INTRODUCTION
The human small intestine is a multi-layered organ with complex cellularity. The intestinal mucosa is characterised by villus forming connective tissues with highly specialized surface lining epithelial cells. The epithelium is highly dynamic and interacts with the underlying mesenchyme and the luminal content. In order for these diverse functions to be performed, spatially distinct compartments of epithelial differentiation are found along the crypt-villus axis (CVA). Stem cells located in the crypts are responsible for the high throughput of surface lining cells within a few days. Histomorphologically, the CVA is a very characteristic mucosal feature and fundamental in explaining the structure and function of the surface lining epithelia. At present, four important cell types are defined, namely absorptive (enterocytes), mucosecreting (goblet cells), enteroendocrine, and Paneth cells[1]. Some other epithelial and non-epithelial cell types are found at the mucosal surface, including M-cells, brush/tuft/caveolated cells, and several types of intraepithelial lymphocytes.
Enterocytes are most common in the surface epithelium and are responsible for digestion and absorption of nutrients as well as in forming the intestinal border. They are assisted by mucosecreting/goblet cells which producing several mucine types which are chemically different along the intestinal tract. Goblet cells increase in number from oral to aboral as the stool becomes increasingly compacted. Enteroendocrine cells represent the third important cell type and comprise a highly specialised chemosensory system involved in sensing the energy balance and reserve of an individual. A detailed classification of enteroendocrine subtypes is possible by the specific intestinal hormones that are produced and the assistance of Notch signalling in enteroendocrine cell differentiation[2,3].
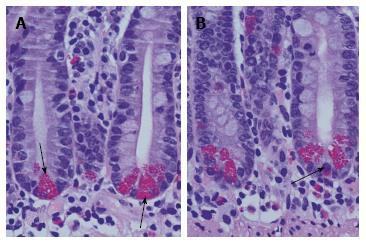
Paneth cells, first described by Gustav Albert Schwalbe in 1872, originate directly from intestinal stem cells[4]. In contrast to other secretory cell types, Paneth cells are physiologically found at the bottom positions of small intestinal crypts (Figure 1). The unique histomorphological feature implicates special functions of Paneth cells in cellular homeostasis as well as in the establishment and configuration of the mucosal barrier as a physical and highly organised immune interface. Recently, some molecular mechanisms underlying secretory disorders of Paneth cells were identified strongly related to intestinal diseases, e.g., ileal Crohn’s disease and necrotising enterocolitis. The important etiopathological findings were addressed and summarised with the term “Paneth’s disease” and introduced in the literature[4].
Figure 1 Normal small intestinal crypts with basal orientated Paneth cells.
A: Paneth cells are characterised by their apical located granules (arrows). Between Paneth cells undifferentiated progenitor cells are found. In the normal Lamina propria mucosae a mixed population of immune cells and stroma resident cells is found; B: Occasionally, Paneth cells at the bottom of small intestinal crypts are mixed up with enteroendocrine cells (arrow). They are characterised by basal located granules. In the upper part of the crypt, a mitotic figure is shown.
In the following paragraphs, important aspects of Paneth cell physiology and pathophysiology are reviewed. The data clearly demonstrate that Paneth cells are a highly specialized cell type strongly involved in assisting to sharpen and maintain of the microbiome as well as in the establishment of the stem cell niche and promotion of cellular renewal and mucosal morphogenesis. Consequently, Paneth cell disorders are involved in the pathophysiology of intestinal diseases.
PANETH AND STEM CELL NETWORK
The small intestinal epithelium renews within 3-6 d. The extraordinary rate of cell renewal is driven by a vigorous proliferation within crypts and a highly dynamic movement of epithelial columns toward the villus tip. The intestinal epithelia descend from a distinct stem cell zone located in small intestinal crypts. The zone consists of Paneth cells and 4 to 6 independent intestinal adult stem cells adjacent to rapidly cycling progenitors in the upper part of intestinal crypts.
