b | 1 ± 0b | 0.79 ± 0.14b | 0.71 ± 0.14b | 1 ± 0b | 1 ± 0b |
| I | 1.64 ± 0.17b | 1.57 ± 0.21b | 1.79 ± 0.2b | 1 ± 0b | 1 ± 0b | 1 ± 0b | 1 ± 0b | 1 ± 0b | |
| 5-FU +AHE2 | J | 1.79 ± 0.14b | 1.71 ± 0.17b | 2 ± 0b | 0.93 ± 0.07b | 1 ± 0b | 0.93 ± 0.07b | 1 ± 0b | 1 ± 0b |
| I | 1.86 ± 0.13b | 1.57 ± 0.19b | 1.93 ± 0.07b | 1 ± 0b | 0.71 ± 0.2b | 0.64 ± 0.22a | 1 ± 0b | 1 ± 0b | |
| 5-FU + ABW1 | J | 1.44 ± 0.16b | 1.25 ± 0.15b | 1.63 ± 0.17b | 1 ± 0b | 0.94 ± 0.19b | 0.88 ± 0.19b | 0.88 ± 0.12b | 0.88 ± 0.12a |
| I | 1.25 ± 0.15b | 1.25 ± 0.15b | 1.63 ± 0.17b | 1 ± 0b | 0.88 ± 0.12b | 0.88 ± 0.12b | 1 ± 0b | 1 ± 0b | |
| 5-FU + ABW2 | J | 1.75 ± 0.13b | 1.19 ± 0.12b | 1.94 ± 0.06b | 1 ± 0b | 0.88 ± 0.15b | 0.63 ± 0.17b | 1.13 ± 0.12b | 1 ± 0b |
| I | 1.75 ± 0.15b | 1.56 ± 0.16b | 1.75 ± 0.15b | 1 ± 0b | 0.94 ± 0.06b | 0.69 ± 0.15a | 1 ± 0b | 1 ± 0b |
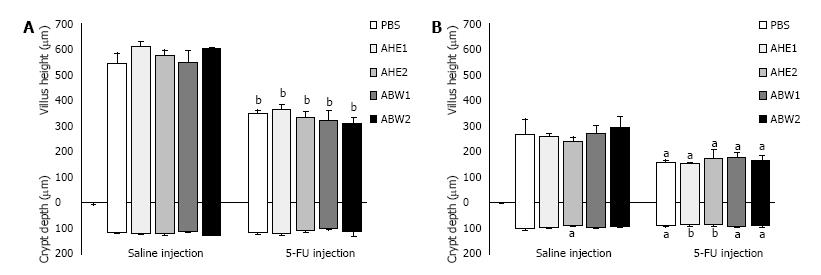
5-FU injection caused a shortening of the villi in the jejunum (34%, P < 0.001) and ileum (40%, P = 0.01) compared to saline controls (Figure 5). However, there was no general reduction in crypt depth in the jejunum although this was noted in the ileum. Saline + AHE2 also caused a reduction in ileal crypt depth in healthy animals in comparison with saline injected control animals (15%, P = 0.02). However, AHE2 caused neither beneficial nor adverse effect on ileal crypt depth when administered with 5-FU.
The present study describes the first investigation into the effect of selected almond production by-products on chemotherapy-induced mucositis. Whilst the data do not identify any beneficial effects of the extracts at the doses selected, they do confirm the safety of the agents in an in-vivo model. This is an important finding given the controversy surrounding the use of bitter almonds as chemotherapeutic agents identified in a recent Cochrane review[17], and the common confusion between the properties of bitter and sweet almonds. Importantly, investigations conducted as part of this work have identified burrowing behaviour as a promising method of determination of animal affective state. To our knowledge this is the first example of a measure of affective state being successfully used in an animal model of chemotherapy-induced toxicity.
The chemotherapeutic agent 5-FU typically causes greater damage to the small intestine than the more distal regions of the gut. This is presumably due to the increased cell-turnover rate which is a characteristic feature of the small intestine[3]. The main histological findings of villus shortening and crypt disruption caused by 5-FU administration were typical, and in concordance with other studies[27-29].
