Published online Aug 15, 2017. doi: 10.4291/wjgp.v8.i3.127
Peer-review started: December 20, 2016
First decision: March 6, 2017
Revised: March 31, 2017
Accepted: May 18, 2017
Article in press: May 19, 2017
Published online: August 15, 2017
Processing time: 240 Days and 3.3 Hours
To assess the use of serum levels of angiopoietin-1 (Ang1), Ang2 and tumor necrosis factor-α (TNFα) as predictive factors for small bowel angiodysplasia (SBA).
Serum samples were collected from patients undergoing capsule endoscopy for any cause of obscure gastrointestinal bleeding (OGIB) or anaemia. Based on small bowel findings patients were divided into 3 groups: (1) SBA; (2) other bleeding causes; and (3) normal, according to diagnosis. Using ELISA technique we measured serum levels of Ang1, Ang2 and TNFα and compared mean and median levels between the groups based on small bowel diagnosis. Using receiver operator curve analysis we determined whether any of the factors were predictive of SBA.
Serum samples were collected from a total of 120 patients undergoing capsule endoscopy for OGIB or anaemia: 40 with SBA, 40 with other causes of small bowel bleeding, and 40 with normal small bowel findings. Mean and median serum levels were measured and compared between groups; patients with SBA had significantly higher median serum levels of Ang2 (3759 pg/mL) compared to both other groups, with no significant differences in levels of Ang1 or TNFα based on diagnosis. There were no differences in Ang2 levels between the other bleeding causes (2261 pg/mL) and normal (2620 pg/mL) groups. Using Receiver Operator Curve analysis, an Ang2 level of > 2600 pg/mL was found to be predictive of SBA, with an area under the curve of 0.7. Neither Ang1 or TNFα were useful as predictive markers.
Elevations in serum Ang2 are specific for SBA and not driven by other causes of bleeding and anaemia. Further work will determine whether Ang2 is useful as a diagnostic or prognostic marker for SBA.
Core tip: Small bowel angiodysplasia (SBA) is an important cause of obscure gastrointestinal bleeding and anaemia but can be difficult to diagnose. This paper assesses the use of novel serum angiogenic factors associated with SBA as potential diagnostic aids. The study has identified a cut-off serum level of Ang2 of 2600 pg/mL which may be useful in predicting patients with the condition. Further studies will be required to determine its use in clinical practice but it may represent a major advancement in the identification of a diagnostic and prognostic marker for angiodysplasia, and also in determining the underlying pathophysiology of the condition.
- Citation: Holleran G, Hussey M, Smith S, McNamara D. Assessment of serum angiogenic factors as a diagnostic aid for small bowel angiodysplasia in patients with obscure gastrointestinal bleeding and anaemia. World J Gastrointest Pathophysiol 2017; 8(3): 127-132
- URL: https://www.wjgnet.com/2150-5330/full/v8/i3/127.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v8.i3.127
Small bowel angiodysplasia (SBA) accounts for over 50% of cases of obscure gastrointestinal bleeding (OGIB) and iron deficiency anaemia (IDA)[1]. It is more common in elderly patients and in those with multiple comorbidities including chronic kidney disease (CKD), cardiovascular disease and chronic obstructive pulmonary disease (COPD)[2]. The clinical presentation of SBA varies from those who remain asymptomatic or with mild IDA only, to those with acute life threatening overt haemorrhages, with a proportion of patients developing a more chronic and refractory form of SBA, suffering from recurrent bleeding episodes[3-5].
The majority of SBAs are diagnosed by visualisation of characteristic mucosal vascular lesions by small bowel capsule endoscopy (SBCE) or device assisted enteroscopy (DAE). One of the difficulties in both diagnosing and following up patients with SBA is that almost half of patients do not notice any overt gastrointestinal bleeding, with their condition deteriorating silently until they become symptomatic of their anaemia[6,7]. For elderly patients with multiple co-morbidities this can lead to cumulative episodes of cardiac and respiratory decompensation, requiring regular hospital admissions and a resultant poor quality of life. An early and timely diagnosis would allow clinicians the opportunity to arrange more vigilant follow up, and to prevent progression to severe anaemia and subsequent pressure on other organs.