The stem zone model is orientated on the morphological finding of crypt base columnar cells (CBC cells). These undifferentiated cycling cells are intermingled with Paneth cells and are hierarchically followed by Mix cells located directly above the Paneth cells[5-7]. Mix cells are assumed to be strongly amplifying precursors of the different epithelial cell lines including Paneth cells.
In contrast to the stem cell zone model, a +4 position model has been suggested[8]. The model was substantiated by the finding that severe radiation sensitivity exists in the +4 position[9]. In this location, active cell cycling is found and radiation sensitivity indicates sufficient protection of the stem cell compartment from genetic damage. In the proposed model, injured +4 position stem cells are replaced by earlier generations of transit amplifying (TA) cells with a better repair capacity and asymmetric segregation of old and new DNA strands[10]. Some parallels exist between the both models including definition of a slow and a rapid cycling cell type and an assisting role of Paneth cells in maintaining stem cell behaviour.
Maturing Paneth cells migrate downward into small intestinal crypts, where they reside for 3-6 wk[11]. Paneth cells escape from the crypt bottom by cellular fragmentation and phagocytosis from lamina propria mucosae infiltrating macrophages. There is experimental evidence that Wnt signalling and the expression of Wnt target genes are essential in the configuration and function of the stem cell zone including establishment of rapidly cycling TA cells[4,12-14]. In a current model, an increasing gradient of Wnt activity directed into the small intestinal crypt is proposed reflecting the governing action of adjacent mesenchymal cells that release Wnt proteins. At the base of crypts, β-catenin is enriched in the nuclei of progenitor cells implying a strong response to Wnt signalling. The Wnt gradient is crucial for a graded expression of EphB2 and EphB3 acting as cell-sorting receptors along the CVA[15]. In addition, graded Wnt activity is essential in the differentiation of Paneth cells with accumulation of large granules in the cytoplasm. Terminal differentiation of Paneth cells exists at the crypt bottom, where Wnt activity is maximised and several Wnt target genes are expressed[16]. It has to be stressed that the expression pattern of Wnt target genes, especially Lgr5/Gpr49, is essential in the establishment of the small intestinal crypt stem cell zone including CBC cells interspersed between the maturing Paneth cells[10]. Recently, intestinal organoids were used to visualize the short-range Wnt gradient in the intestinal stem cell niche[17].
Paneth cells are important players in the intestinal stem cell niche, but they are identified as nonessential constituents. In a mouse model deficient in Math1 (Atoh1), which totally and permanently lacks Paneth cells, Lgr5+ CBCs occupied the full crypt base, showed strong proliferation, and generated differentiated progeny over months[18]. The increased proliferation along the CVA was associated with an increase in crypt height in the duodenum (13%) and ileum (15%). Enhanced or unaffected levels of Wnt target transcripts of genes associated with cell proliferation such as CD44, Myc, and Ccnd1 were found. Using a different strategy of intestinal Math1 deficiency, functional intestinal stem cells were found after Paneth cell ablation[19]. In this study, ex vivo and in vivo experiments were performed to clarify the role of mesenchyme in the establishment of the stem cell niche. In the ex vivo setting, Math1-deficient crypt cells were not able to self-renew or survive indicating for extraepithelial, mesenchymal signals necessary for stem cell activities. Using caspase-8 deleted mesenchyme-free organoids, differentiation of Paneth cells was found[20].
The Wnt dependency of Paneth cell differentiation is modified by other signalling cascades including the hierarchical Notch. The expression of Notch receptors is found in CBCs, and Paneth cells express the corresponding ligands[21]. Consequently, Paneth cells are postulated as a source of Notch signalling to maintain CBC functions and crypt activities. It is suggested that some CBC functions unrelated to secretory metaplasia may require a Paneth cell source of Notch signalling.