The presence of crypt lengthening in response to chemotherapeutic agents has been an inconsistent finding in animal studies. A number of studies have reported a decrease in crypt depth in both jejunum and ileum following 5-FU injection[19,30,31], yet another failed to demonstrate any change in crypt depth[32]. Conversely, an increase in crypt length following 5-FU administration has been demonstrated in rodents[28]. Knowledge of the pathogenesis of mucositis suggests that crypt shortening is the most likely response to chemotherapy agents; due to crypt hypoplasia as a result of clonogenic cell death, or numerous signalling events[33]. In the current study, crypt shortening was observed consistently in the ileum but not in the jejunum. Nevertheless, there was clear crypt cell disruption and a reduced number of cells on histological assessment of jejunal tissue. This apparent inconsistency supports the proposal of Mashtoub et al 2013 for studies to evaluate crypt proliferation and apoptosis using techniques such as the proliferative cell nuclear antigen, and TUNEL assay[30]. Whilst in the current study, almond extracts had no beneficial effect on histological parameters, the study time-course was short. Gut damage is typically maximal in rats 3 d after 5-FU administration with regeneration and repair ensuing[30]. Bioactive factors may not prevent this early gut damage but still be beneficial by aiding healing, for example by preserving crypt stem cell microcolonies. Evaluation of such a protective effect would need a longer study and specific evaluation of crypt cell numbers and organisation.
At a biochemical level myeloperoxidase activity can chart the inflammatory process occurring in the chemotherapy-damaged gut. Myeloperoxidase is the most common proinflammatory enzyme and is stored in the azurophilic granules of neutrophilic granulocytes[34]. Therefore, measurement of the enzyme levels provides a reliable marker of neutrophil infiltration and hence acute inflammation[25]. Given the intestinal damage caused by chemotherapeutic agents an increase in MPO levels as a result of concomitant inflammation would be expected. Indeed a large number of animal-based investigations into mucositis support this hypothesis[19,35-37]. However, Keefe et al[38] 2000 suggest that the role of inflammation in mucositis progression is far from established.
Nevertheless, in the current study, an increase in MPO activity was established in both the jejunum and ileum in the 5-FU treated groups implying that inflammation was present. The administered almond extracts failed to moderate this activity and AHE1 actually elevated MPO activity in comparison with chemotherapy controls. This implies an immune-stimulatory effect of AHE 1. One mechanism of this pro-inflammatory activity could be the presence of omega-6 polyunsaturated fatty acid (ω-6 PUFA), linoleic acid, at greater levels in this extract[39]. Indeed, sunflower oil with a highly imbalanced ω-6 PUFA to ω-3 PUFA ratio resulted in a pro-inflammatory response in mice[40]. Furthermore, in previous studies a greater intake of ω-6 PUFA increased cytokine-induced inflammation via Prostaglandin E2 production[41]. However, given that in the current study a similar elevation was not observed in the ileum, and that the histological data did not demonstrate any detrimental effects of AHE1 on mucosal structure, this finding is unlikely to be clinically significant. The pro-inflammatory nature of almond hull extracts should however be evaluated further if future investigations into this product are performed, especially since AHE2 also caused a reduction in crypt depth in healthy animals.
Patient symptoms arising as a result of the barrage of pathophysiological changes occurring in the alimentary tract in mucositis typically include reduced food consumption, and hence body weight decrease[29,31]. Clinical features in the current study were classic although we observed increased urine output in the absence of polydipsia. This has not been previously reported in mucositis studies. This effect was common to all chemotherapy-treated animals and was not therefore linked to extract treatment. This observation could have resulted from a generalised stress response of psychogenic origin, or as a result of kidney changes due to drug metabolism[42]. However, there was considerable inter-individual variation in fluid intake and urine output, and recording of these parameters may have been subject to error due to excessive leakage of bottles or contaminants in the urine collection chambers. Furthermore, there were no generalised increases in liver or kidney weights to suggest organ pathology except in the group receiving ABW2 which demonstrated an increase in liver weight. However, it would be valuable to perform confirmatory serum biochemistry tests in future studies.