SBCE is the most sensitive diagnostic tool for SBA, however there are limitations to its use. Firstly, although SBAs can bleed significantly, when not bleeding they are generally < 8 mm in size and can be difficult to detect depending on their location, peristaltic activity or intraluminal contents at the time of passage of the capsule, leading to the potential for false negative studies. Secondly, we are as yet unsure as to the significance of the detection of non-bleeding SBAs by SBCE. Not all SBAs will bleed, however it has been shown that once they do bleed, the likelihood of repeated bleeding is significantly increased[8]. In addition, SBCE is generally used to detect SBAs and guide treatment via argon plasma coagulation using DAE. However there are high rates of re-bleeding from previously treated lesions, along with sporadic growth of de novo lesions[9,10]. This means that patients are often dependent on multiple repeat SBCEs on each bleeding episode to direct further treatment, with a resultant economic burden, increase in waiting lists for the procedure and a delay in diagnosis and treatment for patients with SBA. As there are no reliable or useful clinical predictors of SBA activity, it would be helpful if there were some other form of non-invasive marker of disease in order to guide management and predict prognosis for patients.
We have previously published our findings identifying abnormalities in serum angiogenic factors in patients with SBA, specifically the detection of higher levels of angiopoietin-2 (Ang2), and lower levels of angiopoietin-1 (Ang1) and Tumour Necrosis factor α (TNFα) in patients with SBA compared to non-bleeding controls[11]. If specific for SBA, these factors could potentially be used as a diagnostic aid for diagnosis and management of SBA and reduce our dependence on SBCE.
This study aims to determine whether previously identified abnormalities in serum levels of angiogenic factors are specific for SBA or induced by OGIB or anaemia and assess whether serum levels of these factors could be used to predict a diagnosis of SBA in a cohort of patients with OGIB or IDA prior to SBCE.
Ethical approval was obtained from our institutions research and ethics committee and any patient over the age of 18 years who was undergoing a SBCE for either IDA or OGIB was invited to participate. IDA was defined as haemoglobin (Hb) of < 11.5 g/dL in females and < 13 g/dL in males along with a serum ferritin of < 14. OGIB was defined as the presence of overt or occult (faecal occult blood positivity), and all patients had previously undergone at least one upper and lower endoscopy which had failed to identify a source of their anaemia or bleeding. On the day of their SBCE approximately 10 mL of blood was drawn from participants via standard phlebotomy technique. Plasma samples were analysed routinely for Hb level and serum samples were left to clot for 30 min before undergoing centrifugation for 15 min at 1000 rpm. The resultant supernatant was extracted and stored in aliquots at -80 °C for batch analysis.
SBCE was carried out in a routine fashion as previously reported and videos were reported by gastroenterologists trained in SBCE[12]. Based on the report of their SBCE patients were divided into 3 groups: (1) SBA; (2) any other cause of bleeding; and (3) normal. A diagnosis of SBA was made using the classification published by Saurin et al with only definite or P2 lesions being included in the study. Other causes of bleeding included potential malignancies/polyps, any form of enteritis or active bleeding which was not determined to have been from and SBA on subsequent investigations. Recruitment continued until serum samples had been stored on a minimum of 40 patients in each group.
Serum levels of Ang1, Ang2 and TNFα were measured using commercially available solid-phase sandwich enzyme-linked immunosorbent assay (ELISA) kits (R and D systems, Minneapolis, MN, United States). Samples were prepared in duplicate and results were read at 450 nm absorbance. The intra-assay coefficients of variation (CV) were calculated as an average of all of the individual CVs for the sample concentration duplicates analysed by ELISA.
Results of all assays and patient demographics were expressed as a mean and/or median and compared between groups using the Student t-test, Mann-Whitney U test, univariate/multivariate logistic regression analysis or a relative risk (RR) ratio as appropriate. All analyses were performed using SPSS version 22 (SPSS Inc., Chicago, IL, United States). The potential for the use of serum Ang1, Ang2 or a ratio of Ang1/Ang2 as a diagnostic marker or SBCE screening tool for SBA was explored using receiver operator characteristic (ROC) curve analysis.
Of the group overall (n = 120), 58% (n = 70) were male, with a mean age of 63 years (18-93). Patients in the normal group (n = 40) were significantly younger than both the abnormal (n = 40) and angiodysplasia group (n = 40) with a mean age of 55 years (18-81) vs 60 years (19-92) and 73 years (53-93) respectively. There was no difference in gender between the groups with 42% (n = 17), 67% (n = 27), and 65% (n = 26) being male in each group respectively. The specific findings in the abnormal group included; non-specific enteritis n = 20, active bleeding n = 6, polyp/possible malignancy n = 5, denuded coeliac mucosa n = 2, Meckel’s diverticulum n = 2, dieulafoy lesions n = 2, non-specific mucosal erythema not consistent with SBA n = 3.