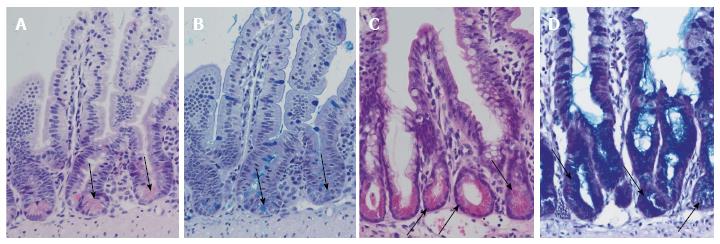
Inhibition of Notch by pharmacological inactivation of the γ-secretase, an enzyme that is involved in Notch signalling, is already established. In dibenzazepine (DBZ) - treated wild-type black-6 mice, a significant increase in secretory differentiated cells located throughout small intestinal crypts is found. After DBZ treatment, small intestinal crypts are lined by orthotopic Paneth cells and transdifferentiated cells with morphological features mixed from Paneth cells and mucus-retaining cells (Figure 2). The change in secretory differentiation is called secretory metaplasia.
Figure 2 Differentiation of Paneth cells and Notch inhibition.
Normal ileal mouse mucosa with normal crypts and Paneth cells: HE staining (A) and alcian-PAS staining (B). Arrows indicate Paneth cells. Ileal mouse tissues after treatment with dibenzazepine show an increase in secretory cells in the crypts with differentiation of Paneth cell-like epithelia (arrows): HE staining (C) and alcian-PAS staining (D).
PANETH CELL METAPLASIA
In distinct intestinal disorders, the physiology of Paneth cells is disturbed. In general, true injuries/diseases of Paneth cells should be separated from such intestinal disorders, where differentiation of Paneth cells is necessary to compensate cellular stress or misdirected mucosal differentiation/tissue homeostasis. The important histomorphological findings include loss, transdifferentiation or metaplasia of Paneth cells.
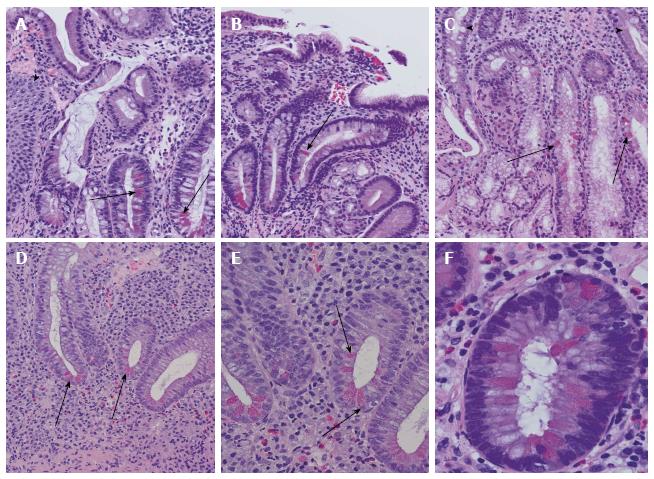
Metaplasia is defined as the occurrence of differentiated cells in a histomorphological location, where they are physiologically not found. Paneth cell metaplasia is seen throughout the gastro-intestinal tract, but frequently manifests in the stomach and is associated with different intestinal injuries (Figure 3). In addition, Paneth cell metaplasia is found in extra-gastro-intestinal sites, like the lung and tracheobronchial system, pancreatico-biliary tract, and the uro-genital tract, but is a rarity in the nasopharyngeal system. It has been postulated that Notch may be involved in forms of pre-cancerous, metaplastic conditions in the stomach, which typically results from a loss of parietal cells and chronic inflammation. The expression of Math1, which is physiologically not found in normal gastric mucosa, has been shown to be upregulated in intestinal metaplasia of the stomach[22,23].
Figure 3 Examples of Paneth cell metaplasia throughout the intestinal tract.