Burrowing activity has been described as a “luxury” behavior since it is non-essential and therefore provides an indication of positive well-being[43]. This category of behaviors is usually the first to be reduced in the event of environmental challenge[44]. Furthermore, the results from a variety of studies have concluded that such complex behaviors may mirror “activities of daily living (ADL)” in humans. Such activities, which include eating, bathing and dressing[45], are often negatively affected by an array of clinical conditions[46,47]. Previous literature using the rat model has demonstrated that burrowing activity or propensity to perform the behavior is reduced in painful conditions[48,49] and as a result of sickness behavior due to cytokine release[50]; both conditions in existence in mucositis. Few studies have evaluated burrowing behavior in gastro-intestinal disease models although Jirkof et al[51] 2013 successfully demonstrated a decrease in burrowing behavior in a mouse colitis model. The finding of a change in propensity to burrow following 5-FU injection in the current study is significant since it identifies burrowing behavior as a possible tool to evaluate rodent affective state. This is of particular importance in this disease model since mucositis is a self-limiting condition, and the key treatment outcome is to improve patient well-being. Measurement of burrowing may therefore provide a simple method of improving the translational validity of this animal model by providing an objective way to assess the subjective state of how the patient “feels”. Therapeutic success would then be evidenced by normalization of burrowing activity to baseline levels.
The current study refined the practical technique for burrowing behavior assessment utilized in Whittaker et al[23] 2015. In that study, burrowing behavior both before and after administration of chemotherapy was determined to have an inherently large inter and intra-individual variability. This impacted on interpretation of the results. It was also clear that there was a learning phase to the behavior such that propensity to burrow increased with experience. To counteract these issues the current study introduced a training phase where the technique of social facilitation was used[52]; pairs of rats were introduced to the test cage on a number of occasions prior to recording of burrowing values. Furthermore, animals were selected into their treatment groups to create similar baseline burrowing group means as described by Deacon 2006[24]. Future studies should build on the results from the current study by incorporation of a positive control group utilizing a drug which provides known symptomatic relief against mucositis.
Interest in almond extracts as potential therapeutic candidates for mucositis is primarily based on their high anti-anti-oxidant status[53], since other potent anti-oxidants such as grapeseed extract have reduced intestinal damage in animal models of mucositis[19,54]. Additionally, almond consumption has been associated with lower levels of C-reactive protein, interleukin-6 and E-selectin which represent biomarkers and an adhesion molecule involved in the inflammatory response[53]. These data imply an inflammatory action of almonds which would be expected to reduce the severity of mucositis symptoms. However, in the current study despite the extracts showing moderate anti-oxidant activity as determined by the L-ascorbic acid equivalent test[55], they failed to evoke measurable effects on the outcomes evaluated. It is possible that the dosages evaluated were too low to cause an affect or that bioactive components are degraded in an in-vivo model system. Alternatively, the oral gavage administration method for the extracts may have reduced their bioavailability in comparison to dietary exposure. Dietary exposure allows the chemical to interact with the mucosal surfaces in the oral cavity. Chemicals absorbed via this route avoid a first-pass metabolism by the gut wall and the liver. This avoidance of first-pass metabolism leads to a comparatively higher bioavailability[56]. Furthermore, oral gavage dosing-induced stress can lead to increased heart rate, blood pressure and activation of the hypothalamic-pituitary axis. These changes have the potential to confound results. However, it has been demonstrated that these stress effects are generally short-lived or inconsistent in rats in comparison to mice; with blood pressure and heart rate generally returning to normal an hour after gavage[57], and corticosterone rises being inconsistently seen[58,59]. Stress-induced effects were therefore unlikely to have caused a significant confounding effect on the results of this study, but bioavailability may have been influenced by the administration method. Future studies could identify methods to protect bioactive components of almond extracts from degradation, for instance microencapsulation, or could investigate use of different administration routes which are not subject to first-pass metabolism.
In conclusion, this study provides the first investigation into the use of by-products of the almond production process as a potential therapeutic for chemotherapy-induced mucositis. No definitive evidence was found for the extracts producing a beneficial effect at the dosages employed. Importantly, this study is the first to identify a measure of affective state in an animal model of mucositis. This finding has the potential to improve assessment of therapeutic effect in future animal studies of mucositis.