However there were significant differences in Hb levels with patients with normal findings having a higher mean Hb level at the time of SBCE 12.0 g/dL in females and 14.0 g/dL in males) compared to both the abnormal (11.3 g/dL in females and 12.9 g/dL in males) and Angiodysplasia (11.0 g/dL in females and 11.3 g/dL in males) groups P values both < 0.02. There was no difference between Hb levels in the abnormal and Angiodysplasia groups, P = 0.832.
Ang1 and Ang2 and TNFα levels
The median serum levels, ranges and P values compared between the groups is shown in Table 1. Patients with SBA have significantly higher serum levels of Ang2 than patients with other causes of bleeding or anaemia. When Ang2 levels were controlled for anaemia across the groups it was not found to be a confounding factor. Although there was a trend towards lower levels of both Ang1 and TNFα, these were not found to be statistically significant. There were no differences in any of the factors when compared between the other cause of bleeding and normal groups. The ratio of Ang1/Ang2 was found to be significantly lower in the SBA group compared to the other groups and again there was no difference in the ratios between the other bleeding cause and the normal groups.
| SBA (n = 40) | Abnormal (n = 40) | Normal (n = 40) | |
| Ang1 (pg/mL) | |||
| Median (range) | 40976 (974-97511) | 44770 (2660-101930) | 47639 (17899-95173) |
| P value vs SBA | 0.33 | 0.04 | |
| Ang2 (pg/mL) | |||
| Median (range) | 3759 (1915-14731) | 2261 (842-14000) | 2620 (686-8850) |
| P value vs SBA | < 0.004 | < 0.003 | |
| Ratio of Ang1/Ang2 | |||
| Median | 11.4 | 20.2 | 19 |
| P value vs SBA | < 0.006 | < 0.001 | |
| TNFα (pg/mL) | |||
| Median (range) | 5.76 (0.35-40) | 9.76 (0.46-58) | 10.14 (0.35-38) |
| P value vs SBA | 0.12 | 0.13 |
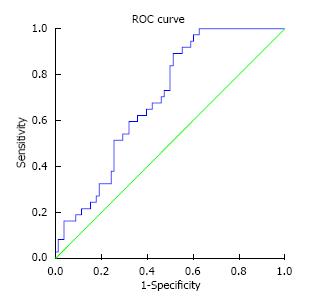
We went on to assess the potential use of serum Ang2 as a diagnostic tool for SBA by using ROC curve analysis as demonstrated in Figure 1. This established a cutoff serum Ang2 level of 2600 pg/mL with an area under the curve (AUC) of 0.695, (standard error 0.048, 95%CI: 0.601-0.789, P = 0.001). Table 2 outlines the sensitivity, specificity, and predictive values of this cut-off level. Using the RR model, a serum level of 2600 pg/mL had a RR of SBA of 2.22 (P = 0.012, 95%CI: 1.20-4.11). Although the positive predictive value for SBA was only 45%, 53% of the false positive patients did clinically significant findings (including 2 potential malignancies, 2 Meckel’s diverticulae and an actively bleeding Dieulafoy’s lesion) all of which would warrant urgent further investigation, making the true false positive rate for clinically significant findings only 26%.
| True positive n = 33 | False positive n = 40 | Sensitivity = 85% |
| Specificity = 50% | ||
| False negative n = 7 | False negative n = 40 | Positive predictive value = 45% |
| Negative predictive value = 85% |
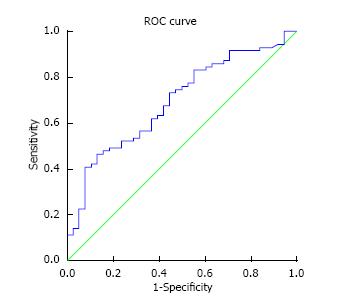
ROC curve analysis was also performed using the Ang1/Ang2 ratio which gave an area under the curve of 0.692, (standard error 0.052, 95%CI: 0.51-0.793, P = 0.001) (Figure 2). As the AUC was slightly lower than that for Ang2 alone curve (AUC 0.695 vs 0.692 respectively) it was felt that no benefit would be conferred by the need for the measurement of two markers and further analysis was not performed.
Due to advances in medical care, particularly cardiovascular and stroke medicine, patients are living longer with a dependency for anticoagulant and antiplatelet therapy, meaning that SBA is increasing in incidence in our aging population. Unfortunately no treatment has yet been shown to prevent re-bleeding from SBAs in the longer term, however there have been suggestions that the earlier diagnosis and intervention of some form of directed treatment prior to respiratory or cardiac decompensation due to anaemia, may improve the longer term outcome for patients.