A: Paneth cell metaplasia (arrows) in Barrett mucosa. Squamous epithelia of the oesophagus are marked with an arrowhead; B: Chronic atrophic gastritis with Paneth cell metaplasia (arrow); C: Paneth cell metaplasia (arrows) of Brunner’s gland. In the upper part, small intestinal mucosa and secretory ducts are shown (arrowheads); D: Colon mucosa in ulcerative colitis with disturbed crypt architecture, increased numbers of stroma infiltrating inflammatory cells, and Paneth cell metaplasia (arrowheads); E: Higher magnification of ulcerative colitis-associated Paneth cell metaplasia (arrows) in colon mucosa as demonstrated in (D); F: Paneth cell metaplasia (arrows) in tubular adenoma of the colon with low-grade dysplasia.
Paneth cell loss is a hallmark of special types of Crohn’s disease and is also found in acute inflammation like graft vs host disease (GvHD) grade II and III and ischemic tissue damage[20,24]. The phenomenon of transdifferentiation, whereby Paneth cells are replaced by lysozyme-producing mucus cells, is described in inflammatory small intestinal disorders and in celiac disease. There is some phenomenological evidence that Paneth cell transdifferentiation could be due to re-programmed stem cells[25].
At present, it is well established that the molecular framework underlying the process of regular Paneth cell differentiation and maturation strongly depends on Wnt activity[16]. In contrast to the crypt physiology, the role of Wnt signalling in the development of Paneth cell metaplasia is only marginally elucidated. However, there is some evidence of Wnt activity as a driving force in metaplasia. In an experimental study with the constitutive expression of a β-catenin-Lef1 fusion protein under control of a lung-endoderm-specific promoter from the surfactant protein C gene transgenic lungs included cells expressing marker genes strongly characteristic of intestinal Paneth cells[26]. The data strongly supports the view that hyperactive Wnt signalling could be crucial in stem cell lineage commitment and the generation of intestinal metaplasia. In the lung study, where lysozyme was used as a marker protein of mature Paneth cells, increased Wnt activity was associated with diminished lysozyme expression in metaplastic cells[26]. This interesting finding is highly suggestive that Wnt activity is an important prerequisite for Paneth cell metaplasia, but additional factors and supportive signalling mechanisms are necessary. The auxiliary components necessary for Paneth cell differentiation and maturation probably differs between tissues. For example, inflammation seems to induce Paneth cell metaplasia/hyperplasia in the colon, but not in the small intestine[27]. The intestinal microbiome is suggested as an important variable in signalling activities and the expression level of several intestinal pathways and regulates phenomena including inflammation and cellular differentiation.
PANETH CELLS AND ANTIMICROBIAL PEPTIDES
In the intestine, a plethora of microbes is found including autochthonous long-term colonisers and allochthonous transient microorganisms. One important function of commensal microbiota is host defence through the promotion of the mucosal immune system and inhibition of pathological microbes. Another function is the nutrition of the host, because the bacteria have the capacity to ferment components of the diet. The synthesised short-chain fatty acids (SCFAs), vitamins, and amino acids are essentials in organising host physiology and energy balance. SCFAs act as signalling molecules and highly specialised free fatty acid receptors (FFARs) exist in the intestinal epithelium. FFARs contributes to the chemosensory intestinal system and differ in their molecular structure, ligand specificity, and functional properties[28]. There is evidence that SCFAs are able to induce glucagon-like peptide-1 release from intestinal cells. The molecular link is important to display the integral role of the intestinal microbiome in the regulation host’s energy homeostasis. Metabolic disorders and obesity are associated with changes in the gut microbiota[29].
In the intestine, a complex molecular system is established to maintain microbiome - host homeostasis and to shape the composition of microbes colonising the intestine. For this purpose, Paneth cells sufficiently express and secrete different types of antimicrobial peptides (AMPs) which are important host-defence substances in the communication between host and microbiome. The secretory capacity of Paneth cells is reflected by the cytoarchitecture with apical clustering of large secretory granules. Ultrastructurally, the endoplasmic reticulum (ER) is hyperplastic and associated with a well-developed Golgi network. Upon prosecretory stimuli like bacterially derived Toll-like receptor ligands the cytosolic calcium content increases via KCa3.1, a calcium-activated potassium channel, and leads to granule secretion[30-33].