As cancer incidence rises and with it the incidence of chemotherapy-induced toxicity, the need to identify novel treatment approaches for chemotherapy-induced mucositis grows. There has been mounting interest in the use of plant-based nutraceuticals as therapeutics in cancer and to relive symptoms resulting from chemotherapy treatment. Based on pre-clinical literature demonstrating anti-cancer properties of fruits from the Prunus genus, and the moderate procyanidin content of almonds, almond extract was investigated to determine whether it reduced mucositis severity in rats. Furthermore, they employed burrowing as a novel behavioural measure of affective state in order to validate this technique in a chemotherapy-induced mucositis model.
To the knowledge, this is the first study to successfully employ a behavioural measure of affective state (as opposed to pain alone) in an animal model of intestinal mucositis. This has potential to improve the translational validity of animal models used in this area of research.
This is the first study to successfully use a behavioural measure of affective state, in a chemotherapy-induced mucositis model.
The finding that burrowing behaviour was decreased in animals with mucositis provides researchers using this animal model with a simple method of identifying affective state which may be replicated in the human patient. This is important given the self-limiting nature of the mucositis condition where the primary therapeutic goal is reduction of pain and discomfort, and improvement of quality of life. To date, animal studies of mucositis have failed to measure this primary outcome. Future studies should build on the results from the current study by incorporation of a positive control group utilizing a drug which provides known symptomatic relief against mucositis.
5-fluorouracil is a widely utilised chemotherapy drug used to treat a number of cancer forms including colo-rectal cancer, breast cancer and cervical cancer amongst others. It may be administered alone or in combination with other chemotherapy drugs, such as methotrexate.
This interesting study shows that the severity of mucositis caused by 5-fluorouracil in rats was not ameliorated oral intake of almond extracts that contain phenolics and have anti-oxidant activity. A second aspect of the work is the use of burrowing behaviour by the rats as a monitor of animal well-being and severity of mucositis.
Grateful thanks are extended to Ms K. Lymn for her assistance with the animal study.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Australia
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Correa L, Grant G S- Editor: Qi Y L- Editor: A E- Editor: Lu YJ
| 1. | Sonis ST, Eilers JP, Epstein JB, LeVeque FG, Liggett WH, Mulagha MT, Peterson DE, Rose AH, Schubert MM, Spijkervet FK. Validation of a new scoring system for the assessment of clinical trial research of oral mucositis induced by radiation or chemotherapy. Mucositis Study Group. Cancer. 1999;85:2103-2113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 2. | Clarke JM, Pelton NC, Bajka BH, Howarth GS, Read LC, Butler RN. Use of the 13C-sucrose breath test to assess chemotherapy-induced small intestinal mucositis in the rat. Cancer Biol Ther. 2006;5:34-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Duncan M, Grant G. Oral and intestinal mucositis - causes and possible treatments. Aliment Pharmacol Ther. 2003;18:853-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 168] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 4. | Diddens H, Teufel T, Niethammer D. High-dose methotrexate therapy with leucovorin rescue: in vitro investigations on human osteosarcoma cell lines. Cancer Chemother Pharmacol. 1987;20:128-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 5. | Avritscher EB, Cooksley CD, Elting LS. Scope and epidemiology of cancer therapy-induced oral and gastrointestinal mucositis. Semin Oncol Nurs. 2004;20:3-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | Pico JL, Avila-Garavito A, Naccache P. Mucositis: Its Occurrence, Consequences, and Treatment in the Oncology Setting. Oncologist. 1998;3:446-451. [PubMed] |