Although SBCE is the most sensitive diagnostic tool for SBA and is far less invasive than conventional small bowel endoscopy, it is still not widely available in all centres, does carry some risks including false negative studies, and remains a relatively expensive test. Thus the availability of alternative diagnostic and prognostic tools may prove extremely useful in reducing the dependence on SBCE and directing treatment for patients at an earlier stage. As the clinical course of SBA is so unpredictable and patients are often unaware of re-bleeding episodes a non-invasive predictive marker which could be used in addition to and to direct further investigation and treatment would be of great advantage.
Our previously published findings of higher levels of Ang2 and lower levels of Ang1 in serum and tissue levels of patients with SBA offers a credible hypothesis for the pathophysiology of SBA as excess Ang2 has been shown to lead to the development of enlarged and weakened blood vessels, and Ang1 is required to ensure blood vessel stabilisation and maturity. However, one of the main concerns regarding our initial findings was that our comparison of serum factors in patients with SBA vs non-bleeding controls may have meant that the detected abnormalities in Ang levels may have been driven by OGIB or anaemia overall and not were not specific for SBA. One of the main objectives of this study therefore was to determine the specificity of these findings for SBA compared to OGIB and IDA of varied causes. This study has shown that the elevation of serum levels of Ang2 is specific for SBA and is not a response to bleeding or anaemia of any other cause. In contrast, our results showed that abnormalities in serum levels of Ang1 is not specific for SBA and may be related to other factors such as OGIB or anaemia overall. Interestingly however there was still a significant difference in the ratio of Ang1/Ang2 levels between SBA and non-SBA patients.
The second aim of this study was to determine whether measuring serum levels of these factors could be used as a diagnostic or screening tool to predict patients likely to have SBA and to facilitate earlier detection. The difficulty in establishing a diagnostic tool is defining a cut off level with adequate sensitivity and specificity levels to make them clinically useful. Using ROC curve analysis we determined that an Ang2 level of 2600 pg/mL with a sensitivity level of 85% and a negative predictive value of 85%, was the most clinically appropriate cut-off level. Although an AUC of 0.7 is not particularly accurate for a diagnostic tool, the purpose of this screening tool is to identify patients likely to have SBA. Therefore the most important aspects of this tool were the sensitivity and negative predictive value for a diagnosis of SBA. Although the ratio of Ang1/Ang2 differed significantly from other causes of OGIB, we found no diagnostic benefit to measuring both serum factors, making the test more economically feasible if translated to clinical practice. Further validation of the use of Ang2 at this cut-off level in a larger group of patients with OGIB and IDA are needed to ensure its accuracy as a potential predictive tool, however it offers a potentially cheap and non-invasive marker to aid in the diagnosis of SBA.
Although it is unlikely that the measurement of serum angiogenic factors will replace our need for SBCE, particularly as this is generally useful to guide route of approach for subsequent DAE, they are likely to have a role in assisting diagnosis and in follow up. It was beyond the scope of this study to assess the ability of serum angiogenic factors as biomarkers of disease activity as serum was taken from patients at a single point in time and it is likely that the majority were not actively bleeding at the time. An interesting assessment and a necessity in determining whether serum Ang2 levels could be used as a prognostic marker would be to take serial measurements from patients with known SBA and establish whether levels correlate with changes in disease course, around the time of active bleeding, or following a definitive treatment intervention.
A recognised weakness of our study is that the patient’s with a normal SBCE had lower mean Hb levels than the other groups. All patients at the time of referral were anaemic, however due to significant waiting times for SBCE in our unit a significant proportion of these patients may have had a spontaneous recovery of their anaemia by the time they underwent SBCE. This is likely to be the case in clinical practice as patients will receive some empiric treatment with iron or red cell transfusions and future prospective studies may determine whether a Hb level alone could be used as a predictive or prioritisation tool in milder cases of anaemia, with serum Ang2 reserved for currently anaemic patients only. In addition, our study relied on SBCE being the gold standard for diagnosis of small bowel causes of OGIB, however as mentioned earlier the sensitivity of SBCE is not 100% and there is a possibility that some of the patients in the normal will include those with false negative studies which may have impacted Ang2 levels.
In conclusion, this study has validated our previous findings showing an elevated serum Ang2 in patients with SBA. In addition it has shown that this association is specific for SBA and is not driven by bleeding or anaemia of other causes. We have identified the potential use of serum Ang2 as a diagnostic aid in SBA, and developed a basis for further work to examine its use as an indicator of disease activity. In the future these findings may lead to a more timely diagnosis for patients with SBA and provide a more accessible and non-invasive mode of follow up.