Defensins are the major AMP family and contain six cysteines involved in intramolecular disulphide bonds[34,35]. Two major subfamilies of defensins exist. The α-defensins are strongly synthesised by Paneth cells and neutrophils, whereas β-defensins with constitutive HBD-1 and inducible HBD-2 are found in different types of cells including enterocytes[36]. In mice, α-defensins are named cryptdins or cryptidins which consist of six major isoforms. Among those isoforms, variant 4 is the most microbicidal peptide[37]. In addition to α-defensins, Paneth cell granules contain lysozyme as another potent host-defence molecule. The biosynthesis of active α-defensins depends on proteolytic processing. In murine Paneth cells, matrix metalloprotease 7 (MMP7) is found cleaving defensin precursor molecules to active α-defensins[38,39]. Disturbed proteolytic activity as found in MMP7 knockout mice is associated with a hampered intestinal microbiome and an increased distribution of bacterial pathogens[40]. In contrast to mice, trypsin is found in human Paneth cells as an activating protease for prodefensins and the antimicrobial protein Reg3A[41]. The human α-defensins type 5 (HD5) and 6 (HD6) are essential in host protection from intestinal pathogens. Using a HD5 transgenic mouse model with physiological defensin levels, animals were resistant to enteric Salmonella infections due to reduced viability of bacteria, diminished bacterial translocation, and increased survival after lethal Salmonella infections[42]. In the same model, evidence of a direct role of α-defensins in regulating and shaping the small intestinal microbiome was shown[40]. In the intestine of HD5 transgenic animals the number of commensals was changed with an increase in Bacteroides, a decrease in Firmicutes, and the loss of segmented filamentous bacteria (SFB), designated Candidatus arthromitus, accompanied by an absence of Th17 cell differentiation. SFB are host specific intestinal symbionts belonging to the Clostridiaceae displaying differentiation of filaments that interact intimately with the surface lining intestinal epithelia. Secreted proteins are expressed by SFB including different ADP-ribosyltransferases and a myosin-cross-reactive antigen, all probably involved in modifying the host response and post-natal maturation of the gut immune system[43,44]. Recently, an SFB-host cell co-culturing system was established producing viable infectious particles, which can colonise intestinal mucosa with the induction of an immune response[45]. Regional variation in the expression and secretion of Paneth cell AMPs along the intestinal tract are well-balanced by the colonising activity of SFB with the establishment and shaping the intestinal microbiome[46].
The α-defensin HD6 secreted by human Paneth cells acts in a way that is completely different to HD5. The HD6 is antibacterial by the configuration of self-assembled peptide nanonets and formation of nanofibrils[47,48]. Targeted bacteria surrounded by the fibrils and nanonets are not able to invade the intestinal mucosa. The HD6 self-assembling function is essentially based on the presence of histidine-27, which forms a salt bridge necessary for multimerising the peptide. The important Paneth cell produced α-defensins HD5 and HD6 can be further classified by their unique functions. The antimicrobial HD5 activity with disruption of bacterial membranes is called “harpoon” activity, whereas HD6 represents so-called “net forming” activities[49]. In summary, HD5 acts antimicrobially and exhibits lectin-like properties, whereas HD6 entraps microbes and blocks host cell invasion[50,51].
In addition to defensins, Paneth cells are able to secrete other AMPs including lysozyme, secretory phospholipase A2 (sPLA2), RegIII, angiogenin 4, and cathelicidins[52-54]. Among the AMPs, RegIII proteins are important in antibacterial defence, and murine RegIIIγshares 65% identity with human RegIIIα. RegIII proteins belong to the family of C-type lectin regenerating islet-derived proteins and bind glycan chains of peptidoglycans on the cell wall of gram-positive bacteria. In contrast to other C-type lectin AMPs as mannose-binding lectin, the complement recruitment domains are not constantly expressed in RegIII suggesting a direct anti-bactericidal function[55].