| 7. | Keefe DM, Sonis ST, Bowen JM. Emerging drugs for chemotherapy-induced mucositis. Expert Opin Emerg Drugs. 2008;13:511-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Vizzotto M, Porter W, Byrne D, Cisneros-Zevallos L. Polyphenols of selected peach and plum genotypes reduce cell viability and inhibit proliferation of breast cancer cells while not affecting normal cells. Food Chem. 2014;164:363-370. [RCA] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 9. | Olsson ME, Gustavsson KE, Andersson S, Nilsson A, Duan RD. Inhibition of cancer cell proliferation in vitro by fruit and berry extracts and correlations with antioxidant levels. J Agric Food Chem. 2004;52:7264-7271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 228] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 10. | Ramos S, Alía M, Bravo L, Goya L. Comparative effects of food-derived polyphenols on the viability and apoptosis of a human hepatoma cell line (HepG2). J Agric Food Chem. 2005;53:1271-1280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 97] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 11. | Fujii T, Ikami T, Xu JW, Ikeda K. Prune extract (Prunus domestica L.) suppresses the proliferation and induces the apoptosis of human colon carcinoma Caco-2. J Nutr Sci Vitaminol (Tokyo). 2006;52:389-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Alasalvar C, Shahidi F. Tree Nuts: Composition, Phytochemicals, and Health Effects. Boca Raton, Fl, USA: CRC Press 2009; . |
| 13. | Soriano-Hernandez AD, Madrigal-Perez DG, Galvan-Salazar HR, Arreola-Cruz A, Briseño-Gomez L, Guzmán-Esquivel J, Dobrovinskaya O, Lara-Esqueda A, Rodríguez-Sanchez IP, Baltazar-Rodriguez LM. The protective effect of peanut, walnut, and almond consumption on the development of breast cancer. Gynecol Obstet Invest. 2015;80:89-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Rajaram S, Connell KM, Sabaté J. Effect of almond-enriched high-monounsaturated fat diet on selected markers of inflammation: a randomised, controlled, crossover study. Br J Nutr. 2010;103:907-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 97] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 15. | Yada S, Lapsley K, Huang G. A review of composition studies of cultivated almonds: Macronutrients and micronutrients. J Food Compos Anal. 2011;24:469-480. [RCA] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 132] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 16. | Wirthensohn MG, Chin WL, Franks TK, Baldock G, Ford CM, Sedgley M. Characterising the flavour phenotypes of almond (Prunus dulcis Mill.) kernels. J Hortic Sci Biotech. 2008;83:462-468. [DOI] [Full Text] |
| 17. | Milazzo S, Horneber M. Laetrile treatment for cancer. Cochrane Database Syst Rev. 2015;CD005476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Milbury PE, Chen CY, Dolnikowski GG, Blumberg JB. Determination of flavonoids and phenolics and their distribution in almonds. J Agric Food Chem. 2006;54:5027-5033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 182] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 19. | Cheah KY, Howarth GS, Bastian SE. Grape seed extract dose-responsively decreases disease severity in a rat model of mucositis; concomitantly enhancing chemotherapeutic effectiveness in colon cancer cells. PLoS One. 2014;9:e85184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 20. | Thomas DJ, Caffrey TC. Lipopolysaccharide induces double-stranded DNA fragmentation in mouse thymus: protective effect of zinc pretreatment. Toxicology. 1991;68:327-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | National Health and Medical Research Council. Australian code for the care and use of animals for scientific purposes. Canberra: Australian Government 2013; . |
| 22. | Andrews N, Legg E, Lisak D, Issop Y, Richardson D, Harper S, Pheby T, Huang W, Burgess G, Machin I. Spontaneous burrowing behaviour in the rat is reduced by peripheral nerve injury or inflammation associated pain. Eur J Pain. 2012;16:485-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 134] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 23. | Whittaker AL, Lymn KA, Nicholson A, Howarth GS. The assessment of general well-being using spontaneous burrowing behaviour in a short-term model of chemotherapy-induced mucositis in the rat. Lab Anim. 2015;49:30-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Deacon RM. Burrowing in rodents: a sensitive method for detecting behavioral dysfunction. Nat Protoc. 2006;1:118-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 224] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 25. | Krawisz JE, Sharon P, Stenson WF. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology. 1984;87:1344-1350. [PubMed] |