Small bowel angiodysplasia (SBA) accounts for over 50% of cases of obscure gastrointestinal bleeding and anaemia. Very little is known about the underlying pathophysiology of the condition which significantly limits improvements in diagnostic aids, prognostic markers and focussed treatments. The authors have previously identified an association between SBA and abnormalities in serum levels of angiopoietin-1 (Ang1), angiopoietin-2 (Ang2) and tumour necrosis factor-α (TNFα). Whether these factors were specifically associated with SBA or were driven by overall gastrointestinal bleeding or anaemia, and whether they could be used as diagnostic aids for the condition were not clear from the authors initial study and required further assessment.
At present diagnosis of SBA is via small bowel endoscopy only in the form of capsule or device assisted endoscopy, both of which have very limited access worldwide. In addition there is currently no specific treatment for angiodysplasia, the development of which is limited by a deficient knowledge f the underlying pathophysiology. The overarching aim of our work is to identify angiogenic factors associated with the condition which may be useful both as diagnostic and prognostic markers and as future treatment targets.
This paper has identified Ang2 as a potential serum diagnostic marker for SBA in patients with obscure gastrointestinal bleeding and anaemia. It may be useful in expediting diagnosis and improving outcome.
Further work will need to be done to validate these findings prior to their use in clinical practice but Ang2 may be useful as a diagnostic aid in patients with obscure gastrointestinal bleeding. It may also be a useful treatment target for anti-angiogenic therapies.
Ang1 and Ang2 are both angiogenic factors known to be involved in blood vessel formation. TNFα is an inflammatory cytokine which also has a role in vessel formation.
This preliminary study is an advance in the understanding of the pathophysiology of angiodysplasias of the small intestine and may be a useful clinical tool to be verified in a larger series of patients.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Ireland
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Vega J S- Editor: Gong ZM L- Editor: A E- Editor: Lu YJ
| 1. | Pasha SF, Hara AK, Leighton JA. Diagnostic evaluation and management of obscure gastrointestinal bleeding: a changing paradigm. Gastroenterol Hepatol (N Y). 2009;5:839-850. [PubMed] |
| 2. | Holleran G, Hall B, Hussey M, McNamara D. Small bowel angiodysplasia and novel disease associations: a cohort study. Scand J Gastroenterol. 2013;48:433-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 3. | Richter JM, Christensen MR, Colditz GA, Nishioka NS. Angiodysplasia. Natural history and efficacy of therapeutic interventions. Dig Dis Sci. 1989;34:1542-1546. [PubMed] |
| 4. | Holleran G, Hall B, Zgaga L, Breslin N, McNamara D. The natural history of small bowel angiodysplasia. Scand J Gastroenterol. 2016;51:393-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 5. | Jackson CS, Gerson LB. Management of gastrointestinal angiodysplastic lesions (GIADs): a systematic review and meta-analysis. Am J Gastroenterol. 2014;109:474-483; quiz 484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 126] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 6. | Igawa A, Oka S, Tanaka S, Kunihara S, Nakano M, Aoyama T, Chayama K. Major predictors and management of small-bowel angioectasia. BMC Gastroenterol. 2015;15:108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 7. | DeBenedet AT, Saini SD, Takami M, Fisher LR. Do clinical characteristics predict the presence of small bowel angioectasias on capsule endoscopy? Dig Dis Sci. 2011;56:1776-1781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Ueno S, Nakase H, Kasahara K, Uza N, Kitamura H, Inoue S, Mikami S, Matsuura M, Chiba T. Clinical features of Japanese patients with colonic angiodysplasia. J Gastroenterol Hepatol. 2008;23:e363-e366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Rahmi G, Samaha E, Vahedi K, Delvaux M, Gay G, Lamouliatte H, Filoche B, Saurin JC, Ponchon T, Rhun ML. Long-term follow-up of patients undergoing capsule and double-balloon enteroscopy for identification and treatment of small-bowel vascular lesions: a prospective, multicenter study. Endoscopy. 2014;46:591-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 10. | May A, Friesing-Sosnik T, Manner H, Pohl J, Ell C. Long-term outcome after argon plasma coagulation of small-bowel lesions using double-balloon enteroscopy in patients with mid-gastrointestinal bleeding. Endoscopy. 2011;43:759-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 11. | Holleran G, Hall B, O’Regan M, Smith S, McNamara D. Expression of Angiogenic Factors in Patients With Sporadic Small Bowel Angiodysplasia. J Clin Gastroenterol. 2015;49:831-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Liao Z, Gao R, Xu C, Li ZS. Indications and detection, completion, and retention rates of small-bowel capsule endoscopy: a systematic review. Gastrointest Endosc. 2010;71:280-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 561] [Cited by in RCA: 475] [Article Influence: 31.7] [Reference Citation Analysis (0)] |