The AMP LL-37 belongs to the cathelicidin family and acts in a similar way as HD5 by puncturing holes in microbial membranes. LL-37 mediates antimicrobial activities in addition to immunological functions via various cellular receptors[56]. LL-37 is an inducible AMP, because LL-37 expression increases in the presence of SCFAs. Consequently, the protein is predominantly found in the transit-amplifying zone in colonic crypts, when compared with small intestinal mucosa[57]. In quantitative terms, the ratio of HD5 to HD6 is about 3:1, whereas HD5 expression levels are higher by a factor of up to 100 than those of lysozyme and sPLA2[4].
Paneth cells are secretory cells with the excretion of a plethora of molecules including several types of AMPs[50,52-54]. The secretory function depends on a well-adapted ER, which is controlled and smoothed by autophagy. Disruption of autophagy by the deletion of the unfolded protein response transcription factor X-box binding protein-1 results in ER stress, Paneth cell impairment, and spontaneous inflammation resembling special variants of Crohn’s disease[58]. There is experimental evidence that lipids and lipid metabolising enzymes are involved in the signalling cascade for exocytosis of granules from Paneth cells[59]. Expression profiling revealed a panel of target genes and related proteins including lipoprotein lipase, apolipoprotein A-IV, stearoyl-CoA desaturase-1, adiponectin, and leptin. Mapped pathways include PPAR signalling, statin pathway, adipocytokine signalling, and polyunsaturated fatty acid biosynthesis. It has been speculated that the lipid-associated exocytosis is an additive mechanism to the chemosensory system of FFARs and enteroendocrine cells to perform synergisms between the intestinal microbiome and host metabolism.
The release of AMPs from Paneth cells into the intestinal mucus and lumen is initiated by stimulation of pattern recognition receptors (PRRs) located on intestinal surfaces. The class of PRRs includes Toll-like receptors (TLRs) activated by lipopolysaccharide and nucleotide-binding oligomerisation domain-containing molecules (NODs), which recognise muramyldipeptide. Independently of PPRs, bacterial cell wall glycolipids are able to stimulate the release of defensins by Paneth cells. The myeloid differentiation primary response gene (MyD88) acts as an important cytoplasmic TLR limiting bacterial penetration into the intestinal mucosa[4].
In summary, Paneth cells are essential for the establishment of a mucus layer enriched with AMPs resting on the surface lining epithelia. The composition of AMPs differs along the intestine, implicating diverse functions in communication with the intestinal microbiome.
PANETH CELLS AND INTESTINAL DISEASES
High numbers of Lamina propria mucosae infiltrating leukocytes with different types of specialised lymphocytes, plasma cells, mast cells, monocytes, and eosinophiles is a very characteristic histomorphological feature of intestinal mucosa. The balanced correspondence of the immune cells with the intestinal microbiome via the surface epithelium is a prerequisite for intestinal homeostasis. In consequence, an inflammatory response with a further increase in leukocytes including neutrophiles is very common in intestinal disorders and is established in the majority of intestinal diseases. There are remarkable differences in the quantity and quality of infiltrating leukocytes between the underlying intestinal disorders. The differences of leukocytes are helpful to classify the basal disease using morphological, immunohistochemical, and functional techniques.