| 26. | Howarth GS, Francis GL, Cool JC, Xu X, Byard RW, Read LC. Milk growth factors enriched from cheese whey ameliorate intestinal damage by methotrexate when administered orally to rats. J Nutr. 1996;126:2519-2530. [PubMed] |
| 27. | Leocádio PC, Antunes MM, Teixeira LG, Leonel AJ, Alvarez-Leite JI, Machado DC, Generoso SV, Cardoso VN, Correia MI. L-arginine pretreatment reduces intestinal mucositis as induced by 5-FU in mice. Nutr Cancer. 2015;67:486-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 28. | Yeung CY, Chan WT, Jiang CB, Cheng ML, Liu CY, Chang SW, Chiang Chiau JS, Lee HC. Amelioration of Chemotherapy-Induced Intestinal Mucositis by Orally Administered Probiotics in a Mouse Model. PLoS One. 2015;10:e0138746. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 108] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 29. | Mashtoub S, Feo B, Whittaker AL, Lymn KA, Martinez-Puig D, Howarth GS. Oral Nucleotides Only Minimally Improve 5-Fluorouracil-Induced Mucositis in Rats. Nutr Cancer. 2015;67:994-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Mashtoub S, Tran CD, Howarth GS. Emu oil expedites small intestinal repair following 5-fluorouracil-induced mucositis in rats. Exp Biol Med (Maywood). 2013;238:1305-1317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 31. | Reinke D, Kritas S, Polychronopoulos P, Skaltsounis AL, Aligiannis N, Tran CD. Herbal substance, acteoside, alleviates intestinal mucositis in mice. Gastroenterol Res Pract. 2015;2015:327872. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 32. | Wang H, Brook CL, Whittaker AL, Lawrence A, Yazbeck R, Howarth GS. Effects of Streptococcus thermophilus TH-4 in a rat model of doxorubicin-induced mucositis. Scand J Gastroenterol. 2013;48:959-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 33. | Al-Dasooqi N, Gibson RJ, Bowen JM, Keefe DM. Matrix metalloproteinases: key regulators in the pathogenesis of chemotherapy-induced mucositis? Cancer Chemother Pharmacol. 2009;64:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 34. | Schultz J, Kaminker K. Myeloperoxidase of the leucocyte of normal human blood. I. Content and localization. Arch Biochem Biophys. 1962;96:465-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 310] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 35. | Tooley KL, Howarth GS, Lymn KA, Lawrence A, Butler RN. Oral ingestion of streptococcus thermophilus diminishes severity of small intestinal mucositis in methotrexate treated rats. Cancer Biol Ther. 2006;5:593-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 54] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 36. | Mauger CA, Butler RN, Geier MS, Tooley KL, Howarth GS. Probiotic effects on 5-fluorouracil-induced mucositis assessed by the sucrose breath test in rats. Dig Dis Sci. 2007;52:612-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 37. | Miyamoto H, Kanayama T, Horii K, Kawai T, Tsuchimochi T, Shigetomi T, Shibamoto Y, Shibuya Y. The relationship between the severity of radiation-induced oral mucositis and the myeloperoxidase levels in rats. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;120:329-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 38. | Keefe DM, Brealey J, Goland GJ, Cummins AG. Chemotherapy for cancer causes apoptosis that precedes hypoplasia in crypts of the small intestine in humans. Gut. 2000;47:632-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 280] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 39. | Maguire LS, O’Sullivan SM, Galvin K, O’Connor TP, O’Brien NM. Fatty acid profile, tocopherol, squalene and phytosterol content of walnuts, almonds, peanuts, hazelnuts and the macadamia nut. Int J Food Sci Nutr. 2004;55:171-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 329] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 40. | Masi LN, Martins AR, Rosa Neto JC, do Amaral CL, Crisma AR, Vinolo MA, de Lima Júnior EA, Hirabara SM, Curi R. Sunflower oil supplementation has proinflammatory effects and does not reverse insulin resistance in obesity induced by high-fat diet in C57BL/6 mice. J Biomed Biotechnol. 2012;2012:945131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 41. | Kuehl FA, Egan RW. Prostaglandins, arachidonic acid, and inflammation. Science. 1980;210:978-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 538] [Cited by in RCA: 504] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 42. | Sodikoff CH. Laboratory Profiles of Small Animal Disease- A guide to laboratory diagnosis. St Louis: Mosby 1995; . |