In addition to resting Paneth cells, infiltrating leukocytes are an important transient source for AMPs. They are assisted by metaplastic Paneth cells, found in the large intestine and stomach. In intestinal inflammation, sPLA2, which is physiologically not found in the colon, is expressed by metaplastic Paneth cells[60,61]. There are experimental data demonstrating a direct role of the microbiome in regulating the defensin production and secretion. Using Nod2 knockout mice in co-housing experiments, the wild-type microbiome was able to regulate defensin secretion to physiological levels[62]. The inflammation-related expression profile of AMPs includes some characteristics indicating a defined intestinal disease[63,64]. For example, β-defensin types 2, 3, and 4 are increased in ulcerative colitis but not in Crohn’s disease[65]. In summary Nod2 is highly expressed in ileal Paneth cells that essentially contribute to the regulation of ileal microbiota through the secretion of AMPs[66].
As outlined above, Paneth cells are the main source of AMPs and acts in stabilising the stem cell zone. Consequently, Paneth cells are investigated as a site of origin for intestinal inflammation. This point of view seems of high relevance concerning the pathogenesis of inflammatory bowel diseases (IBD). A fundamental feature of ileal Crohn’s disease is a reduced expression of HD5 and HD6. The finding is sometimes paralleled by a reduced Paneth cell number, but may be also a result of an injured microbiome[4,36,67].
An in-detail analysis of Paneth cells in Crohn’s disease revealed subgroups of the disease characterised by unique molecular, morphological, and clinical features. The clinical phenotype of ileal Crohn’s disease is especially associated with Paneth cell injuries. There was first evidence from loss-of-function mutations in NOD2 (SNP 13), a gene that is strongly expressed by Paneth cells[66,68-70]. Subsequently, the molecular mechanisms underlying several injuries of Paneth cells were described and linked to (ileal) Crohn’s disease. One important finding was low Wnt signalling activity in ileal Crohn’s disease with diminished Tcf-4 and reduced secretion of defensins[71,72]. Another milestone was the identification of defective granule exocytosis from Paneth cells with diminished levels of defensins due to abnormal autophagy in homozygosity for the risk allele autophagy related 16-like 1[59,73]. The molecular characterisation of disturbed autophagy and unresolved ER stress due to genetically and environmentally controlled dysfunction of unfolded protein response (UPR) was the breakthrough in understanding the underlying mechanisms for the threshold for the development of (ileal) Crohn’s disease[58,59,74].
In addition to Paneth cells, enterocytes are an additional source to produce AMPs including Reg proteins, defensins, and cathelicidins[50,52-54]. This fact is of high relevance to physiologically compensate the perinatal period prior to the establishment of Paneth cells and to pathologically balance Paneth cell disorders[75]. Paneth cells are found in the prenatal small intestine, but the number of cells is highly variable. In view of this observation, insufficient Paneth cell maturation is in discussion as an important variable in the pathogenesis of necrotising enterocolitis. The multifactorial disease is preferentially found in premature infants. Clinically, a sudden onset and high mortality in babies born after 35 weeks’ gestation are characteristic.
At present, two different pathophysiological models for the development of necrotising enterocolitis are in discussion; the top-down and the bottom-up hypothesis[76]. In the top-down scenario, bacterial invasion is found in the villus that triggers thrombosis of small arteries via activation of platelet-activating factor (PAF). In contrast to the top-down scenario, bacterial translocation at the crypt basis with consecutive activation of Paneth cells and arterial thrombosis are the key points in the bottom-up model. In very preterm neonates, the time period between birth and onset of the necrotising enterocolitis is frequently long. In this time maturation of the intestinal immune system is found, a pre-requisite for the initiation and development of enterocolitis[77]. In animal models, toxic Paneth cell ablation and gastral infection with Klebsiella pneumoniae is able to induce necrotising enterocolitis in a bottom-up scenario[78]. The variable number of Paneth cells found in human necrotising enterocolitis is discussed as enterocolitis associated necrobiosis and does not contradict the bottom-up model[79,80]. It has to be stressed that the immense damage to intestinal tissues in necrotising enterocolitis is not plausible from the thrombosis of small arteries. Therefore, a two-hit model has been proposed including at first ischemic damage of the intestinal mucosa. In a second step, reperfusion is found with uncontrolled activation of signalling cascades and enzymes aggravating the tissue damage[81].
Barrett mucosa is frequently found in the oesophageal junction and is due to chronic gastroesophageal reflux. Histomorphologically, the epithelial cells display intestinal differentiation with goblet cells and aberrant mucus retention[82]. In contrast to complete intestinal metaplasia, brush border and Paneth cells are not commonly found in Barrett mucosa. However, there is evidence of Paneth cell metaplasia in Barrett oesophagus (Figure 3A). The phenomenon is found in about 30% of the cases[83]. The frequency of Paneth cells in Barrett mucosa is inversely correlated to the development of epithelial dysplasia. In long-segment Barrett oesophagus, the metaplasia is more frequent than in short-segment lesions. The persistence of Barrett mucosa when Paneth cell metaplasia is found could be due to a dys-balanced Notch and Wnt signalling[84,85]. There is no evidence that Paneth cell metaplasia is associated with the progression of Barrett mucosa to dysplasia and adenocarcinoma[83].
In GvHD the intestinal tract is frequently affected and Paneth cell loss is a hallmark of the acute inflammation associated with changes in the composition of the microbiome[86,87]. Due to the loss of Paneth cells a striking disease of α-defensins is found favouring a shift from commensal to pathologic microbiota[88]. The dysbiotic shift is assumed to be crucial for severe septic complications in GvHD. In particular, the diminished number of Paneth cells correlates with higher disease severity and poor treatment response in patients suffering from GvHD[89]. Enteroendocrine cells have been recently discovered as sensors of the intestinal microbiome. The cells are involved in the pathogenesis of intestinal disorders and express specific receptors which can respond to bacterial products[90]. In germ-free mice, the number of enteroendocrine cells is drastically reduced[91], whereas an increase in microbiome metabolites such as in GvHD is inductive for secretion of immunomodulatory enteroendocrine hormone peptides[92]. The inductive mechanisms could be a pathophysiological link for hyperplasia of enteroendocrine cells in GvHD.
PANETH CELLS AND MORPHOGENESIS
Crypt fission, the division of a single crypt into two daughters, is fundamental in intestinal tissue expansion and morphogenesis, but is also found in tumourigenesis driving the clonal expansion of mutant adenomatous crypts[93-96]. A specific cellular arrangement in the intestinal stem cell niche is observed that controls crypt fission. In a recent model, Paneth cells and CBCs (Lgr5+) are both importantly involved in crypt morphogenesis[97]. The findings summarise the data from intestinal organoids, where Paneth cells are essentially involved in crypt budding[98,99], and as mice lacking intestinal Paneth cells, which are able to sufficiently repair crypt injuries[21,100]. The morphogenic activities of Paneth cells depend on Wnt signalling and redundant sources contribute[101,102]. Important for the crypt fission model is the observation that Paneth cells adhere to their substrate more strongly than other crypt cells. In view with this strong evidence is given that so-called “mislocalised” Paneth cells change the symmetry of fission in determining the site of fission[97].
CONCLUSION
Paneth cells are important secretory cells in the small intestinal mucosa. They are found as metaplastic cells in several other locations. Important secretory products of Paneth cells belong to the AMPs. They are essential for the dialogue between the microbiome and the host. In addition to Paneth cell functions in controlling the microbiome, the cells are functionally and structurally involved in forming the stem cell zone of small intestinal crypts and involved in morphogenesis of the CVA. In the last decade, molecular mechanisms of different Paneth cell injuries have been identified and correlated with intestinal diseases. There is evidence from these experiments that ileal Crohn’s disease as well as necrotising enterocolitis are strongly associated with diseased Paneth cells. The important role of Paneth cells in intestinal physiology and pathophysiology strongly supports the view that these cells are the guardian of small intestinal crypts.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Germany
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Hokama A, Wejman J, Xu WX S- Editor: Kong JX L- Editor: A E- Editor: Lu YJ