| 43. | Jirkof P. Burrowing and nest building behavior as indicators of well-being in mice. J Neurosci Methods. 2014;234:139-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 302] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 44. | Boissy A, Manteuffel G, Jensen MB, Moe RO, Spruijt B, Keeling LJ, Winckler C, Forkman B, Dimitrov I, Langbein J. Assessment of positive emotions in animals to improve their welfare. Physiol Behav. 2007;92:375-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 758] [Cited by in RCA: 842] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 45. | Lindahl-Jacobsen L, Hansen DG, Wæhrens EE, la Cour K, Søndergaard J. Performance of activities of daily living among hospitalized cancer patients. Scand J Occup Ther. 2015;22:137-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 46. | Lau W, Dykstra C, Thevarkunnel S, Silenieks LB, de Lannoy IA, Lee DK, Higgins GA. A back translation of pregabalin and carbamazepine against evoked and non-evoked endpoints in the rat spared nerve injury model of neuropathic pain. Neuropharmacology. 2013;73:204-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 47. | Torres-Lista V, Giménez-Llort L. Impairment of nesting behaviour in 3xTg-AD mice. Behav Brain Res. 2013;247:153-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 48. | Jirkof P, Cesarovic N, Rettich A, Nicholls F, Seifert B, Arras M. Burrowing behavior as an indicator of post-laparotomy pain in mice. Front Behav Neurosci. 2010;4:165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 123] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 49. | Goebel A, Stock M, Deacon R, Sprotte G, Vincent A. Intravenous immunoglobulin response and evidence for pathogenic antibodies in a case of complex regional pain syndrome 1. Ann Neurol. 2005;57:463-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 50. | Teeling JL, Felton LM, Deacon RM, Cunningham C, Rawlins JN, Perry VH. Sub-pyrogenic systemic inflammation impacts on brain and behavior, independent of cytokines. Brain Behav Immun. 2007;21:836-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 121] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 51. | Jirkof P, Leucht K, Cesarovic N, Caj M, Nicholls F, Rogler G, Arras M, Hausmann M. Burrowing is a sensitive behavioural assay for monitoring general wellbeing during dextran sulfate sodium colitis in laboratory mice. Lab Anim. 2013;47:274-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 52. | McLinden KA, Kranjac D, Deodati LE, Kahn M, Chumley MJ, Boehm GW. Age exacerbates sickness behavior following exposure to a viral mimetic. Physiol Behav. 2012;105:1219-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 53. | Kamil A, Chen CY. Health benefits of almonds beyond cholesterol reduction. J Agric Food Chem. 2012;60:6694-6702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 54. | Cheah KY, Howarth GS, Yazbeck R, Wright TH, Whitford EJ, Payne C, Butler RN, Bastian SE. Grape seed extract protects IEC-6 cells from chemotherapy-induced cytotoxicity and improves parameters of small intestinal mucositis in rats with experimentally-induced mucositis. Cancer Biol Ther. 2009;8:382-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 55. | Kim DO, Lee CY. Comprehensive study on vitamin C equivalent antioxidant capacity (VCEAC) of various polyphenolics in scavenging a free radical and its structural relationship. Crit Rev Food Sci Nutr. 2004;44:253-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 250] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 56. | Vandenberg LN, Welshons WV, vom Saal FS, Toutain P, Myers JP. Should oral gavage be abandoned in toxicity testing of endocrine disruptors? Environ Health. 2014;13:46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 107] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 57. | Bonnichsen M, Dragsted N, Hansen AK. The welfare impact of gavaging laboratory rats. Anim Welf. 2005;14:223-227. |
| 58. | Brown AP, Dinger N, Levine BS. Stress produced by gavage administration in the rat. Contemp Top Lab Anim Sci. 2000;39:17-21. [PubMed] |
| 59. | Turner PV, Vaughn E, Sunohara-Neilson J, Ovari J, Leri F. Oral gavage in rats: animal welfare evaluation. J Am Assoc Lab Anim Sci. 2012;51:25-30. [PubMed] |